Abstract
Cerebello-thalamo-cortical circuit has been indicated important for tremor in Parkinson’s disease (PD), but the role of dentate nucleus (DN) in parkinsonian tremor remains unclear. To investigate whether DN plays a role in PD tremor, we recruited 50 PD and 29 age-matched health controls (HC). The patients were divided into tremor-dominant (TD) and non-tremor-dominant (NTD) groups. We collected resting-state fMRIs data for each subject. The bilateral DN was then chosen as the region of interest to examine PD tremor-related network changes, as well as its correlation with tremor severity. Voxel-wise functional connectivity analysis revealed that the bilateral DN had higher connectivity with the bilateral cerebellar anterior lobe, and had lower connectivity with the bilateral prefrontal cortex in TD compared to the HC and NTD groups. Functional connectivity of the bilateral DN with the bilateral cerebellar posterior lobe was also higher in TD than NTD group. Functional connectivity between the bilateral DN and the bilateral cerebellar posterior lobe showed positive correlation with tremor severity, while that between the bilateral DN and the bilateral prefrontal cortex displayed negative correlation. Our study demonstrates higher dentato-cerebellar connectivity and lower dentato-prefrontal connectivity in TD patients, which might be involved in the pathogenesis of PD tremor. And we conclude that DN might be associated with the pathogenesis of PD tremor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tremor is one of the cardinal symptoms of Parkinson’s disease (PD). Previous studies report it to be present in up to 80 % of patients with autopsy-proven PD [1]. However, the neural substrate underlying PD tremor remains largely unknown. Increasing evidence indicated the involvement of the cerebello-thalamo-cortical circuit in the pathophysiology of PD tremor [2–5]. The cerebellum, as an important component in the cerebello-thalamo-cortical circuit, has been indicated to be associated with PD tremor in different studies. Hyperactive cerebellum, for instance, was found in PD tremor-related pattern (PDTP) by FDG PET studies [6]. It has been suggested that dysfunction of basal ganglia triggers tremor while disturbance of cerebellum or primary motor cortex (M1) drives tremor by manipulating its amplitude [3, 7, 8]. As a major efferent nucleus in the cerebellum, dentate nucleus (DN) connects with the motor cortex via the ventrolateral nuclei of thalamus [containing the ventral intermediate nucleus (VIM)] and red nuclei of the brainstem, and connects with the subthalamic nucleus (STN) via pons [9]. Deep brain stimulation (DBS) of Vim, STN, or the locations inside or in proximity to the dentato-rubro-thalamic tract has been proven effective in reducing PD tremor [5, 10], which implies the participation of DN in PD tremor.
However, the role of DN in the pathophysiology of PD tremor remains unclear. Resting-state functional magnetic resonance imaging (R-fMRI), a non-invasive and promising imaging technique, can be used to measure not only focal brain activity but also functional connectivity between different brain regions [11, 12], and it has been proven helpful for our understanding of pathophysiology of a disease state. With such application, Liu and colleagues reported that the posterior lobe of cerebellum showed higher connectivity with the bilateral DN in PD compared to healthy subjects, while this connectivity was relatively lower in tremor-dominant subtype of PD (TD) compared to non-tremor-dominant (NTD) subtype [13]. However, this investigation did not report different DN functional connectivity with brain regions in addition to the cerebellum in the TD subtype compared to the NTD subtype. Thus, more studies are needed to further investigate the role of DN in PD tremor-related networks.
In the present study, we used resting-state fMRI to investigate functional connectivity of DN in PD patients with tremor, and explore the correlation between DN functional connectivity and the tremor severity. We hypothesized that the functional connectivity of DN may underlie the pathophysiology of PD tremor.
Methods
Participant
Participants were recruited from Beijing Tiantan Hospital, Capital Medical University. Patients were diagnosed in accordance with the United Kingdom Parkinson’s Disease Society Brain Bank (UKPDBB) criteria [14], by two experienced experts on movement disorders. Exclusion criteria were: (1) diagnosis uncertain for PD or suspicious of parkinsonism syndrome (vascular, drug induced, toxin induced, post-infectious parkinsonism), multiple system atrophy, corticobasal ganglionic degeneration, or progressive supranuclear palsy; (2) a history of stroke, moderate to severe head trauma, hydrocephalus, brain surgery or brain tumor; (3) a contraindication for MRI scan (pacemaker implanted, etc.); (4) inability to cooperate or communicate; (5) Mini-Mental State Exam (MMSE) score ≤24. Health controls were chosen with normal neurological statue and movement function, absence of neurological or psychiatric disease, as well as MMSE >24. All procedures were approved and supervised by the ethics committee of Beijing Tiantan Hospital, in accordance with the Declaration of Helsinki. Informed consent was obtained either from the participants or their closest relatives.
Demographic information, including age at assessment, disease duration, Hoehn and Yahr (H–Y) stage, Unified Parkinson’s Disease Rating Scale (UPDRS) in the off state and levodopa daily dosage, were collected for each patient. In our current study, NTD represented posture instability/gait difficulty (PIGD) subtype. The TD and NTD groups were defined by UPDRS, where the ratio of the mean UPDRS tremor scores [UPDRS item 16, tremor; item 20, rest tremor (face, right upper extremity, left upper extremity, right lower extremity, left lower extremity); item 21, action tremor (right upper extremity, left upper extremity)] to the mean UPDRS PIGD scores (UPDRS item 13, falling; item 14, freezing; item 15, walking; item 29, gait; item 30, postural stability) was calculated. Patients with a ratio >1.5 were classified as TD and ratio <1 as NTD. Additionally, patients who had a positive mean in the numerator and zero in the denominator were classified as TD; patients with a zero in the numerator and a positive mean in the denominator were classified as NTD [15].
Data acquisition
fMRIs were performed on a 3T MR scanner (Trio system; Siemens Magnetom scanner, Erlangen, Germany). High-resolution anatomical images were acquired with 3D-MPRAGE sequence (TR = 2530 ms, TE = 3.39 ms, 128 axial slices, 1.33 mm thickness, FOV = 256 mm). BOLD data were acquired with gradient-echo echo-planar sequences (TR = 2000 ms, TE = 30 ms, 33 axial slices, 3.5 mm thickness, flip angle = 90°, FOV = 220 mm, voxel size = 4 × 4 × 5 mm3). Two hundred and forty volumes were obtained from 79 individuals (25 TD, 25 NTD, 29 HC).
Before scanning, patients were washed off with levodopa, i.e. without taking levodopa drugs for at least 12 h. Each individual was asked to stay static, keep their eyes closed and think of nothing during scanning.
Data preprocessing
fMRIs data were processed with Data Processing Assistant for Resting-State fMRI V2.3 (DPARSF V2.3, http://rfmri.org/dparsf_v2_3) [16]. Data were initially transformed into NIFTI format, and the first 10 volumes were excluded for magnetization stabilization. The remaining images were slice-time corrected, and aligned to the first image of each session for motion correction. Subjects with head motion parameter greater than 3 mm maximum translation in x, y, or z, or 3° of maximum rotation about three axes were excluded. The remaining imaging data were spatially normalized with Montreal Neurological Institute (MNI) template, re-sampled to 3-mm isotropic voxels and smoothed with Gaussian filter of 4 mm of full width at half maximum (FWHM). Resulting data were then detrended and temporally band-pass filtered (0.01–0.08 Hz) to reduce low-frequency drifts and physiological high-frequency respiratory and cardiac noise. For functional connectivity analysis, regression of nuisance covariates derived from the head motion, the white matter signal, the global mean signal, and the cerebrospinal fluid signal was performed.
Each subject’s head motion parameters were examined. Since correlation analysis is sensitive to gross head motion, we further evaluated effect of head motion via the mean framewise displacement (FD) derived with Jenkinson’s relative root mean square (RMS) algorithm, which was initiated by Jenkinson [17] and was further used by other studies [18, 19]. Parameters were averaged for each subject and then compared among groups by ANOVA analysis.
Definition of regions of interest (ROIs)
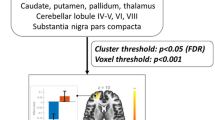
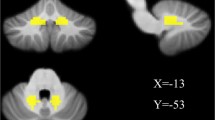
We chose the bilateral DN as ROIs for functional connectivity analysis. The centers of the ROIs were defined as (−16.5, −60, −38) on the left and (17, −59, −38) on the right according to a probabilistic 3D atlas of the human cerebellar DN in terms of MNI coordinate [20]. The diameters of the ROIs were defined as 3.6 mm on the left and 4 mm on the right. According to Dimitrova’s description, such ROI definition could reach a 61–71 % overlap for the bilateral DN of all 63 healthy subjects [20] (Fig. 1).
Functional connectivity analysis
Functional connectivity was analyzed using the REST Toolkit (http://restfmri.net/forum/REST_V1.8). A seed reference time course was obtained by averaging the time courses within each ROI. Correlation analysis was carried out between the seed reference and the whole brain in a voxel-wise manner. The individual correlation coefficients were further transformed to z values using the Fisher r-to-z transformation. One-sample t test with levodopa dosage as a covariate was applied to investigate functional connectivity within each group. The correction threshold was determined as p < 0.05 and cluster size >5400 mm3, which corresponded to a corrected p < 0.05 by Monte Carlo simulations with the program AlphaSim in AFNI (http://afni.nih.gov/afni/docpdf/AlphaSim.pdf). To examine the differences in the DN functional connectivity among the three groups, a one-way analysis of covariance (ANCOVA) was performed in a voxel-wise manner with levodopa daily dosage treated as a covariant factor. The statistical significant level was set at p < 0.05 and cluster size >2160 mm3, which corresponded to a corrected p < 0.05. To determine inter-group differences in DN functional connectivity, a subsequent post hoc analysis with the general linear model (GLM) was further performed (within a mask showing significant group differences in the ANCOVA analysis). The statistical significant level was set at p < 0.05 and cluster size >1080 mm3, which corresponded to a corrected p < 0.02. Finally, we correlated UPDRS tremor score with DN functional connectivity in PD patients (within a mask showing significant group differences in ANCOVA). The statistical significant level was set at p < 0.05 and cluster size >540 mm3, which corresponded to a corrected p < 0.05.
Results
Demographics
Three groups were age and gender matched. Levodopa dosage was significantly lower in TD group (p = 0.006 with Mann–Whitney Test), in keeping with clinical observation that NTD requires larger levodopa dosage to improve motor function. As for other parameters, no significant difference was detected (Table 1).
Head motion
Head motion influences the measurement of the intrinsic functional connectivity. To evaluate the intra-group differences in head motion, we applied mean framewise displacement (FD) Jenkinson measurement and ANOVA analysis. The Jenkinson parameters were 0.118 ± 0.080 (range 0.036–0.381) mm in HC group, 0.093 ± 0.050 (range 0.029–0.195) mm in TD group, and 0.094 ± 0.048 (range 0.034–0.202) mm in NTD group. There was no significant difference in head motion among three groups (p = 0.401). Therefore, TD, NTD and HC groups in the present study were similar in head motion characteristics.
Functional connectivity results within each group
In HC group, the DN positively connected with the bilateral cerebellar anterior and posterior lobes, the bilateral thalamus, the bilateral striatum (putamen and caudate), the bilateral globus pallidus and the brainstem; and negatively connected with the bilateral prefrontal cortex, the bilateral middle occipital lobe and the bilateral inferior parietal lobule (Fig. 2).
Brain regions that functionally connected with the bilateral DN in HC, TD and NTD groups. The statistical significant level was set at p < 0.05 and cluster size >5400 mm3. Cool color represents negative correlation and warm color represents positive correlation. The left side of the image corresponds to the right side of the brain in axial orientation; Slice coordinates according to MNI space are shown in the upper right corner of the slices, indicating Z axis in axial orientation
In TD and NTD groups, DN functional connectivity showed similar pattern with HC group in the bilateral cerebellar anterior and posterior lobes, the bilateral thalamus, the bilateral globus pallidus, the bilateral inferior parietal lobule and the bilateral middle occipital lobe. In addition, DN had scarce functional connectivity with the putamen and caudate in TD and NTD groups (Fig. 2).
ANCOVA analysis for intra-group differences in the DN functional connectivity
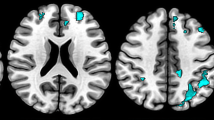
The ANCOVA results revealed that the bilateral DN had different functional connectivity with the bilateral cerebellar anterior and posterior lobes, the bilateral prefrontal cortex, the bilateral caudate nucleus, and the right parieto-occipital area among TD, NTD and HC groups (Fig. 3).
Brain maps for differences in the DN functional connectivity among TD, NTD and HC groups. The statistical threshold was set at p < 0.05 and cluster size >2160 mm3, which corresponds to a corrected p < 0.05. Brain regions in the bilateral cerebellar anterior and posterior lobe, the bilateral prefrontal cortex, the bilateral caudate nucleus and the right parieto-occipital area showed significantly different functional connectivity with the bilateral DN among TD, NTD and HC groups
Post hoc analysis for inter-group difference in the DN functional connectivity
Compared to the controls, the TD patients exhibited higher functional connectivity between the bilateral DN and the bilateral cerebellar anterior lobe, the left inferior temporal lobe, the right inferior frontal lobe, and the right parahippocampal, as well as lower functional connectivity between the bilateral DN and the left caudate, the right thalamus, the bilateral prefrontal cortex, and the right superior parietal lobule (p < 0.02, corrected) (Table 2; Fig. 4).
Brain maps for inter-group differences in the DN functional connectivity. The statistical threshold was set at p < 0.05 and cluster size >1080 mm3, which corresponds to a corrected p < 0.02. Warm colors illustrate regions showing higher functional connectivity in the former group. a Comparison between the TD and HC groups. b Comparison between the TD and NTD groups. c Comparison between the NTD and HC groups. For the details of the regions, see Table 2
Compared to the NTD group, the TD patients displayed higher functional connectivity of the bilateral DN with the left inferior temporal gyrus, the bilateral cerebellar anterior and posterior lobes, as well as lower functional connectivity between the bilateral DN with the bilateral prefrontal cortex, and the right superior parietal lobule (p < 0.02, corrected) (Table 2; Fig. 4).
In comparison between the NTD and HC groups, NTD group had higher DN functional connectivity with the right inferior frontal gyrus and the left superior temporal gyrus, and had lower DN functional connectivity with the left cerebellar posterior lobe, the right prefrontal cortex, the left caudate, and the left fusiform gyrus compared to the HC group.
Correlation of DN functional connectivity with tremor severity
In PD patients (including the TD and NTD groups), the functional connectivity of the bilateral DN with the left cerebellar posterior lobe (r = 0.67), and with the left cerebellar posterior lobe (r = 0.63) showed positive correlation with the UPDRS tremor score. And the functional connectivity of the bilateral DN with the bilateral prefrontal cortex, and with the right superior occipital lobe showed negative correlation with the UPDRS tremor score (Table 3; Fig. 5).
Correlations of DN functional connectivity with UPDRS tremor score in PD patients. For the brain map, the statistical threshold was set at p < 0.05 and cluster size >540 mm3, which corresponds to a corrected p < 0.05. The scatter plots displayed correlation between the DN functional connectivity of the peak voxels and the tremor score in PD patients
Discussion
The current study investigated resting-state functional connectivity of the dentate nucleus (DN) in PD patients with tremor. Our main findings include: (1) DN functional connectivity with the bilateral cerebellar anterior lobe was higher in TD group compared to the HC and NTD groups, and that with the bilateral cerebellar posterior lobe was higher in TD group compared to the NTD group. (2) DN functional connectivity with the bilateral prefrontal cortex was lower in TD compared to the HC and NTD groups. (3) The UPDRS tremor score positively correlated with the connectivity between the DN and the bilateral cerebellar posterior lobe, whereas it negatively correlated with the connectivity between the bilateral DN and the bilateral prefrontal cortex in PD patients. These findings emphasize the dysfunction of brain motor network in PD tremor, particularly in the cerebellum and the bilateral prefrontal cortex.
Our results showed higher DN functional connectivity with the bilateral cerebellum in TD, which we postulated to be associated with PD tremor as a compensatory effect to overcome impaired motor network. Hyperactivation in the cerebellum has been suggested to compensate for defective basal ganglia [4, 21, 22]. Previous evidence has indicated that tremor itself might be a compensatory symptom as TD patients have relatively benign nigrostriatal degeneration [23], displayed slower progression, and symptom tends to decrease with disease progression in some patients [24]. More recently, hypothesis is raised where the alteration of the cerebello-thalamo-cortex circuit is indicated to lead to PD tremor as a compensatory effect [3, 7, 8, 25]. In consistence with such hypothesis, alteration of DN functional connectivity was observed in our study. Differently, we show here enhanced connectivity within the cerebellum, i.e. higher functional connectivity between the DN and the bilateral cerebellar anterior and posterior lobes. In addition, higher dentato-cerebellum connectivity positively correlated with the UPDRS tremor score in PD patients. No other study yet has demonstrated correlation between tremor severity and the DN functional connectivity with the bilateral cerebellar posterior lobe. In contrast to our finding, a previous study found lower connectivity between DN and the posterior lobe of cerebellum in TD group compared to NTD patients [13]. Such conflict lies possibly in different sample size and disease duration between the two studies. In summary, we postulated higher coupling between the DN and the cerebellar posterior lobe may be associated with PD tremor. And we appealed more investigations of dentato-cerebellar connectivity in PD patients, such as its reaction towards levodopa or DBS treatment to provide additional evidence.
We also found that the functional connectivity of the DN with the bilateral prefrontal cortex was significantly lower in TD group compared to the HC and the NTD groups, which negatively correlated with tremor severity in PD patients (Fig. 4; Table 2). Lower coupling between the striatum and prefrontal area has been frequently observed in PD [11, 26, 27], while lower connectivity between the DN and the prefrontal cortex has not been reported in PD patients. Allen and colleagues reported functional connectivity between the cerebellum and the prefrontal cortex in healthy subjects using fMRI [28], which was consistent with our findings. The prefrontal cortex can be subdivided into the dorsolateral prefrontal cortex (DLPFC), pre-motor cortex (PMC) and supplementary motor area (SMA), where DLPFC (Brodmann area 46) and PMC (Brodmann area 6) displayed prominent altered functional connectivity with the bilateral DN in TD group as in our study. The DLPFC plays critical role in attention, motor preparation and motor monitoring [29]. The PMC is important in the motor learning and the temporal organization of sequential movements [30, 31]. Previous resting-state fMRI study has demonstrated decreased functional connectivity of the subthalamus nucleus with the DLPFC and the PMC in PD patients [11], and also between the pre-SMA and PMC [32], which may be responsible for PD motor dysfunction. Thus, we postulated that decoupling between the DN and the prefrontal cortex may be associated with the movement disturbance in PD, which may in turn advance or set on compensatory physiological alteration to generate PD tremor.
In addition to the prefrontal cortex, we also found that the parieto-occipital area (Mainly in the Brodmann area 7) displayed lower functional connectivity with the DN in TD group than the HC and NTD groups. Besides, functional connectivity between the Brodmann area 7 and the bilateral DN showed negative correlation with the tremor score. The Brodmann area 7 is believed to play a role in visuo-motor coordination (e.g., in reaching to grasp an object) and participates in the motor preparation and monitoring [33, 34]. Previous study has demonstrated functional coherence between the DN and the parieto-occipital area in vivo [28]. Liu and colleagues further showed that PD patients have lower functional connectivity between the DN and Brodmann area 7 [13], which is consistent with our findings. Therefore, we speculate that the lower functional connectivity between the DN and the parieto-occipital area may be associated with PD motor dysfunction. However, such speculation needs further explorations.
We acknowledged some limitations in our study. First, there was a mismatch of levodopa dosage between the TD and NTD groups. Such a mismatch is a clinical reflection that NTD patients always require higher drug dosage to maintain motor function. As we treated levodopa daily dosage as a covariate in statistical analyses and also our patients were off levodopa for at least 12 h before scanning, the confounding from mismatched levodopa dosage could be reduced. Second, only moderate correlation between connectivity and tremor score was found in PD, which could be attributed to the narrow range of the tremor score as our patients with at most moderate tremor, to avoid the disturbance of MRI signal. Third, overestimation of correlation strengths can be found in scatter plots by calculating the peak correlation from just these regions that survive correlation analysis [35], thus more caution should be exercised in interpreting the plotted figures. Finally, it has been suggested that functional connectivity estimates are particularly vulnerable to motion artifact and that group differences in connectivity could be influenced by confounding differences in head motion between groups [36]. It is likely that data pre-procession such as exclusion of subjects with excessive head motion and regression of head motion parameters will lessen the effects of head motion artifact. In addition, Zeng et al. pointed out that head motion itself may underlie neurological basis in brain imaging [37]. Nevertheless, the effects from individual head motion should still be included as limitation.
Conclusion
Our study demonstrates higher dentato-cerebellar connectivity and lower dentato-prefrontal connectivity in TD patients, which might be involved in the pathogenesis of PD tremor. We conclude that DN might be associated with the pathogenesis of PD tremor. Whether the DN has the potential to be an effective target for treatment of PD tremor is worth future investigations.
References
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39
Ni Z et al (2010) Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol 68(6):816–824
Helmich RC et al (2011) Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69(2):269–281
Lewis MM et al (2011) Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson’s disease. Neuroscience 177:230–239
Coenen VA et al (2014) Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery 75(6):657–669 (discussion 669–70)
Mure H et al (2011) Parkinson’s disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage 54(2):1244–1253
Hallett M (2012) Parkinson’s disease tremor: pathophysiology. Parkinsonism Relat Disord 18(Suppl 1):S85–S86
Hallett M (2014) Tremor: pathophysiology. Parkinsonism Relat Disord 20(Suppl 1):S118–S122
Bostan AC, Dum RP, Strick PL (2010) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107(18):8452–8456
Mehanna R, Lai EC (2013) Deep brain stimulation in Parkinson’s disease. Transl Neurodegener 2(1):22
Wu T et al (2012) Basal ganglia circuits changes in Parkinson’s disease patients. Neurosci Lett 524(1):55–59
Wu T et al (2009) Changes of functional connectivity of the motor network in the resting state in Parkinson’s disease. Neurosci Lett 460(1):6–10
Liu H et al (2013) Altered resting-state functional connectivity of the dentate nucleus in Parkinson’s disease. Psychiatry Res 211(1):64–71
Hughes AJ et al (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Jankovic J et al (1990) Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 40(10):1529–1534
Biswal BB et al (2010) Toward discovery science of human brain function. Proc Natl Acad Sci USA 107(10):4734–4739
Jenkinson M et al (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17(2):825–841
Maximo JO et al (2013) Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci 7:605
Liu C et al (2014) Abnormal intrinsic brain activity patterns in patients with subcortical ischemic vascular dementia. PLoS One 9(2):e87880
Dimitrova A et al (2006) Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage 30(1):12–25
Yu H et al (2007) Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35(1):222–233
Wu T et al (2010) Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain 133(Pt 8):2394–2409
Paulus W, Jellinger K (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50(6):743–755
Lees AJ (2007) Unresolved issues relating to the shaking palsy on the celebration of James Parkinson’s 250th birthday. Mov Disord 22(Suppl 17):S327–S334
Helmich RC et al (2012) Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 135(Pt 11):3206–3226
Hacker CD et al (2012) Resting state functional connectivity of the striatum in Parkinson’s disease. Brain 135(Pt 12):3699–3711
Jokinen P et al (2013) Cognitive slowing in Parkinson’s disease is related to frontostriatal dopaminergic dysfunction. J Neurol Sci 329(1–2):23–28
Allen G et al (2005) Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. Neuroimage 28(1):39–48
Owen AM, Evans AC, Petrides M (1996) Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex 6(1):31–38
Halsband U, Freund HJ (1990) Premotor cortex and conditional motor learning in man. Brain 113(Pt 1):207–222
Halsband U et al (1993) The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 116(Pt 1):243–266
Wu T et al (2011) Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp 32(9):1443–1457
Seitz RJ et al (1997) Representations of graphomotor trajectories in the human parietal cortex: evidence for controlled processing and automatic performance. Eur J Neurosci 9(2):378–389
Jancke L et al (2001) The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb Cortex 11(2):114–121
Vul E, Pashler H (2012) Voodoo and circularity errors. Neuroimage 62(2):945–948
Van Dijk KR, Sabuncu MR, Buckner RL (2012) The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59(1):431–438
Zeng LL et al (2014) Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci USA 111(16):6058–6062
Acknowledgments
We appreciate Beijing Municipal Science and Technology Commission, Beijing Health System, Beijing Nature Science Foundation, Beijing Municipal Commission of Education, National Key Technology Research and Development Program of the Ministry of Science and Technology of China, and National Science Foundation of China for the financial support. This research was supported by Beijing Municipal Science and Technology Commission, China (Grant No. Z111107058811012), High Level Technical Personnel Training Project of Beijing Health System, China (Grant No. 2011-3-022), Beijing Nature Science Foundation and Beijing Municipal Commission of Education, China (Grant No. kz20120025028), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03), Beijing Institute for Brain Disorders (BIBD-PXM2013_014226_07_000084), and the National Science Foundation of China (Grant Numbers 81271429).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflict exists among authors.
Ethical standard
All procedures were approved and supervised by the ethics committee of Beijing Tiantan Hospital, in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained either from the participants or their closest relatives.
Additional information
H. Ma and H. Chen contributed equally to the work.
Rights and permissions
About this article
Cite this article
Ma, H., Chen, H., Fang, J. et al. Resting-state functional connectivity of dentate nucleus is associated with tremor in Parkinson’s disease. J Neurol 262, 2247–2256 (2015). https://doi.org/10.1007/s00415-015-7835-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7835-z