Abstract
Non-motor symptoms are gaining relevance in Parkinson’s disease (PD) management but little is known about their progression and contribution to deterioration of quality of life. We followed prospectively 707 PD patients (62 % males) for 2 years. We assessed non-motor symptoms referred to 12 different domains, each including 1–10 specific symptoms, as well as motor state (UPDRS), general cognition, and life quality. Hoehn & Yahr (H&Y) stage was used to categorize patient status (I–II mild; III moderate; IV–V severe). We found that individual non-motor symptoms had variable evolution over the 2-year follow-up with sleep, gastrointestinal, attention/memory and skin disturbances (hyperhidrosis and seborrhea) becoming more prevalent and psychiatric, cardiovascular, and respiratory disorders becoming less prevalent. Development of symptoms in the cardiovascular, apathy, urinary, psychiatric, and fatigue domains was associated with significant life-quality worsening (p < 0.0045, alpha with Bonferroni correction). During the observation period, 123 patients (17 %) worsened clinically while 584 were rated as stable. There was a fivefold greater increase in UPDRS motor score in worse compared with stable patients over 24 months (p < 0.0001 vs. baseline both in stable and worse group). The total number of reported non-motor symptoms increased over 24 months in patients with motor worsening compared to stable ones (p < 0.001). Thirty-nine patients died (3.4 % of patients evaluable at baseline) with mean age at death of 74 years. Deceased patients were older, had significantly higher H&Y stage and motor score, and reported a greater number of non-motor symptoms at baseline. In conclusion, overall non-motor symptom progression does not follow motor deterioration, is symptom-specific, and only development of specific domains negatively impacts quality of life. These results have consequences for drug studies targeting non-motor features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-motor symptoms (NMS) are gaining increasing relevance in the management of Parkinson’s disease (PD) and the use of dedicated questionnaires and scales may facilitate their clinical recognition and assessment [1, 2]. In our PRIAMO cohort study, we initially reported that among the 1,072 patients screened, 98 % complained of at least 1 NMS while the average number of NMS per patient was 6. In addition, we found a negative correlation between presence of NMS and quality of life, further emphasizing their clinical relevance [3], a finding similar to other large clinical surveys [4–6]. NMS may also show a variable response to medications. A recent study demonstrated improvement in PD depressive symptoms after therapy with the dopamine agonist pramipexole compared to placebo [8]. Moreover, switching advanced PD patients from oral to continuous duodenal levodopa infusion improved sleep, bladder function, pain, sexual dysfunction, and depression while cognition did not change, suggesting that NMS impairment is variably driven by dopaminergic denervation [7].
Overall, interpretation of these results is limited by the scarce amount of data on individual NMS rate of progression, small number of study patients and short-term prospective observation period. More data are available on cognitive dysfunction with two recent studies reporting that almost 50 % of PD may develop dementia after 15-year follow-up [9], with prevalence increasing to 80–90 % by the age of 90 years [10]. Cognitive changes may be associated with PD neuropsychiatric features, particularly depression and psychosis, which have been shown to be highly prevalent at all disease stages and to contribute significantly to disability [11].
In the current manuscript, we are now reporting for the first time 2-year prospective assessment of NMS in a large cohort of PD patients. In our PRIAMO cohort, in addition to assessment of NMS progression, we also explored their relationship with motor features and quality of life as well as the relevance for life expectancy. The main advantage of the PRIAMO PD cohort is represented by the number of patients investigated using a naturalistic approach, the involvement of both academic institutions and hospital-based services, and the even distribution of participating sites across all Italian regions.
Patients and methods
Study population
The PRIAMO study (PaRkInson And non MOtor symptoms) is an Italian multicenter naturalistic survey aimed at assessing NMS in a large cohort of patients affected by different types of parkinsonism, consecutively enrolled between July 2005 and June 2006 at 55 participating centers (see Appendix 1). It consisted of a cross-sectional phase (results have been published previously [3]) and of the here reported prospective assessment. The methodological issues and the baseline features of these patients have been described in our previous papers [3, 12]. We are here now presenting data only for PD patients who completed baseline visit, the first follow-up within 12 ± 4 months from baseline and the last visit after additional 12 ± 4 months from first follow-up.
Data collection and methods
All patients underwent the same evaluation at each visit. We assessed NMS using a semi-structured interview, which explores symptoms referred to 12 different domains (gastrointestinal, pain, urinary, cardiovascular, sleep, fatigue, apathy, attention/memory, skin, respiratory, psychiatric, and miscellaneous other symptoms). Each domain included between one and ten discrete symptoms that patients were asked to report as “present/absent” with reference to the last month prior to the visit. Details of symptoms listed for each domain were published previously [12, 13]. A domain was counted as “present” if at least one symptom was reported. At each visit we recorded the Hoehn & Yahr scale (H&Y) [13], the Unified Parkinson Disease Rating Scale part III (UPDRS-III) [14], the Mini-Mental State Examination (MMSE) [15], the Frontal Assessment Battery (FAB) [16], the 39-item Parkinsons’ Disease Questionnaire (PDQ-39) [17], and the Hamilton Depression Scale (HAM-D; [18]).
Statistical analysis
Descriptive statistics consisted of mean and standard deviation (SD) when statistical distribution of quantitative variables was not skewed. In all other cases, non-parametric statistics, i.e., median and interquartile range (IQR), were applied. The evolution of NMS was evaluated with regard to the 12 domains: patients complaining of ≥1 symptom within a domain were considered NMS-domain positive. The proportion of patients who remained stable and developed a non-motor domain during the 24-month follow-up (incidence) or did not have a non-motor domain any longer at the 12 or 24 month follow-up (regression) was calculated in order to assess NMS evolution.
Moreover, both the overall number of NMS and the number of positive domains reported by patients were calculated. For each scale, only the fully completed evaluations were considered for statistical analysis, i.e., no re-coding or interpolation of missing items was performed. Missing responses at the MMSE were considered equal to 0, according to McDowell and Newell [19]), unless all items were missing. For MMSE and FAB, age- and education-adjusted scores were calculated and a cut-off of 23.8 and 13.48, respectively, were used, according to Italian normative data [20, 21]. Presence of cognitive impairment was defined as MMSE score ≤23.8 and frontal dysfunction as FAB score ≤13.48. UPDRS-III, HAM-D, and PDQ-39 total scores were calculated by summing single items scores. Changes in the H&Y score between the visits were used to stratify patients in worse and stable. More specifically, patients moving from H&Y 1–2 (rated as “mild”) at baseline to H&Y 3 (rated as “moderate”) or H&Y 4–5 (rated as “severe”) at either follow-up visit or from H&Y 3 (moderate) to H&Y 4–5 (severe) at either follow-up visit were defined “worse” while all other patients were defined “stable”.
Comparisons were performed by paired t test for mean values and Fisher’s exact test for frequency distribution. We used ANOVA, or non-parametric ANOVA, for variables with asymmetrical distribution to assess the effect of disease severity on clinical evolution. The significance threshold was set to 0.05 and corrected with Bonferroni’s formula in case of multiple comparisons accepting of k significance tests as statistically significant those with p values smaller than 0.05/k. Data were analyzed using SAS for Windows, release 9.1. Project management including data management, quality control and statistical analysis was performed by MEDIDATA (Modena, Italy).
Results
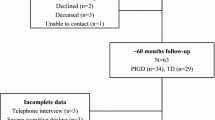
A total of 707 (66 % of 1,072 evaluable patients at baseline) PD patients (439 males: 62 % and 268 females: 38 %) completed all prospective assessments. We compared general clinical characteristics the 707 patients followed-up for 24-months to the 365 patients who did not have complete follow-up. The patients who completed the PRIAMO study were younger and had less severe clinical conditions at baseline as shown by lower H&Y and UPDRS scores and by a lower number of NMS and NMS domains at baseline (p, t test <0.0001 for all the comparisons). No significant gender differences were present. The complete patients’ flow of our PRIAMO study is summarized in Fig. 1.
The mean (±SD) H&Y score changed from 1.97 (0.75) at baseline to 2.09 (0.77) after 12 months and to 2.23 (0.80) after 24 months (both p < 0.0001 vs. baseline: ANOVA repeated measures). Based on H&Y stage, 123 (17 %) patients worsened over the 2-year follow-up, whereas the remaining 584 (83 %) were rated as clinically unchanged. The proportion of patients with mild disease severity decreased from 70 % at baseline to 58 % at 24-month follow-up visit and the proportion of patients with moderate and severe disease severity increased over time (from 26 to 34 % and from 4 to 8 %, respectively).
The mean UPDRS-III scores in the whole PD cohort as well as in stable and worse patients are listed in Table 1. Over 2 years, the mean increase in UPDRS-III score was of 3.39 points in the whole population, but 9.93 in the subgroup of patients presenting clinical worsening. Almost 90 % of our patients were already on dopaminergic medications (levodopa or dopamine agonist alone or in combination) at baseline increasing to 95 % at the end of the 2-year observation period. Given the naturalistic design of the PRIAMO study, we did not record individual medication doses.
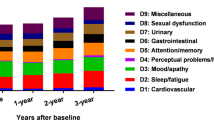
There was no change in number of NMS domains over the entire follow-up period (p, ANOVA repeated measures >0.05) while the total number of NMS increased significantly (p, paired t test <0.01) only at the 24-month visit compared to baseline (no difference between baseline and the 12-month visit, p paired t test >0.05) (Table 2). NMS of pain, gastro-intestinal, urinary, sleep, and psychiatric domains were consistently observed during the entire study period, with an approximately 40 % frequency at all visits. Overall, NMS had a variable evolution over the 2-year observation period with domains like sleep, gastrointestinal, attention/memory disturbances and skin (hyperhidrosis and seborrhea) becoming more prevalent and psychiatric, cardiovascular, and respiratory becoming less prevalent. Figure 2 depicts the difference between the incidence and the remission of non-motor symptoms over 24 months. There was no difference between stable and worse patients in incidence and remission of the different NMS domains over the follow-up for all NMS domains (p, Chi-square test >0.0045 (alpha with Bonferroni’s correction = 0.05/11)). However, in worse patients, the most frequent newly incident NMS were in the sleep, apathy and attention/memory domains (Table 3).
The mean MMSE was 27.63 (SD 2.69) at baseline, 27.46 (3.31) at 12 months, and 27.19 (3.75) at 24 months. The latter value was lower compared to baseline (p, paired t test < 0.0003), though the difference may not to be clinically meaningful. Forty-six patients (7 %) with a normal MMSE score at baseline reached or fell below the cut-off value (≤23.8) at 24-month assessment. No change was observed in the mean FAB score (p, ANOVA repeated measures >0.05) as well as the HAM-D score (p, ANOVA repeated measures = 0.03) throughout the study period.
During the 2-year follow-up, 39 PD patients died (3.4 %; 26 males, 13 females), 21 (1.8 %) during the first year and 18 (1.6 %) during the second year. Table 4 compares age and disease characteristics of deceased patients with the patients who were alive at 24-month follow-up visit. Deceased patients were older, had significantly higher H&Y and UPDRS-III scores, and presented a greater number of NMS at baseline. Mortality was higher among PRIAMO PD patients with age between 60 and 74 years versus overall Italian population as established for year 2005 by the Italian Institute of Statistics (ISTAT) [22].
A total of 377 patients completed the PDQ-39 questionnaire at all visits. There was no mean change in total PDQ-39 score over 2 years although total score increased (reflecting worsening quality of life) in patients who developed symptoms in cardiovascular, apathy, psychiatric and fatigue domains during the 24-month observation, compared to patients with regression of the same symptoms in these domains [Mann–Whitney test, p < 0.0045 (alpha with Bonferroni’s correction = 0.05/11)]. Conversely, incidence or regression of NMS in urinary, skin, pain, gastrointestinal, respiratory, attention/memory, and sleep domains did not affect PDQ-39 score (Figs. 3, 4).
Median (IQR) PDQ-39 total score change from baseline to 24-month follow-up in patients with newly incident or regressed NMS domains (IQR interquartile range). A positive change in PDQ-39 means a worsening in quality of life while a negative change indicates an improvement in quality of life (median total score at 24-month follow-up minus median total score at baseline). *Mann–Whitney test, p < 0.004
Discussion
The main objective of our PRIAMO study was to assess prospectively the progression of NMS in a large cohort of PD patients at different disease stages selected from both academic and hospital-based services. In our previous cross-sectional observation, we found that overall NMS number increases along with disease motor severity and duration. We used a new questionnaire that did not undergo formal clinimetric validation but delivered consistent results with other cohort studies where NMS were evaluated using specific scales or questionnaire [3]. However, the results of our current prospective assessment indicate that NMS progression is variable and domain specific, which means that it often follows a different pattern compared to motor features. More specifically, although NMS increased in number only in patients showing clinical motor progression, there were domains becoming more and other less prevalent. Finally, only the development of NMS in specific domains contributed to worsening quality of life. These findings further highlight the relevance of NMS for PD [9], but also indicate that their assessment is complementary to motor evaluation if one wants to measure PD progression [23, 24]. The uneven development of discrete NMS domains in our PRIAMO cohort suggests a non-linear progression that is relevant for planning future trials targeting specific NMS and possibly neuroprotection [25].
The occurrence of NMS that are concomitantly increasing and decreasing in frequency in the same patient population is not easily explained and would probably require additional investigation. In the PRIAMO study, we only recorded presence or absence of NMS and did not rate their severity except for cognition and mood where we used specific scales. Our interpretation is that by applying the questionnaire, treating neurologists became aware of NMS presence and may have adjusted medications’ doses or regimens. Indeed a recent survey suggested that NMS are often undeclared by patients unless a specific questionnaire is administered [26], while randomized studies indicate that NMS like sleep disturbances or depressive symptoms can be improved by dopamine agonists [8, 27]. This may be reflected particularly in the stable group where adjustment in dopaminergic therapy possibly contributed in stabilizing clinical motor conditions. The observation that NMS with remitter greater than incident number included cardiovascular symptoms (which included two symptoms lightheadedness/dizziness during the postural changes, fall because of syncope) or psychiatric features (which incorporated ten symptoms including anxiety, panic attacks, depression, and hallucinations) which may benefit from optimization of dopaminergic therapy would be consistent with this hypothesis. It must be stressed that given the naturalistic nature of our PRIAMO study, we did not specifically record medication doses and therefore we cannot establish with certainty the presence of this relationship. Interestingly, presence or absence of symptoms in the cardiovascular and psychiatric domains had a great impact on quality of life, confirming their relevance for PD management. By contrast, skin domain, which in our study included two NMS hyperhidrosis and seborrhea, showed the greatest progression in terms of new incident patients but had little impact on quality of life.
We did not observe significant mean changes in MMSE and frontal lobe function assessed using the FAB. Given the large number of participating sites, which included both academic institutions as well as hospital-based services, we had to limit our cognitive evaluation to relatively simple tests. Moreover, among our initial exclusion criteria there was MMSE < 24 meaning that our PRIAMO cohort did not include patients who had significant cognitive abnormalities at baseline and may be at greater risk to develop dementia. Nonetheless, in 7 % of our patients, the MMSE fell below the MMSE cut-off for dementia in PD [28]. Moreover, apathy (which included loss of interest in surrounding matters, loss of interest in activities of daily living, awareness deficit) as well as attention/memory were the most frequent newly incident NMS domains in patients showing clinical worsening confirming their key-role for disease progression. Presence of these NMS domains contributed also significantly to patients’ quality of life.
One interesting observation from our study regards the assessment of mortality rate in our patients. Overall, we observed a higher mortality in our PRIAMO cohort compared to the Italian population in the same age range [22] and more importantly a similar death rate in male and female patients indicating the PD has greater impact on women life expectancy (which is significantly higher than men in the control population). Interestingly, the number of NMS in patients who died during follow-up was higher at baseline compared to other patients, confirming that end-stage PD is also associated with widespread degeneration in addition to motor disability.
We had a relatively high number of dropouts during the follow-up period (34 % of the total initial patients). This is due to the naturalistic nature of the study and to the exclusion of patients who did not have complete follow-up data for the 2 years.
In conclusion, we reported for the first time a naturalistic prospective assessment of NMS in a large cohort of PD patients evaluated at multiple centers in Italy. The observation of a variable course as well as contribution to quality of life of different NMS domains may serve as basis for planning future studies targeting disturbances beyond motor features and possibly also for defining appropriate outcome measures in future neuroprotective trials. Further studies using specific scales are warranted to assess prospectively both NMS occurrence and severity and how they are modified by current and future therapies.
References
Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P et al (2006) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord 21:916–923
Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, Macphee G et al (2007) Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 22:1623–1629
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E et al (2009) The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 24:1641–1649
Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P et al (2007) The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 22:1901–1911
Simuni T, Sethi K (2008) Non-motor manifestations of Parkinson’s disease. Ann Neurol 64(Suppl 2):S65–S80
Beiske AG, Loge JH, Rønningen A, Svensson E (2009) Pain in Parkinson’s disease: prevalence and characteristics. Pain 141:173–177
Honig H, Antonini A, Martinez-Martin P, Forgacs I, Faye GC, Fox T et al (2009) Intrajejunal levodopa infusion in Parkinson’s disease: a pilot multicenter study of effects on nonmotor symptoms and quality of life. Mov Disord 24:1468–1474
Barone P, Poewe W, Albrecht S et al (2010) Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 9:573–580
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-L-dopa responsive problems dominate at 15 years. Mov Disord 20:190–199
Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D (2008) Dementia and survival in Parkinson disease: a 12-year population study. Neurology 70:1017–1022
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474
Antonini A, Colosimo C, Marconi R, Morgante L, Barone P (2008) The PRIAMO Study: background, methods and recruitment. Neurol Sci 29:61–65
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Fahn S, Elton R, Members of the UPDRS Development Committee (1987) The unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds) Recent developments in Parkinson’s disease, vol 2. Macmillan Health Care Information, Florham Park, pp 153–63, 293–304
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: a Frontal Assessment Battery at bedside. Neurology 55:1621–1626
Peto V, Jenkinson C, Fitzpatrick R, Greenhall R (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4:241–248
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
McDowell I, Newell C (1996) Measuring health: a guide to rating scales and questionnaires. Oxford University Press, New York
Measso GF, Cavarzeran F, Zappalà G, Lebowitz BD, Pirozzolo FJ, Amaducci LA et al (1993) The Mini-Mental State Examination: normative study of a random sample of Italian population. Dev Neuropsychol 9:77–85
Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML et al (2005) The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci 26:108–116
Istituto Nazionale di Statistica (2008) Tavole di mortalità della popolazione residente
Poewe W, Mahlknecht P (2009) The clinical progression of Parkinson’s disease. Parkinsonism Relat Disord 15:S28–S32
Schrag A, Dodel R, Spottke A, Bornschein B, Siebert U, Quinn NP (2007) Rate of clinical progression in Parkinson’s disease. A prospective study. Mov Disord 22:938–945
Maetzler W, Liepelt I, Liepelt I, Berg D (2009) Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol 8:1158–1171
Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S et al (2010) The non-declaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord 25:697–701
Trenkwalder C, Kies B, Rudzinska M et al (2011) Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 26(1):90–99
Hoops S, Nazem S, Siderowf AD et al (2009) Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73(21):1738–1745
Conflicts of interest
A. Antonini has received honoraria for consulting services and symposia from Abbott, Boehringer Ingelheim, GSK, Lundbeck, UCB, Novartis and Merck Serono; P. Barone has received honoraria for consulting services and symposia from Boehringer Ingelheim; R. Marconi has received honoraria for consulting services and symposia from Boehringer Ingelheim; L. Morgante has received honoraria for consulting services and symposia from Boehringer Ingelheim; C. Colosimo has received honoraria for consulting services and symposia from Boehringer Ingelheim; and all other authors have nothing to disclose.
Ethical standards
The study was approved by the Ethic Committee of each individual institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the PRIAMO study group.
The members of the PRIAMO study group are given in Appendix 1.
Appendices
Appendix 1: The PRIAMO study group
First name | Last name | Unit | Hospital | Town |
|---|---|---|---|---|
Salvatore | Zappulla | Neurologia | Ospedale Umberto I | Enna |
Clelia | Pellicano | Clinica Neurologica | Ospedale Sant’Andrea, II Facoltà di Medicina e Chirurgia, “Sapienza” Università di Roma | Roma |
Sara | Meoni | Clinica Neurologica I | Day Hospital Dip. Scienze Neurologiche e Psichiatriche | Firenze |
Marianna | Capecci | Clinica di Neuroriabilitazione | Ospedale Umberto I | Ancona |
Natalia | Caravona | Centro Parkinson Dipartimento di Neurologia e Psichiatria e Centro di Ricerca per le Malattie Sociali (CIMS) | “Sapienza” Università di Roma | Roma |
Gianni | Pezzoli | Centro Parkinson | Istituti Clinici di Perfezionamento | Milano |
Vittorio | Petretta | Neurologia e Stroke | A.O.R.N. San Giuseppe Moscati | Avellino |
Massimo | Pederzoli | Neurologia | Ospedale Civile | Vimercate |
Fulvio | Pepe | Neurologia | Fondazione Poliambulanza | Brescia |
Marianna | Amboni | IDC-Hermitage-Capodimonte | Napoli | |
Daniela | Frosini | Centro Parkinson | Azienda Ospedaliero-Universitaria Pisana | Pisa |
Sergio | Zanini | Clinica Neurologica | Policlinico Universitario Udine | Udine |
Giampiero | Volpe | Neurofisiopatologia Dip. Neuro Orto Traumatologia | Presidio Ospedaliero S. Luca | Vallo della Lucania |
Gilda | Di Brigida | Dipartimento Neuroscienze, Oftalmologia e Genetica | Università degli Studi di Genova | Genova |
Marco | Di Giovanni | U.O. di Neurologia | Ospedale Regionale | Aosta |
Roberto | L’Erario | Neurologia | Ospedale Civile San Bortolo | Vicenza |
Giuseppe | Ciacci | Neurologia | Policlinico Le Scotte | Siena |
Antonio | Cannas | U.O.Neurologia | Policlinico Universitario di Monserrato | Monserrato, Cagliari |
Luisa | Giglia | Neurologia | Azienda Ospedaliera S. Elia | Caltanissetta |
Alfredo | Petrone | Neurologia | Presidio Ospedaliero Annunziata | Cosenza |
Stefano | Amidei | Neurologia | Ospedale Ramazzini | Carpi |
Giorgio | Trianni | Neurologia | P.O. F. Ferrari | Casarano |
Giovanni | Cossu | Centro Parkinson -U.O.Neurologia | Azienda Ospedaliera G.BROTZU | Cagliari |
Maria | Bloise | Dip.to Scienze Neurologiche | Policlinico Umberto I Univ.La Sapienza | Roma |
Chiara | Logi | U.O Neurologia | Ospedale Versilia | Camajore |
Francesco | Soleti | Clinica Neurologica | Università Cattolica S. Cuore Policlinico Gemelli | Roma |
Michele | Abrignani | U.O. Neurologia | Ospedale di Marsala ASP TP/2 | Marsala |
Rossana | Scala | Neurologia | Ospedale S. Maria Loreto Nuovo | Napoli |
Franco | Pennisi | Neurologia | Ospedale di Castelvetrano | Castelvetrano |
Lucia | Grasso | Neurologia | Ospedale della Misericordia | Grosseto |
Francesca | Preda | Neurologia Dip. Neuroscienze applicate alla clinica | Ospedale Sant’Anna | Ferrara |
Giacomo | Gurgone | U.O. Neurologia | Az. Osp. S.Giovanni di Dio | Agrigento |
Mario | Zappia | Clinica Neurologica I | Policlinico Universitario | Catania |
Stefania | Lanfranchi | Neurologia | Ospedale S. Antonio Abate Gallarate | Gallarate |
Tania | Avarello | Centro per lo studio delle M.Extrapiramidali | O.R. Villa Sofia | Palermo |
Francesca | Morgante | Dipartimento di Neuroscienze, Scienze psichiatriche ed Anestesiologiche | Università di Messina | Messina |
Paolo | Stanzione | Dept. Neuroscience | Università di Roma Tor Vergata | Roma |
Augusto | Scaglioni | Div. Neurologia | Ospedale di Vaio | Fidenza |
Sabina | Capellari | Centro per lo studio e la cura delle malattie extrapiramidali | Dipartimento Scienze Neurologiche Università di Bologna | Bologna |
Monia | Blundo | U.O. Neurologia | P.O. Guzzardi | Vittoria |
Lucia | Antonutti | Clinica Neurologica | Ospedale di Cattinara | Trieste |
Pasqualino | Simone | Neurologia | Ospedale Casa Sollievo della Sofferenza | San Giovanni Rotondo |
Paola | Soliveri | Neurologia I | Istituto Nazionale Neurologico C. Besta | Milano |
Biagio | Troianello | Neurologia | Istituto Clinico Città di Brescia | Brescia |
Mattia Anna | Iellamo | U.O. Neurologia | Ospedale G. Iazzolino | Vibo Valentia |
Alessandro | Mauro | Neurologia | Istituto Scientifico San Giuseppe | Piancavallo |
Maurizio | Zibetti | Dipartimento Neuroscienze | Università degli Studi di Torino | Torino |
Giuseppe | Nastasi | Neurologia | Az. Osp. Papardo | Messina |
Appendix 2
Steering committee
- Angelo Antonini:
-
Department for Parkinson’s disease IRCCS San Camillo, Venezia
- Paolo Barone:
-
Scuola Medica Salernitana, Università di Salerno, Salerno; IDC-Hermitage-Capodimonte, Napoli
- Carlo Colosimo:
-
Università La Sapienza, Roma
- Roberto Marconi:
-
Ospedale della Misericordia, Grosseto
- Letterio Morgante:
-
Dipartimento di Neuroscienze, Scienze Psichiatriche ed Anestesiologiche, Università di Messina, Italy.
Sponsorship
-
Tania Corbetta, Arina Dumitriu, Boehringer Ingelheim, Milan, Italy.
Project management, statistical analyses, and data management
-
Simona Sgarbi, project leader MediData Studi e Ricerche, Modena
-
Andrea Rapisarda, clinical project manager MediData Studi e Ricerche, Modena
-
Sara Rizzoli, Lucia Simoni, statisticians MediData Studi e Ricerche, Modena
-
Luca Zanoli, clinical data manager MediData Studi e Ricerche, Modena
-
Alessandra Manfredi, clinical operation specialist MediData Studi e Ricerche, Modena, Italy.
Rights and permissions
About this article
Cite this article
Antonini, A., Barone, P., Marconi, R. et al. The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. J Neurol 259, 2621–2631 (2012). https://doi.org/10.1007/s00415-012-6557-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6557-8