Abstract
Transcranial Doppler ultrasonography (TCD) is used widely to evaluate dynamic cerebral autoregulation (dCA). However, the validity of TCD-determined dCA remains unknown because TCD is only capable of measuring blood velocity and thus only provides an index as opposed to true blood flow. To test the validity of TCD-determined dCA, in nine healthy subjects, dCA was evaluated by transfer function analysis (TFA) using cerebral blood flow (CBF) or TCD-measured cerebral blood velocity during a perturbation that induces reductions in TCD-determined dCA, lower body negative pressure (LBNP) at two different stages: LBNP − 15 mmHg and − 50 mmHg. Internal carotid artery blood flow (ICA Q) was assessed as an index of CBF using duplex Doppler ultrasound. The TFA low frequency (LF) normalized gain (ngain) calculated using ICA Q increased during LBNP at − 50 mmHg (LBNP50) from rest (P = 0.005) and LBNP at − 15 mmHg (LBNP15) (P = 0.015), indicating an impaired dCA. These responses were the same as those obtained using TCD-measured cerebral blood velocity (from rest and LBNP15; P = 0.001 and P = 0.015). In addition, the ICA Q-determined TFA LF ngain from rest to LBNP50 was significantly correlated with TCD-determined TFA LF ngain (r = 0.460, P = 0.016) despite a low intraclass correlation coefficient. Moreover, in the Bland–Altman analysis, the difference in the TFA LF ngains determined by blood flow and velocity was within the margin of error, indicating that the two measurement methods can be interpreted as equivalent. These findings suggest that TCD-determined dCA can be representative of actual dCA evaluated with CBF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Middle cerebral artery blood velocity (MCA V), measured using transcranial Doppler ultrasonography (TCD) is used worldwide as an index of cerebral blood flow (CBF), however, this concept is based on the premise that vessel diameter of the MCA is constant. Adding to the validity of TCD measures, some previous studies have demonstrated that changes in MCA V were tightly related to that of blood flow measured via duplex Doppler ultrasound in the upstream artery of the MCA during supine exercise and carbon dioxide stimulation; i.e. internal carotid artery [1, 2]. Thus, it has been thought that change in TCD-measured MCA V is a valid measure of CBF. However, more recent studies, utilizing sophisticated imaging technology, have demonstrated that MCA diameter can be altered during severe physiological conditions (e.g. hypo- and hypercapnia, rhythmic handgrip exercise) [3,4,5,6], indicating the possibility that the TCD-measured MCA V signal does not always accurately reflect true CBF in the MCA.
Dynamic cerebral autoregulation (dCA) in humans is an important mechanism for maintaining adequate CBF in response to transient changes in arterial blood pressure (ABP) [7, 8]. In many previous investigations, the dCA has been evaluated non-invasively by the thigh-cuff occlusion release technique [9,10,11] and transfer function analysis (TFA) during resting conditions as well as during various perturbations [12,13,14]. Notably, almost all previous studies used the TCD-measured MCA V as an index of CBF as opposed to measuring CBF directly in order to evaluate dCA. In the thigh-cuff occlusion release technique, dCA-induced change in MCA V during acute hypotension was similar for that determined by internal carotid artery blood flow (ICA Q) [15], supporting the use of MCA V to identify dCA instead of using CBF (i.e., directly measured ICA Q). The TFA evaluates dCA by the relationship between beat-to-beat spontaneous oscillations of MCA V and arterial blood pressure (ABP) via frequency domain analysis and does not require evoked changes in ABP [12,13,14]. In the comparison of dCA assessed by TFA in ICA and MCA, studies [16, 17] have reported that dCA evaluated by TFA using both MCA V and ICA blood velocity provide similar results. However, the TCD signal data may not assess CBF accurately because it is not capable of assessing vessel diameter. Moreover, relative changes in TFA parameters, especially TFA gain, should be evaluated to estimate dCA [18], however, in these previous studies, the experimental condition was not varied (only resting condition) and therefore dCA was not manipulated to fully evaluate the validity of TFA using MCA V. More importantly, it has been reported that the steady-state MCA diameter was unchanged [19], while a variation of cerebral arterial diameter may occur during more extreme alterations in steady-state hemodynamics [4,5,6, 20, 21]. It is possible that even small variations of cerebral arterial diameter should affect frequency domain analysis, such as TFA to identify dCA. Thus, the validity of the dCA evaluated by TFA using TCD-measured cerebral blood velocity instead of using CBF remains unclear.
TCD-determined TFA is used worldwide as the standard for dCA assessment, however, under these backgrounds, the limitation of TCD technique (using blood velocity instead of flow) may affect dCA assessment using TFA. Thus, the aim of the present study was to examine the validity of dCA evaluated by TFA using TCD-measured blood velocity during a condition that has been reported to change dCA. The change in TCD-determined dCA should be the same as that of CBF-determined dCA. In this regard, is has been previously reported [13] that orthostatic stress, e.g. lower body negative pressure (LBNP) at − 50 mmHg, attenuates dCA evaluated by TFA using TCD-measured MCA V. Therefore, in the present study, we used LBNP as a physiological stimulus to examine the validity of TFA parameters using TCD-measured MCA V in comparison to dCA assessed using ICA Q measures. Notably, one previous study using magnetic resonance angiography reported that LBNP does not change MCA diameter [19]. Herein, we hypothesized that ICA Q-determined dCA by TFA is attenuated during LBNP at − 50 mmHg, similarly with MCA V-determined dCA. To test this hypothesis, we continuously measured CBF (ICA Q), cerebral blood velocity (MCA V), and ABP at rest and during LBNP to directly compare TFA-determined dCA measures using CBF and cerebral blood velocity.
2 Material and methods
2.1 Subjects
Nine healthy young male adults with a mean age of 24 ± 4 year, height of 171 ± 4 cm, and weight of 64 ± 4 kg were recruited. All subjects were non-smokers, had no known cerebrovascular, cardiovascular, or pulmonary disorders, and were not taking any medications at the time of enrollment. In addition, none were sedentary, nor were any high-level athletes, and the subjects were excluded if they performed either dynamic or static exercise for 30 min/day more than three times a week [14]. Thus, all subjects were considered normal healthy adults. Before the experiment, the subjects were required to abstain from caffeinated beverages for 12 h, and alcohol for 24 h, and also strenuous exercise for 24 h. It has been reported that TFA metrics are impacted up to 6 h following exercise [22]. Furthermore, the subjects were instructed to consume a light meal 3 h prior to the start of the experiment in order to minimize the potential effect of individual meal on cardiorespiratory and cerebrovascular responses [23]. This study was approved by the ethics committee of Kyorin University (Approval Number 723) in accordance with the Declaration of Helsinki. All subjects gave informed consent before commencing the experiments.
2.2 Experimental procedure
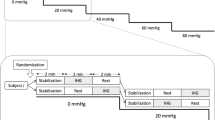
Given the within- and between-day reproducibility of dCA quantified using TFA on spontaneous blood pressure oscillations, all measurements were simultaneously performed within the same day before or after midday for each subject [24, 25]. Upon arrival to the laboratory, the subjects were fitted with an electrocardiogram and finger photoplethysmography as well as TCD probe (see Experimental measurement). The subjects lied in the supine position with their legs placed in the LBNP chamber that was sealed at the level of the iliac crest with a flexible rubber dam. The shoulder joint was maintained in slight abduction, while the arm was flexed slightly with the forearm pronated. The head was immobilized using towels to prevent unwanted movements. Air pressure inside the LBNP chamber was lowered using a vacuum source and controlled with a variable transformer. To familiarize the subjects with LBNP and the vacuum noise, a short-period of LBNP exposure was applied, and then subjects rested at least 20 min before the experimental protocol commenced. Following a 5 min baseline measurement of quiet rest (rest), LBNP was administered in a stepwise approach of graded decreases in pressure according to the following protocol: − 15 mmHg × 7 min (LBNP15), and − 50 mmHg × 7 min (LBNP50). We decided to use the graded LBNP manner rather than randomized approach to be consistent with the previous studies [13, 14] for relatively rapid cardiovascular stabilization during each LBNP level and ensuring stable recordings that are essential for spectral analysis [26]. At each LBNP condition, 5 min measurements started after 2 min exposure for stabilization of cerebrovascular and cardiorespiratory hemodynamics. The previous study has suggested that ABP changes from supine to standing position becomes steady-state within 2 min [27]. Given that the fluid shift from supine to the standing position would vastly exceed that which occurs during LBNP transition from rest to LBNP15 and from LBNP15 to LBNP50, 2 min-LBNP exposure at each LBNP level should be appropriate as a methodology. Then, 5 min of beat-to-beat cerebrovascular and cardiorespiratory data were obtained for the determination of the steady-state mean value and TFA analysis as described below. The absolute values derived for dCA, such as those from TFA, are limited by their inherently high individual variability [28]. This may partly arise from anatomical variability in the diameter of the vessel of interest or the orientation of the vessel to the TCD probe. Therefore, TFA assessments of dCA are better suited to the comparison between different conditions [18]. In other words, in order to identify the validity of TFA assessment, we need to manipulate dCA. A previous study [13] demonstrated that LBNP increases TFA low frequency (LF) gain, indicating an impairment in dCA. For this reason, we used the LBNP to manipulate dCA to examine the validity of TCD-determined TFA data in the present study.
2.3 Experimental measurement
Heart rate (HR) was measured by using a lead II electro-cardiogram (bedside monitor, BMS-3400; Nihon Kohden, Tokyo, Japan). ABP was monitored using volume clamp method with finger photoplethysmography (Finometer MIDI; Finapres Medical Systems, Amsterdam, the Netherlands). End-tidal carbon dioxide (EtCO2) was measured using a nasal capnometer (OLG-3800; Nihon Kohden). Based on a standardized methods [29], MCA V was measured through the right temporal window by using transcranial Doppler ultrasonography (DWL Doppler Box-X; Compumedics, Singen, Germany) equipped with a 2.0 MHz pulsed TCD probe. The TCD probe was fixed and held in measurement position using an adjustable headband (Elastic Headband T; Compumedics) to maintain a constant insonation angle throughout experiment. To determine the ICA Q, the right-side ICA blood velocity and diameter were continuously measured by using a color-coded ultrasound system (Vivid-i; GE Healthcare Systems, Chicago, USA) equipped with a 13.0 MHz linear transducer from baseline and during LBNP conditions. The probe was placed at 1.0–1.5 cm distal to the carotid bifurcation, while the subject’s chin was slightly elevated. For obtaining stable data, the subjects were instructed to keep breathing stable and to avoid swallowing as much as possible during measurements. When measuring ICA blood velocity and diameter, a trained investigator took care of ensuring that the hand-held probe position was stable throughout the experiment at as low as an insonation angle to get the best Doppler signal individually (less than 30 deg. from the artery) for accurately determining blood flow as possible [30, 31]. Moreover, the probe was attached to the same location such that the same vessel image could be acquired in the center of the B-mode images across all conditions as much as possible. The longitudinal vessel image (B-mode) and velocity signal (PW-mode) were continuously and simultaneously measured and then stored on a personal computer at 30 Hz using a capture box (VGA2USB LR; Epiphan Systems, Ottawa, Canada) for offline analysis. ICA time-averaged mean velocity and diameter were analyzed at 30 Hz using a custom-designed edge detecting and wall tracking software (S-13037 version 2.0.1; Takei Scientific Instruments, Niigata, Japan).
2.4 Data acquisition
HR, ABP, EtCO2, and MCA V were recorded at a sampling rate of 1000 Hz using a 16-bit A/D converter (Power Lab 16 s; ADInstruments, Sydney, Australia) and stored on a personal computer. The onset of data acquisition between computers were synchronized via a trigger generator. ICA Q was calculated by multiplying the cross-sectional area with time-averaged mean velocity; ICA Q = [π × (ICA diameter/2)2] × ICA time-averaged mean velocity × 60 (ml/min). MCA cerebral vascular conductance index (MCA CVCi) and ICA cerebral vascular conductance (ICA CVC) were estimated from the ratio of MCA V or ICA Q to mean ABP, respectively. All cardiorespiratory and cerebrovascular variables were averaged over 5-min for each condition.
2.5 Dynamic cerebral autoregulation analysis
According to previous studies [12, 32], mean ABP (MAP), MCA V (MCA Vmean), and ICA Q (ICA Qmean) were obtained across each cardiac cycle, linearly interpolated, and resampled at 4 Hz for spectral analysis of dCA [33]. dCA was calculated as the transfer function gain and phase shift between fluctuations in MAP and MCA Vmean or ICA Qmean [34]. The transfer function gain and phase shift reflect the relative amplitude and time relationship between the changes in MAP and MCA Vmean or ICA Qmean over a specified frequency range. From the temporal sequences of MAP and MCA Vmean or ICA Qmean, the frequency-domain transformations were computed with a fast Fourier transformation algorithm. The transfer function H(f) between the two signals was calculated as H(f) = Sxy(f)/Sxx(f), where Sxx(f) is the autospectrum of changes in MAP and Sxy(f) is the cross-spectrum between MAP and MCA Vmean or ICA Qmean. The transfer function magnitude |H(f)| and phase spectrum |Φ(f)| were obtained from the real part HR(f) and imaginary part HI(f) of the complex function. Moreover, the transfer function H(f) was normalized to the mean values of input (x) and output (y) variables as H′(f) = [Sxy(f)x]/[Sxx(f)y], and the normalized gain (ngain) was calculated as 20 log H′(f) to provide values in decibels. Spectra were calculated using 100-s window length with 50% overlap, and smoothing was accomplished by employing a Hanning window [33]. The transfer function coherence, phase, and ngain were calculated in the very low (VLF: 0.02–0.07 Hz), low (LF: 0.07–0.20 Hz) and high frequency (HF: 0.20–0.30 Hz) ranges, as previously described [35]. The VLF range of both blood flow and blood pressure variability appears to reflect multiple physiological mechanisms that confound interpretation [36]. ABP fluctuations in the HF range are induced primarily by respiration, however, those in the LF range are independent of the respiratory frequency and are dampened by dCA [37]. In the present study, therefore, we focused on the TFA data within LF range for dCA parameters to examine the validity of TCD-determined dCA. Indeed, previous studies employed the TFA parameter within LF range as an index of dCA [13, 36, 38]. More importantly, the previous study [13] demonstrated that LBNP changed only LF gain (increase in LF gain). In the TFA, in all subjects, coherence in LF range assessed by MCA V and ICA Q was greater than 0.40 for each condition, suggesting that there was little effect of signal noise on the validity of transfer function analysis [34, 39]. It is noted that the cut-off value for coherence with a recommended setting for TFA estimation in the present study (5 windows and α = 0.05) is 0.34 [33], thus 0.40 is valid to be the critical value of coherence in the present study.
2.6 Statistical analysis
With reference to data of TFA ngain assessed by MCA V in our pilot study (n = 3), a prior power analysis using expected effect size (Effect size f = 0.602) revealed that a sample of more than 6 subjects was needed to obtain 80% power with 3 conditions (α = 0.05). Thus, the sample size of the present study (n = 9) was sufficient to achieve the desired statistical assurance.
Data are expressed as mean ± SD in the text, tables, and figures. The Shapiro–Wilk’s test was applied to verify the normal distribution for each variable. The distribution normality was confirmed in variables (W ≥ 0.739, P ≥ 0.058), excluding HR, and VLF phase and VLF coherence assessed by MCA V, VLF ngain, HF ngain, LF coherence, and HF coherence assessed by ICA Q (W ≥ 0.688, P ≤ 0.045). To compare normally distributed outcomes between conditions, the one-way repeated measures analysis of variance (ANOVA) with Tukey's honestly significant difference test was applied to identify the significant difference in measurement parameters amongst conditions. Greenhouse–Geisser correction was applied when sphericity assumption was violated. For non-normal variables, non-parametric ANOVA (Friedman test) followed by Wilcoxon matched-pairs tests were performed amongst conditions. Statistical significance was set at P < 0.05. While informative reliance on only the p-value has been reported to potentially result in methodological errors and misinterpretations [40, 41]. Accordingly, the effect sizes (ES) were calculated as eta squared (η2) for one-way ANOVA outcomes, as Cohen’s d for all post-hoc comparisons, and as Kendall’s W for non-parametric ANOVA outcomes using the spreadsheet provided by Lakens [42]. Conservative interpretation guidelines for effect size were defined as small (η2 = 0.01, Cohen’s d = 0.20, Kendall’s W = 0.10), medium (η2 = 0.06, Cohen’s d = 0.50, Kendall’s W = 0.30), and large (η2 = 0.14, Cohen’s d = 0.80, Kendall’s W = 0.50) effect [42, 43]. A Pearson product-moment correlation coefficient was calculated to determine the relationship between absolute values in the LF ngain during rest, LBNP15, and LBNP50 assessed by MCA V and ICA Q. To verify whether LF ngain assessed by TCD-determined cerebral blood velocity (MCA V) matches those values assessed by the actual cerebral blood flow (ICA Q), intraclass correlation coefficient (ICC) estimates and their 95% confident intervals were calculated using a two-way random-effects model (absolute agreement definition) were used to determine the reliability of measurements. Moreover, the absolute difference between changes in MCA V- and ICA Q-determined TFA LF ngain from rest to LBNP15 or LBNP50 were visualized using Bland–Altman plots and 95% upper and lower limits of agreement (LOA; mean difference ± 1.96 SD) and mean difference (or Bias) were computed [44]. All data were analyzed using the SPSS software (SPSS Statistics 25; IBM, Tokyo, Japan).
3 Results
3.1 Cardiorespiratory and cerebrovascular parameters
HR was increased during LBNP50 compared with rest and LBNP15, while MAP was increased during LBNP15 and LBNP50 compared with rest (Table 1 and Fig. 1). EtCO2 during LBNP50 decreased compared with rest and LBNP15. In contrast, during LBNP50 MCA Vmean and ICA Qmean decreased, and consequently MCA CVCi and ICA CVC were also decreased.
Representative individual data of the raw wave and beat-to-beat-variation of arterial blood pressure (ABP), middle cerebral artery blood velocity (MCA V), and internal carotid artery blood flow (ICA Q) at rest and, during − 15 mmHg (LBNP15) and − 50 mmHg (LBNP50) lower body negative pressure. Gray and black lines denote the waveform and mean values, respectively
3.2 Dynamic cerebral autoregulation (dCA)
The group-averaged TFA data is shown in Fig. 2 and Table 2. For calculating TFA phase and ngain assessed by MCA V and ICA Q, both LF coherences were ≥ 0.4. In the TFA between MAP and MCA Vmean, the one-way ANOVA revealed that there was no significant change of LF phase amongst conditions (F2,16 = 2.367, P = 0.126, η2 = 0.189 [large]), whereas LF ngain differed amongst conditions (F2,16 = 9.748, P = 0.002, η2 = 0.175 [large], Fig. 3). Post hoc analysis revealed that LF ngain was larger during LBNP50 compared with rest and LBNP15 (rest vs. LBNP50: P = 0.001, Cohen’s d = 1.325 [large] and LBNP15 vs. LBNP50: P = 0.015, Cohen’s d = 1.037 [large]), but there were no differences between rest and LBNP15 (P = 0.135, Cohen’s d = 0.620 [medium]). In the TFA between MAP and ICA Qmean in LF (Table 2), there was no change of LF phase (F2,16 = 0.500, P = 0.616, η2 = 0.034 [small]) similar to the result of TFA assessed by MCA Vmean. Moreover, there was difference in LF ngain between conditions (F2,16 = 8.152, P = 0.004, η2 = 0.281 [large]). Post hoc analysis revealed that LF ngain was larger during LBNP50 compared with rest and LBNP15 (rest vs. LBNP50: P = 0.005, Cohen’s d = 1.023 [large] and LBNP15 vs. LBNP50: P = 0.015, Cohen’s d = 1.030 [large]), but there were no differences between rest and LBNP15 (P = 0.844, Cohen’s d = 0.276 [small]).
Cross-spectral analysis of the entire spectrum from 0 to 0.3 Hz at rest, during − 15 mmHg (LBNP15) and − 50 mmHg (LBNP50) lower body negative pressure. The group-averaged transfer function phase (upper), normalized gain (ngain, middle) and coherence function (bottom) between mean arterial pressure (MAP) and mean middle cerebral artery blood velocity (MCA Vmean, dotted line), and between MAP and mean internal carotid artery blood flow (ICA Qmean, solid line) are shown
Group-average data combined with dot plot showing the distribution of low frequency ngain assessed by mean middle cerebral artery blood velocity (MCA Vmean, the left panel) and internal carotid artery blood flow (ICA Qmean, the right panel) at rest, and during − 15 mmHg (LBNP15) and − 50 mmHg (LBNP50) lower body negative pressure. *P < 0.05 compared with rest; †P < 0.05 compared with LBNP15
3.3 The agreement and reliability of the dCA evaluation in the TFA between using CBF and CBF velocity data
The relationship between ICA Q- or MCA V-determined LF ngains (95% CI 0.097–0.715, r = 0.460, P = 0.016) among all conditions was significant (Fig. 4). However, the calculated ICC was very low in ngain (95% CI 0.005–0.655, ICC(2,1):0.373, P = 0.007). In the Bland–Altman analysis for changes in LF ngain from rest to LBNP assessed by ICA Q and MCA V, the biases were very low and all plots were within the LOA (change from rest to LBNP15, 95% CI − 0.889–0.557; change from rest to LBNP50, 95% CI − 0.728–0.810, Fig. 5).
Relationship between low frequency normalized gains (LF ngain) determined by mean internal carotid artery blood flow (ICA Qmean) and middle cerebral artery blood velocity (MCA Vmean). Hairlines indicate the 95% CI for the regression line. CI, confidence interval; ICC, intraclass correlation coefficients
Bland–Altman plots of changes low frequency normalized gains (LF ngain) determined by mean internal carotid artery blood flow (ICA Qmean) and middle cerebral artery blood velocity (MCA Vmean) from rest during − 15 mmHg (LBNP15, the left panel) and − 50 mmHg lower body negative pressure (LBNP50, the right panel). The solid line represents the mean difference in ICA Qmean and MCA Vmean, while the dashed lines represent the 95% upper and lower limits of agreement (LOA, mean difference ± 1.96 SD)
4 Discussion
The findings of the present study demonstrated for the first time that dCA evaluated with MCA V measured from TCD can be representative of actual dCA evaluated with direct measures of ICA Q by using TFA. The Pearson correlation between ICA Q- and MCA V-determined LF ngains from rest to LBNP was significant despite a low ICC. Moreover, the Bland–Altman analysis indicated the agreement between changes in dCA evaluated using two TFA quantitative measurements. These findings suggest that TCD-determined dCA at least during orthostatic stress was not affected by its inherent methodological limitation (using velocity instead of flow data).
In the present study, ICA Q was used as an index of CBF because it is difficult/impossible to measure blood flow in the MCA in vivo in humans due to the limitations associated with assessing vessel diameter in this region utilized TCD. The ICA is the upstream artery of the MCA, and changes in MCA V parallels that of ICA Q [1, 2]. Moreover, the diameter of MCA was confirmed to be unchanged during LBNP, particularly lower levels of LBNP as used in this study [19], thus it can be speculated that an alteration in MCA V during orthostatic stress is similar with that of ICA Q. In the present study, indeed, the decrease in MCA V from baseline (e.g., supine rest) to − 50 mmHg LBNP was similar to that of ICA Q (MCA V vs. ICA Q, − 11.2 ± 10.0% vs. − 15.1 ± 9.2%, P = 0.329). However, importantly, the absolute MCA V value is different from that of ICA Q (blood velocity vs. blood flow). Thus, to compare dCA between the different flow parameters (MCA V vs. ICA Q), TFA gain needs to be normalized to baseline values of these parameters as an index of dCA, while TFA phase and coherence are not affected by the different absolute values of MCA Vmean and ICA Qmean. As such, LF ngain was calculated between MCA Vmean or ICA Qmean and MAP from rest to LBNP in order to compare TFA data between different analyses. We found that ngain between MCA Vmean and MAP within the LF range increased during LBNP50 from rest and LBNP15 (rest vs. LBNP50: P = 0.001 and LBNP15 vs. LBNP50: P = 0.015, Fig. 3), indicating that dCA was impaired during orthostatic stress. This finding is consistent with the previous study [34], showing that LBNP at − 50 mmHg increased TFA LF gain between the changes in MAP and MCA Vmean. In this previous report, the authors argued that this impairment of dCA likely contributes to orthostatic intolerance/syncope. Similarly, we found that ICA Q-determined TFA LF ngain was also increased from rest and LBNP15 to LBNP50 (rest vs. LBNP50: P = 0.005 and LBNP15 vs. LBNP50: P = 0.015). In addition, the Pearson correlation between MCA V- and ICA Q-determined LF ngains among all conditions (95% CI, 0.097–0.715, r = 0.460, P = 0.016) was significant (Fig. 4). These findings suggest that changes in dCA determined by TCD-measured cerebral blood velocity reflect that of actual dCA determined by flow data at least during orthostatic stress.
However, the intra-class correlation coefficients between MCA V- and ICA Q-determined ngains were very low (95% CI 0.005–0.655, ICC(2,1): 0.373, P = 0.007, Fig. 4). The change in dCA evaluated using TCD can be representative of actual change in dCA evaluated with direct measures of ICA Q during orthostatic stress, however, these findings indicate that individual value of MCA V-determined LF ngain was not equal to that determined by ICA Q probably because those were calculated by the same method (TFA) using different measurements (velocity and flow). However, caution may be used when dCA is derived using TCD-measured cerebral blood velocity. Regarding the issue for ICC evaluation, a Bland–Altman plot was used to analyze the agreement in a change in dCA in the TFA between two different measurements (velocity vs. flow). In this analysis, the biases were very low and all plots were within the LOA, indicating the agreement between changes in ngain from rest to LBNP evaluated in TFA using two TFA quantitative measurements. In addition to the result of the Pearson correlation, these findings suggest that TCD-determined dCA at least during orthostatic stress was not affected by the measurement limitation in TFA (using velocity instead of flow data).
Several possible mechanisms for the divergent responses between dCA derived from MCA V and ICA Q warrant discussion. Although a previous study demonstrated that the high level of LBNP does not necessarily change the diameter of MCA [19], it is possible that fluctuations of MCA diameter contribute. The variation of cerebral artery diameter may be an effective damping function for blood flow or increase in a fluctuation in MCA V despite no change in average of diameter. Changes in perfusion pressure may cause even small changes in MCA diameter via cerebral autoregulation. For example, increase in perfusion pressure may cause vasoconstriction of the MCA via a myogenic response (i.e., cerebral autoregulation) and consequently, according to Poiseuille’s law, result in relatively large decrease in CBF despite MCA V being maintained. Also, a decrease in perfusion pressure may cause vasodilation of the MCA via cerebral autoregulation and consequently result in relatively large increases in CBF. Thus, cerebral autoregulation-induced MCA vasomotion may acutely enhance dCA for CBF regulation with small change in MCA V, even as the diameter change is small and the average of MCA diameter is unchanged. In addition, variability of blood velocity changes cerebral shear rate which could cause changes in diameter of the cerebral arteries [45]. For example, an increase or decrease in blood velocity changes shear rate, which could increase or decrease the diameter of cerebral artery, respectively, via endothelial function, leading to enhanced dCA for CBF regulation. This transient change in cerebral artery diameter may also increase the variation of CBF compared with that of MCA V. Indeed, one previous study demonstrated that endothelial function contributes to CBF regulation [46]. However, because these are all based on speculation, we need further investigations to identify the underlying mechanism for the future use of TCD data for TFA.
Some limitations to the present study deserve further discussion. First, the data of the current study did not include female and/or elderly individuals. Thus, these results might not be adapted to the general population. It is possible that dCA is influenced by sex- and age-related differences [47, 48]. Further studies including subjects across multiple generations and genders are warranted to clarify this issue. Second, even a slight change in the insonation angle of the probe impacts the reflected signal and alters the calculated CBF value. In the present study, the operators of the Doppler measurement maintained the insonation angle of the probe by checking the blood vessel image as frequently as possible. However, we do not have any technique to reduce this limitation. Lastly, the TFA LF coherence assessed by ICA Q was smaller than that assessed by MCA V, thus the validity of the two measurements in TFA may be different. The different coherence between two TFA data using ICA Q and MCA V) may affect the comparison results. Thus, for example, in order to increase TFA LF coherence, the previous studies have used a squat-stand maneuver [49] or oscillatory LBNP [25] as the methodological approach for sufficient reliability. Also, in the present study, we could not neglect the possibility that these technical issues may cause a poor validity of analyzed data (e.g. ngain comparing between ICA Q and MCA V). Further studies using various techniques and methodological approaches to increase TFA coherence may be needed to increase the validity of the findings of the present study.
5 Conclusion
We found that ICA Q-determined TFA LF ngain was increased from rest and LBNP15 to LBNP50, similarly with those of MCA V-determined TFA LF ngain. In addition, the relationship between MCA V- and ICA Q-determined TFA LF ngains was statistically significant. These findings suggest that dCA evaluated using TCD can be representative of actual dCA evaluated with direct measures of ICA Q during orthostatic stress. Moreover, in the Bland–Altman analysis, the difference in the TFA LF ngains determined by blood flow and velocity was within the margin of error, indicating that the two measurement methods can be interpreted as equivalent. Accordingly, the findings of the present study demonstrated that TCD-determined dCA was not affected by the measurement limitation (using velocity instead of flow data) and thus indicates that this traditional technique can be used as the standard approach for assessing dCA.
Data availability
The data that supports the findings of this study are available within the article.
References
Hellström G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol. 1996;81:413–8.
Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, et al. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol. 2012;590:3277–90.
Ainslie PN, Hoiland RL. Transcranial doppler ultrasound: Valid, invalid, or both? J Appl Physiol. 2014;117:1081–3.
Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol. 2014;117:1090–6.
Coverdale NS, Lalande S, Perrotta A, Shoemaker JK. Heterogeneous patterns of vasoreactivity in the middle cerebral and internal carotid arteries. Am J Physiol - Hear Circ Physiol. 2015;308:H1030–8.
Verbree J, Bronzwaer ASGT, Ghariq E, Versluis MJ, Daemen MJAP, Van Buchem MA, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol. 2014;117:1084–9.
Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–559.
Brassard P, Labrecque L, Smirl JD, Tymko MM, Caldwell HG, Hoiland RL, et al. Losing the dogmatic view of cerebral autoregulation. Physiol Rep. 2021;9:1–11.
Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol. 2012;97:1272–80.
Ogoh S, Sørensen H, Hirasawa A, Sasaki H, Washio T, Hashimoto T, et al. Dynamic cerebral autoregulation is unrelated to decrease in external carotid artery blood flow during acute hypotension in healthy young men. Exp Physiol. 2016;101:1040–9.
Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–7.
Ogoh S, Nakata H, Miyamoto T, Bailey DM, Shibasaki M. Dynamic cerebral autoregulation during cognitive task: effect of hypoxia. J Appl Physiol. 2018;124:1413–9.
Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol. 1998;85:1113–22.
Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effects of head-down-tilt bed rest on cerebral hemodynamics during orthostatic stress. J Appl Physiol. 1997;83:2139–45.
Ogoh S, Lericollais R, Hirasawa A, Sakai S, Normand H, Bailey DM. Regional redistribution of blood flow in the external and internal carotid arteries during acute hypotension. Am J Physiol Integr Comp Physiol. 2014;306:R747–51.
Nogueira RC, Saeed NP, Bor-Seng-Shu E, Teixeira MJ, Robinson TG, Panerai RB. The carotid artery as an alternative site for dynamic autoregulation measurement: an inter-observer reproducibility study. Med Eng Phys. 2016;38:690–4.
Chi NF, Ku HL, Wang CY, Liu Y, Chan L, Lin YC, et al. Dynamic cerebral autoregulation assessment using extracranial internal carotid artery Doppler ultrasonography. Ultrasound Med Biol. 2017;43:1307–13.
Ichikawa D, Miyazawa T, Horiuchi M, Kitama T, Fisher JP, Ogoh S. Relationship between aerobic endurance training and dynamic cerebral blood flow regulation in humans. Scand J Med Sci Sport. 2013;23:e320–9.
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–8.
Verbree J, Bronzwaer AGT, van Buchem MA, Daemen MJAP, van Lieshout JJ, van Osch MJP. Middle cerebral artery diameter changes during rhythmic handgrip exercise in humans. J Cereb Blood Flow Metab. 2017;37:2921–7.
Al-Khazraji BK, Shoemaker LN, Gati JS, Szekeres T, Shoemaker JK. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J Cereb Blood Flow Metab. 2019;39:1204–14.
Burma JS, Copeland P, Macaulay A, Khatra O, Wright AD, Smirl JD. Dynamic cerebral autoregulation across the cardiac cycle during 8 hr of recovery from acute exercise. Physiol Rep. 2020;8:1–15.
Washio T, Sasaki H, Ogoh S. Transcranial Doppler-determined change in posterior cerebral artery blood flow velocity does not reflect vertebral artery blood flow during exercise. Am J Physiol Circ Physiol. 2017;312:H827–31.
Burma JS, Copeland P, Macaulay A, Khatra O, Smirl JD. Comparison of diurnal variation, anatomical location, and biological sex within spontaneous and driven dynamic cerebral autoregulation measures. Physiol Rep. 2020;8:e14458.
Smirl JD, Hoffman K, Tzeng YC, Hansen A, Ainslie APN. Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J Appl Physiol. 2015;119:487–501.
Brown CM, Dütsch M, Hecht MJ, Neundörfer B, Hilz MJ. Assessment of cerebrovascular and cardiovascular responses to lower body negative pressure as a test of cerebral autoregulation. J Neurol Sci. 2003;208:71–8.
Wieling W, Krediet CTP, Van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci. 2007;112:157–65.
Sanders ML, Elting JWJ, Panerai RB, Aries M, Bor-Seng-Shu E, Caicedo A, et al. Dynamic cerebral autoregulation reproducibility is affected by physiological variability. Front Physiol. 2019;10:1–11.
Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods. 2011;196:221–37.
Thomas KN, Lewis NCS, Hill BG, Ainslie PN. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am J Physiol - Regul Integr Comp Physiol. 2015;309:R707–20.
Radparvar JR, Lim G, Chiem AT. Effect of insonation angle on peak systolic velocity variation. Am J Emerg Med. 2020;38:173–7.
Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–20.
Claassen JAHR, Meel-Van Den Abeelen AS, Simpson DM, Panerai RB, Dorado AC, Mitsis GD, et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab. 2015;36:665–80.
Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol - Hear Circ Physiol. 1998;274:H233–41.
Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, et al. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Circ Physiol. 2005;288:H1461–7.
Ogoh S, Fadel PJ, Zhang R, Selmer C, Jans Ø, Secher NH, et al. Middle cerebral artery flow velocity and pulse pressure during dynamic exercise in humans. Am J Physiol Circ Physiol. 2005;288:H1526–31.
Diehl RR, Linden D, Lücke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure a clinical test of autoregulation. Stroke. 1995;26:1801–4.
Ogoh S, Dalsgaard MK, Secher NH, Raven PB. Dynamic blood pressure control and middle cerebral artery mean blood velocity variability at rest and during exercise in humans. Acta Physiol. 2007;191:3–14.
Diehl RR, Linden D, Lücke D, Berlit P. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res. 1998;8:7–12.
Panagiotakos DB. The value of p value in biomedical research. Open Cardiovasc Med J. 2008;2:97–9.
Halsey LG. The reign of the p-value is over: What alternative analyses could we employ to fill the power vacuum? Biol Lett. 2019;15:20190174.
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:1–12.
Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge Academic; 1988.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8:307–10.
Smith KJ, Hoiland RL, Grove R, McKirdy H, Naylor L, Ainslie PN, et al. Matched increases in cerebral artery shear stress, irrespective of stimulus, induce similar changes in extra-cranial arterial diameter in humans. J Cereb Blood Flow Metab. 2019;39:849–58.
Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J Cereb Blood Flow Metab. 2016;36:72–94.
Patel N, Panerai RB, Haunton V, Katsogridakis E, Saeed NP, Salinet A, et al. The Leicester cerebral haemodynamics database: normative values and the influence of age and sex. Physiol Meas. 2016;37:1485–98.
Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, et al. Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep. 2019;7:1–12.
Claassen JAHR, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol. 2009;106:153–60.
Acknowledgments
The authors appreciate the time and effort expended by the volunteer subjects and staffs. Also, the authors would like to thank Prof. Paul Fadel (University of Texas at Arlington) for his helpful scientific comments for improving this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was in part supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant Number 15H003098).
Author information
Authors and Affiliations
Contributions
HW and SO conception and design of research; HW, TW, SS, AH, RS, SS, SO performed experiments; HW analyzed data; HW and SO interpreted results of experiments; HW prepared figures; HW, MB, SO drafted manuscript; all authors edited and revised manuscript; all authors approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest related to the subject matter or materials discussed in this article. All data of the present study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Ethical approval
This study was approved by the ethics committee of Kyorin University (Approval Number 723) in accordance with the Declaration of Helsinki.
Informed consent
All subjects provided written informed consent prior to participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Watanabe, H., Washio, T., Saito, S. et al. Validity of transcranial Doppler ultrasonography-determined dynamic cerebral autoregulation estimated using transfer function analysis. J Clin Monit Comput 36, 1711–1721 (2022). https://doi.org/10.1007/s10877-022-00817-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00817-1