Abstract
Background
Adequate cerebral blood flow (CBF) is mainly governed by neurovascular coupling (NC) which adapts local CBF to underlying cortical activity, and cerebral autoregulation (CA) that tends to maintain constant CBF despite changes in arterial blood pressure (BP). Since it was suggested that resistance vessels play an important role in both mechanisms, we investigated their regulative interplay by performing a functional transcranial Doppler (f-TCD) test under different orthostatic conditions.
Methods
Fifteen healthy volunteers performed a visual reading test stimulation task after stabilized in sitting, supine and upright position on a tilt table. Simultaneously, BP and heart rate (HR) were recorded by a photoplethysmographic method and CBF velocity was measured with TCD in left posterior cerebral artery, and, as a reference, also in right middle cerebral artery. Evoked flow velocity (FV) responses were evaluated by a control system approach for systolic and diastolic data. Parameters studied were baseline FV with eyes closed, stable FV under stimulation (gain), oscillatory feature (natural frequency) and damping (attenuation) of the control system model, rate time, and also systolic and diastolic BP and HR. ANOVA test was used for comparing the values of variables in different postural settings, inferring statistical significance at a p < 0,05 level.
Results
Although there was a significant variation on the different orthostatic conditions in systolic (p = 0,027) and diastolic (p = 0,001) BP and HR (p = 0,0001), there was no significant change in the basal or evoked CBF velocities.
Conclusions
An intact CA compensates the different orthostatic conditions completely thus allowing an independent regulation of NC according to the metabolic needs of cortical stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain circulatory physiology is not yet fully understood and there are still competing theories about the modulating role of myogenic, neurogenic and metabolic mechanisms [13]. Independently from their biological realisation two fast regulating principles of cerebral perfusion are well accepted. The cerebral autoregulation maintains stable cerebral blood flow for a wide range of systemic blood pressure as long as the fluctuations remain between 50–150 mmHg mean blood pressure [12]. The neurovascular coupling adapts local cerebral blood flow in accordance with the metabolic needs and activity of the underlying cortex [7]. It is assumed that the small arteriolar resistance vessels are the effector structures of both mechanisms [15]. Also, from a system-theory point of view similar effectors have been suggested because both mechanisms show near to identical response times [1]. However, since both mechanisms are continuously active they interfere biologically and functionally at least at the level of the resistance vessels. Consequently, the question arises if the interplay might affect the regulative properties in such a way that the regulative compensation of one mechanism affects the properties of the other. Although this question appears in first sight theoretical, the question of interference gains relevance in many areas of brain circulatory investigations. The neurovascular coupling mechanism is fundamental for many functional activation tests as performed with techniques such as MRI, PET, SPECT and transcranial Doppler (TCD). By performing cortical activation tasks it is intended only to modulate the neurovascular coupling. In practice, the obtained results depend on both mechanisms: functional activation studies with transcranial Doppler are normally performed in a sitting position whereas for technical reasons all MRI investigations are performed in a supine position. Therefore, the functional tests are performed in different orthostatic conditions with different compensative levels of cerebral autoregulation. Although the cerebral autoregulation will compensate for the orthostatic difference it is to the best of our knowledge unknown if the change in the tone of the resistance vessels might influence the results of the neurovascular coupling.
To investigate this issue, we studied the regulative properties of the neurovascular coupling under different stable orthostatic conditions in healthy young volunteers.
Subjects and Methods
This study was performed in Hospital S. João, a 1200-bed university hospital in Oporto. The local institutional ethical committee approved the study. After information and instruction each volunteer gave informed consent to participate in the study.
Subjects
Fifteen healthy young adult volunteers (8 male and 7 female) with the mean age of 26.8 ± 8.8 years, ranging from 18 to 48 years, were included. These volunteers lacked any cardiovascular risk factors and did not take any medication, except for birth control pills. They abstained from caffeine more than 12 hours before the tests. Previously to the study, all volunteers performed a cervical and transcranial duplex scan, with a HDI 5000 device (Philips, USA). Normal findings and a good temporal acoustic bone window to ensure a good acquisition of velocity curves during the whole test were required as an inclusion criterion.
Hemodynamic monitoring and reading test
The study was carried out in a quiet room with a constant temperature of approximately 22°C. Systolic and diastolic blood pressure and heart rate were monitored with a non-invasive finger cuff Finapres device (model 2300; Ohmeda, Englewood, CO, USA) holding the finger at heart level. A hand support was used to permit a constant position throughout the tests in the three different postural conditions [11]. Following previously described approaches for insonation through the temporal transcranial ultrasonic bone window [3], 2 MHz pulsed wave Doppler monitoring probes were positioned in order to record the flow velocity in the P2 segment of the left posterior cerebral artery (PCA) with a Multidop T2 Doppler device (DWL, Sipplingen, Germany). Also the M1 segment of the right middle cerebral artery (MCA) was insonated as a reference for monitoring non-specific effects in the test situation. The probes were mounted on an individually fitted headband. Beat-to-beat peak systolic and end diastolic blood flow velocities were recorded. The recordings were considered as technically adequate when the blood flow velocities could be detected bilaterally in good conditions, with a clear envelope of the velocity spectrum during the whole cardiac cycle.
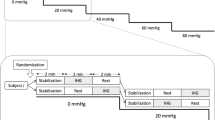
The visual evoked paradigm consisted of 10 cycles, each with a resting phase of 20 s with closed eyes and a stimulating phase of 40 s of silent reading text columns of a news magazine. Changes between phases were signalled acoustically using a tone. One test set had a total duration of 10 minutes, and was repeated in each position - supine, sitting and standing tilted in a tilt table (70° head-up, with an electrically-driven table, with a footboard support). The headband with the probes was kept in the same position during the whole study. The reading test and its reliability were validated against a checkerboard stimulation paradigm [16]. Continuous beat-to-beat recordings of systolic and diastolic arterial blood pressure and peak systolic and end diastolic cerebral blood flow velocities were stored together with the calculated heart rate on the Doppler device.
Data analysis
To become independent from the insonation angle, the comparison of evoked blood flow data was made after transforming the absolute data into relative changes of flow velocity, separately for the peak systolic and end diastolic blood flow velocities, using the following form:
where ΔBFV refers to the relative increase in blood flow velocity, VStim the peak systolic or the end diastolic blood flow velocity during stimulation, and VavR the averaged peak systolic or end diastolic blood flow velocity during rest five to zero seconds before the stimulation started.
Ten cycles of 20 s rest (closed eyes) and 40 s stimulation (silent reading) were averaged for each volunteer at each position.
A control system approach [15] was used to quantify the averaged relative evoked flow changes of each volunteer in each postural setting. Peak systolic and diastolic blood flow velocities were evaluated separately. According to control system theory any regulative process, which convolutes an input signal to an output signal, can be approximated by a mathematical polynom [9]. In the present case the input signal is the change between conditions of rest and activation, which is expressed mathematically as a unit-step function changing from zero to one, according to conditions of rest and activation. The output of the assumed regulative system is the measured flow velocity response. Knowing the input and output signal a mathematical polynom with a distinct order of the numerator and denominator can be approximated which sufficiently describes the input-output relation. The minimal control system model, which describes the main dynamic features of the flow velocity response, is assumed as the model of the system. A proportional derivative second order lag system with dead time (PDT2) model was found sufficient to describe the flow velocity response evoked by visual stimulation. The features of this model are explained in more detail in the following.
The input and output data were analysed by using a commercial software tool for identifying and specifying control systems (Vascochecker, DWL, Sipplingen, Germany), which transformed the relative blood flow averaged velocity curves of each volunteer into terms of a second-order linear system with the following equation G(s):
where K represents the gain, Tv the rate time, ω the undampened natural angular frequency (natural frequency), and ξ the attenuation parameter of the system [9].
These parameters describe all dynamic properties of the entire evoked flow curve. The rate time indicates the initial steepness of the flow velocity increase. Natural frequency and attenuation describe oscillatory features of the system; natural frequency is assumed to represent the tonus and the speed of the system, whereas the attenuation describes dampening features of the system such as the elastic properties of the wall vessel [14]. The parameter gain describes the relative flow velocity difference between stable flow conditions of rest and stimulation. The program also calculated the absolute resting peak systolic and end diastolic blood flow velocity with eyes closed, VavR, for the time span five to zero seconds before the stimulation started.
Statistics
ANOVA test was used to compare the resting flow velocities, blood pressure data, heart rate and each of the control system parameters for each of the different postural settings, inferring statistical significance at a p < 0.05 level. Post hoc Fisher tests were performed to compare between the three conditions if significance occurred.
Results
Systemic data:
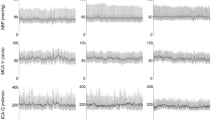
Figure 1 shows the variation of systolic and diastolic systemic blood pressure values and of heart rate in this healthy population in the positions of supine, sitting on a chair and upright in a tilt table at 70°.
Averaged systolic/ diastolic arterial blood pressure of subjects was 111 ± 15 / 68 ± 13 mmHg at supine, 125 ± 17 / 84 ± 15 mmHg at sitting and 123 ± 14 / 83 ± 12 mmHg at tilted-up positions. The mean arterial blood pressure was respectively 81 ± 15, 98 ± 15 and 94 ± 13 mmHg at the three different postural settings. The heart rate changed from 71 ± 11 bpm at supine, 79 ± 13 bpm at sitting and 89 ± 11 bpm at tilted-up position.
There was a significant variation of systemic hemodynamic parameters at the three postural conditions. ANOVA test revealed a significant variation for systolic and diastolic systemic blood pressure and heart rate when comparing the three positions, as expressed in Fig. 1.
The post hoc analysis showed that the arterial pressure had a significant increase in sitting and tilted-up positions compared with lying position. There was no significant variation between sitting and tilted up positions. For heart rate the values were significantly higher in upright position comparing to seat position and also between sitting position and lying position (Table 1).
Cerebrovascular data:
Blood flow velocity monitored in the middle cerebral artery showed no statistical significant differences comparing resting and reading conditions. Neither resting flow velocities in the PCA nor flow velocity in MCA varied with orthostatic conditions.
For each postural condition, Fig. 2a shows the subjects averaged measured and modelled relative changes in peak systolic visual evoked blood flow velocity against time, in seconds, in relation to non-stimulating conditions. The beginning of the stimulation phase represents the zero point of time scale. All the curves showed the characteristic dynamic time course of neurovascular coupling with an initial acceleration phase, and after an overshoot the velocity stabilized at a constant lower level, that ended with closure of the eyes. The modelled curves correspond to a PDT2 system. The same is shown in Fig. 2b for the end diastolic evoked flow curves.
Relative blood flow velocity change in the P2-segment of the posterior cerebral artery due to the reading tests plotted against time. The black curves show the averaged data of the volunteers with the data from supine, sitting and tilted-up positions. Plotted in grey are the modelled blood flow data of the second-order linear system. Recorded and modelled time course of blood flow show near congruency. A – peak systolic evoked flow curve. B – end diastolic evoked flow curve
According to the equation G(s), the parameters rate time, natural frequency, attenuation and gain were calculated. The results of the analysis with mean and SD of the control-system parameter values for sitting, supine ant tilted-up positions are described in Table 2. There was no significant change of resting flow velocity and evoked flow velocity parameters in the cerebral blood flow velocities both for systolic and for diastolic values between sitting, supine and tilt-up positions. The results of the ANOVA test are also given in Table 2.
Discussion
From the results of our Doppler investigation we conclude that in healthy subjects the dynamic regulative properties of the neurovascular coupling mechanism are not affected by the tested orthostatic conditions. It appears that the cerebral autoregulative mechanism allows an operation of the neurovascular coupling independent from the systemic circulation. Furthermore, the compensative range appears wide enough so that both mechanisms do not interfere adversely at the level of the resistance vessels. The near to congruent flow velocity responses indicates a fine tuned interplay between both mechanisms.
A transcranial Doppler approach was utilized in the present investigation because a diagnostic tool with a high temporal resolution and a high flexibility to allow investigations under different orthostatic conditions was needed. Although the Doppler measures rather velocity than flow, there is good evidence that the flow velocity changes correlate closely with flow changes [8, 10]. Calibre effects of the insonated basal cerebral vessels between the different conditions are not likely, as it was shown that basal cerebral vessels remain constant in pressure changes of up to 20 mmHg [2, 5, 17]. We performed a visual stimulation since it does not affect cardiac or systemic hemodynamic parameters and the changes between phases of activation and rest can be carried out easily by opening and closing the eyes. Furthermore, the low spatial resolution of the technique is not a limitation, since the insonated posterior cerebral artery almost exclusively supplies the visual cortex.
A limitation of the non-invasive study is that we did not measure the cerebral perfusion pressure directly, but assumed the change from measurements of systemic blood pressure. According to standardized protocols in the literature we measured the blood pressure at heart level and abstained from Finapres measurements at the brain level. Nevertheless, for the interpretation of data the hydrostatic difference between heart and brain that emerged in the transition from a supine to a sitting or standing position has to be considered. Since the hydrostatic difference affects the arterial as well as venous side of the cerebral circulation simultaneously and in an opposite manner the orthostatic difference should be neutralised. Therefore, changes in arterial blood pressure will increase the cerebral perfusion pressure. However, the transition from a sitting to a standing phase does not have an affect on hydrostatic pressure. Compensating an additional pooling effect of venous blood in the legs the heart rate increases maintaining systemic blood pressure constant. The present data support former studies which showed that physiologic variation in heart rate does not affect the neurovascular coupling [14].
A slight drop in the CO2 partial pressure changing from a supine to a standing position has been reported in literature [6]. It was assumed to be caused by a downward orientation of the diaphragma, increase in tidal volume and functional residual capacity and thus better ventilation [6]. Since the pCO2 level bears a strong effect on the cerebral blood flow [13] such an effect also has to be considered. We abstained from capnography to minimize additional effects in measurements. Mask ventilation itself can affect the respiration, might lead to stress and can affect the cerebral circulation, an effect described as R-waves in the literature [4]. Although it is known that a change in the pCO2 pressure co-directionally changes flow velocity levels as well as the rate time parameter in functional TCD tests, since we did not find such differences we assume that the pCO2 levels remained stable in the present investigation. Unspecific effects, such as changes in attention, systemic blood pressure, or ventilation, due to changing between conditions of rest and activation can be excluded since flow velocity levels in the MCA territory remained nearly constant.
Taken together we conclude that the data from activity-induced flow velocity responses measured under different orthostatic conditions can be compared with each other as long as an intact cerebral autoregulation can be assumed.
Further studies have to follow to investigate the matter in patients with orthostatic intolerance in which regulative properties of the cerebral autoregulation are exhausted and thus regulative properties of the neurovascular coupling can also be affected.
References
Aaslid R (1992) Cerebral hemodynamics. Raven Press, New York
Aaslid R, Lindegaard KF, Sorteberg W, Nornes H (1989) Cerebral autoregulation dynamics in humans. Stroke 20:45–52
Aaslid R, Markwalder TM, Nornes H (1982) Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57:769–774
Diehl RR, Linden D, Lucke D, Berlit P (1998) Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res 8:7–12
Giller CA, Bowman G, Dyer H, Mootz L, Krippner W (1993) Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32:737–741; discussion 741–732
Gisolf J, Wilders R, Immink RV, van Lieshout JJ, Karemaker JM (2004) Tidal volume, cardiac output and functional residual capacity determine end-tidal CO2 transient during standing up in humans. J Physiol 554:579–590
Iadecola C (1993) Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci 16:206–214
Kirkham FJ, Padayachee TS, Parsons S, Seargeant LS, House FR, Gosling RG (1986) Transcranial measurement of blood velocities in the basal cerebral arteries using pulsed Doppler ultrasound: velocity as an index of flow. Ultrasound Med Biol 12:15–21
Melsa JL SD, Rohrs CE (1990) Linear Control Systems. Prentice Hall
Newell DW, Aaslid R (1992) Transcranial Doppler: clinical and experimental uses. Cerebrovasc Brain Metab Rev 4:122–143
Omboni S, Parati G, Frattola A, Mutti E, Di Rienzo M, Castiglioni P, Mancia G (1993) Spectral and sequence analysis of finger blood pressure variability. Comparison with analysis of intra-arterial recordings. Hypertension 22:26–33
Panerai RB (1998) Assessment of cerebral pressure autoregulation in humans–a review of measurement methods. Physiol Meas 19:305–338
Paulson OB, Strandgaard S, Edvinsson L (1990) Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2:161–192
Rosengarten B, Budden C, Osthaus S, Kaps M (2003) Effect of heart rate on regulative features of the cortical activity-flow coupling. Cerebrovasc Dis 16:47–52
Rosengarten B, Huwendiek O, Kaps M (2001) Neurovascular coupling and cerebral autoregulation can be described in terms of a control system. Ultrasound Med Biol 27:189–193
Rosengarten B, Huwendiek O, Kaps M (2001) Neurovascular coupling in terms of a control system: validation of a second-order linear system model. Ultrasound Med Biol 27:631–635
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL (2000) MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31:1672–1678
Acknowledgements
The authors thank DWL for technical support with transcranial Doppler Multidop T2 Doppler device (DWL, Sipplingen, Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 10 June 2006
Rights and permissions
About this article
Cite this article
Azevedo, E., Rosengarten, B., Santos, R. et al. Interplay of cerebral autoregulation and neurovascular coupling evaluated by functional TCD in different orthostatic conditions. J Neurol 254, 236–241 (2007). https://doi.org/10.1007/s00415-006-0338-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0338-1