Abstract
Forensic entomology requires knowledge of the developmental rates of the species that colonize a body after death to estimate the postmortem interval (PMI). These developmental rates may vary depending not only on the species but also on the geographic location due to population differences. Therefore, the objectives of this work were to determine the developmental duration of the forensically important fly Chrysomya megacephala under constant controlled and field condition temperatures and to compare these results, through a meta-analysis, with data reported by other authors on populations from different localities. For this, C. megacephala colonies were established in the laboratory, and the duration of the life cycle was studied at two controlled temperatures (25 °C and 27 °C) and field conditions (27.5 ± 3.2 °C). Analysis of variance was performed to determine differences in developmental time and larval length between constant laboratory temperatures and field conditions. A generalized linear model was performed with predictor variables extracted from the literature (diet, relative humidity, latitude, longitude) to evaluate the effect of population variation on developmental times. The results showed significant differences in developmental times between 25 and 27 °C. As expected, the complete life cycle of C. megacephala was shorter at 27 °C. Finally, the meta-analysis suggested differences between the developmental times of different populations, based on temperature and geographic location. The results of this study provide fundamental developmental data to use C. megacephala in PMI estimations. Finally, we suggest that, when making expert reports, information from local populations should be used to determine a more accurate and reliable PMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The estimation of the postmortem interval (PMI), defined as the time elapsed between death and the finding of the corpse, is a crucial step in a death scene investigation [1]. Knowledge of this time helps to reconstruct the events, determine the circumstances of death, link or rule out suspects, or reinforce the witnesses’ testimony [2]. Legal medicine estimates the PMI by using different techniques based on postmortem changes in soft tissue during the first hours after death, such as the drop in body temperature, the presence of lividity, and cadaveric rigidity [3]. However, when corpses initiate the bloating stage, estimates based on pathological criteria become more complex and less accurate [1]. In times of high temperatures, this stage can be established from 72 h after death [4, 5]. In these cases, another discipline such as entomology is necessary to aid in estimations with more appropriate techniques [6,7,8,9].
Forensic entomology studies the insects and other arthropods that arrive on a dead body [10]. The colonization of the corpse by necrophagous insects starts a few minutes after death, following a predictable sequence. Therefore, the PMI can be estimated using two methods: insect succession and age estimation. Insect succession involves ecological succession and is based on the known arrival sequence of entomofauna in a decomposing body [10,11,12], whereas age estimation is based on the determination of the age of the different developmental stages of insects, especially considering the oldest individuals [13]. In general, the succession method is used when the corpse has been dead for between a month and a year or more, with the estimated window of death broadening as time since death increases. Instead, the age estimation method is used when death occurred less than a month prior to the finding of the corpse and can give a narrower and more precise time interval. Using available information on the development of insects of forensic interest, and temperature data, it is possible to calculate the age of the immature individuals in the corpse and thus estimate the time when the insects initially colonized it [14, 15].
Since insects are unable to regulate their internal body temperature (i.e., they are ectotherms), environmental temperatures strongly alter their metabolism, directly affecting the developmental rates of their life cycles [16]. In this way, the time required to complete their life cycle depends on the physiology of each species and the environmental temperature conditions. Likewise, certain species may present local adaptations depending on the environmental conditions of the locality they inhabit, which leads them to develop physiological, morphological, and behavioral mechanisms that may provide them an advantage in their habitat [17,18,19]. For example, in Austria, Grassberger and Reiter [20] observed that, at 28 °C, the duration of the life cycle of the forensically important fly Lucilia sericata was 12 days, whereas, in the USA, Roe and Higley et al. [21] found that the life cycle of this same species at the same temperature lasted 8 days, and in Ecuador, Pruna et al. [22] found that it lasted 16 days. This is of great importance at the time of estimating the PMI in a criminal investigation since incorrect insect biological information would lead to wrong conclusions.
The most important necrophagous insects used during forensic investigations are blowflies (Diptera: Calliphoridae). Species of this family are usually common, highly abundant, and the first colonizers of a corpse [6]. Among blowflies, the oriental latrine fly Chrysomya megacephala (Fabricius 1974) is commonly found in cadavers in many parts of the world [23,24,25,26]. This species, native to the Oriental and Australasian regions, has rapidly spread through Africa [27, 28] and the New World [24]. Currently, this species is widely distributed throughout the American continent [29], from North America [23], passing through Central America [30] to South America [31,32,33,34] and the Caribbean islands [35, 36]. The reputation of C. megacephala as an early and dominant colonizer of carrion makes it a particularly attractive species to estimate the PMI [24, 37,38,39].
Since one of the methods to estimate the PMI requires knowing the life cycle of the entomological species collected from corpses and because insect development depends directly on the thermal conditions, the objective of this work was to determine the developmental duration of C. megacephala under two constant laboratory temperatures (25 °C and 27 °C) and field condition temperatures (27.5 ± 3.2 °C), in the city of Tapachula in Mexico. We also compared, through a meta-analysis, developmental rates between different populations of C. megacephala worldwide distributed.
Material and methods
Establishment of the colony and collection of eggs
The study was carried out in the facilities of the Centro Regional de Investigación en Salud Pública of the Instituto Nacional de Salud Pública (CRISP/INSP), located in Tapachula, Chiapas, Mexico (14.9N, 92.3W) during April 2018. Tapachula is characterized by the presence of a large variety of tree and bush species, among which Mangifera indica, Theobroma cacao, and Coffea sp. predominate. The region typically experiences a dry season from December to May and a rainy season from June to November (an average of 2500 mm over the season) and has a mean annual temperature of 27.1 °C (with a minimum and maximum of 22.5 °C and 31.4 °C respectively), and an altitude of 177 m.
Adults of C. megacephala were collected using traps baited with beef liver. In the laboratory, specimens were placed individually in transparent tubes for ocular observation and identified using Whitworth’s taxonomic key [40]. The flies were then placed in 300-cm3 cages held under laboratory conditions (controlled temperature of 25 °C and a not controlled mean relative humidity (RH) of 72%) and provided with water and a 50:50 mixture of sugar and powdered milk ad libitum. To obtain eggs, 100 g of beef liver was provided as oviposition substrate. All the eggs were collected the same day within 1 h after deposition from the F1 and F2 generations and then used throughout the study.
Experimental designs
In order to know the developmental time of each of the instar and pupa stages of C. megacephala, we conducted experiments under two different scenarios: two laboratory-controlled temperatures (25 °C and 27 °C, with 72% RH and a photoperiod of 12:12) and field conditions (27.5 ± 3.2 °C). We refer to field conditions as the exposure of specimens to fluctuating outdoor temperatures. A total of 100 eggs of C. megacephala were placed in each of 30 transparent plastic containers of 500 ml capacity, together with 200 g of vermiculite and 200 g of beef liver. Ten containers were used for each of the three temperature assays. The containers were protected with fine mesh cloth fastened with an elastic band. Containers for the field assays were exposed to environmental conditions but were kept inside a mesh cage (1 m × 1 m) and under a roof to prevent invasion by other scavengers and to protect them from the rain. The temperature and RH percentages were recorded every hour with a data logger Omega (Mod: OM-EL-USB-2-LCD-PLUS).
Eggs were monitored visually in person every hour, both during daytime and nighttime, until hatching was observed. After hatching, observations and larval sampling were performed every 12 h. Five larvae were randomly sampled from each of the 10 containers for every time point and temperature condition evaluated. The larvae were killed by immersing in hot water (80–90 °C) for 5 s to prevent shrinkage and then dried with a paper towel before taking measurements. Photographs of each larva were taken on a millimeter scale with the help of a 3.2 Mpx AmScope digital camera and an AmScope SM-4TZZ-144A-3 M tri-ocular stereomicroscope. The images were processed with the ImageJ software [41] to calculate the length of each larva. The larval instar of each specimen was also recorded at each observation based on their posterior spiracular slits. At the pupal stage, no samples were taken, and no measurements or any type of manipulation of the individual were performed. However, the observations continued every 12 h, to record the time of emergence of adults. The same procedure was carried out for the two controlled temperatures and the field assays.
Meta-analysis
A systematic bibliographic search was carried out to analyze the possible effects of population variations on developmental time by comparing different C. megacephala populations from different worldwide locations. The database was built from online searches on Google Scholar, Scielo, Science Direct, PubMed, Scopus, and Web of Science platforms, using the keywords “Chrysomya megacephala,” “development,” “life cycle,” “life table,” “stage,” “temperature,” “growth,” and its relevant combinations. Only publications that included constant temperatures, geographic location, and all developmental stages (from eggs to adults) were used for records.
Data analysis
To determine differences in developmental times and larval lengths, an analysis of variance (ANOVA) was performed between temperatures (25 °C, 27 °C, and field conditions) followed by Tukey’s post-hoc test. To assess differences between the developmental times of different populations recorded by other authors around the world, we fitted generalized linear models (GLMs) with Poisson error structure with a log link function (glm function in the R base package). Models included both the temperature and RH as numerical predictors, the diet (food source used to culture the larvae) as a categorical predictor (with four categories: beef meat, pork meat, liver, and lamb meat), the latitude and the longitude (decimal degrees) as numerical predictors, and the interactions between latitude and longitude as the geographic location of localities [42,43,44] (Table 1). Latitude values were used as distance from the equator independently of whether the study region was located in the northern or southern hemisphere. The developmental time was the dependent variable. Analyses were performed using the R statistical environment [45]. The minimum adequate model was selected using Akaike’s information criterion (AIC).
Results
The data logger recorded hourly temperature and RH data of assays under field conditions. RH was 76.6 ± 11% on average, ranging between 51.5 and 91.9%. Regarding temperature, the minimum and maximum recorded were 22.4 °C and 34.1 °C respectively, being 27.5 ± 3.2 °C on average.
With respect to the complete developmental time of C. megacephala (from egg to adult emergence), significant differences were found between growth at the two constant temperatures evaluated: 25 °C and 27 °C (F = 29; df = 2; Tukey’s test, p < 0.05). The complete life cycle of C. megacephala was shorter at 25 °C than that at 27 °C, and also shorter at 25 °C than that at field condition temperatures (Tukey’s test, p < 0.05) (Table 2). No differences were observed between 27 °C and field conditions (Tukey’s test, p = 0.999). In addition, the developmental time of each stage also depended on the exposure temperature (Table 2). The data were expressed both in hours and in accumulated degree hours (ADH) (Tables 2 and 3) because both are widely used for the estimation of the PMI [46, 47].
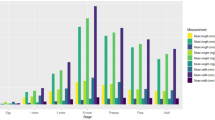
The comparison between the two laboratory temperatures and the field condition temperatures showed differences in the percent time spent by C. megacephala at each instar and other developmental stages (df = 8, F = 61, p < 0.05) (Fig. 1). In particular, no differences were observed between stages L1 (p = 0.999) and L2 (p = 0.999) at 27 °C and field conditions or between L3 at 25 °C and L3 at 27 °C (p = 1). Although the field condition temperatures were similar to the higher controlled temperature evaluated (27 °C), the field temperatures recorded during each stage were different. For example, during stages L2 and L3, the mean temperature in the field was greater than 28 °C (Fig. 1).
Percentage of mean time spent by C. megacephala at each instar of development at two constant temperatures (25 °C and 27 °C) and field condition temperatures (27.5 ± 3.2 °C). Different letters in the same instar indicate statistically significant differences (Tukey’s test, p < 0.05). Symbols represent the mean temperature (°C) for each instar at field conditions, *24.3 ± 1.0; **27.3 ± 2.7; ***28.1 ± 2.4; ****29.8 ± 3.0; *****27.6 ± 3.4
Concerning larval size, significant differences in mean length were found between 25 and 27 °C (Tukey’s test, p < 0.05) but not between field conditions and the two controlled temperatures evaluated (Tukey’s test, p = 0.692 and p = 0.883 respectively; Fig. 2). No differences were found when analyzing the effect of temperature on the mean length of the different instars (df = 4, F = 0.8, p = 0.5; Table 4).
Meta-analysis
In our meta-analysis, we found 14 publications that had examined the development of C. megacephala populations from different locations at different temperatures that included all the stages of the cycle and detailed the coordinates of the sampling place [38, 39, 48,49,50,51,52,53,54,55,56,57,58,59]. Our meta-analysis also included the results of the present study.
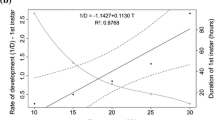
The AIC analysis indicated that the developmental time of C. megacephala was better explained by the temperature and the latitude and longitude (Table 5; Fig. 3). The duration of the life cycle was negatively influenced by the temperature (Z = − 45.55, p < 0.001). The geographic location (given by the interaction between latitude and longitude) was statistically significant (Z = − 2.40, p = 0.0161). Interestingly, the positive significant interaction between the latitude and the developmental time (Z = 3.30, p < 0.001) showed that the higher the latitude, the higher the duration of the life cycle (Z = 2.67, p < 0.01). The same was observed for the longitude. Since not all studies detailed RH information, a linear regression analysis was made between RH and developmental time with the 12 papers that indicated this information. According to this, the RH did not affect the developmental time of C. megacephala (R2 = 0.04, p = 0.22).
Discussion
The objective of our study was to determine the developmental time of C. megacephala under laboratory constant and field temperature conditions. As expected, we found that the life cycle of C. megacephala is longer at lower temperatures. We also found no differences in the developmental time of C. megacephala between similar temperatures under controlled and field conditions. Moreover, we evaluated the effects of population variations on developmental rates. Our results suggested that the duration of the life cycle of C. megacephala depends not only on the temperature but also on the geographic location of the population studied. Data from this study were used to construct the first developmental curve of C. megacephala in Mexico.
Insect development is directly related to environmental temperatures due to the characteristic ectothermy of these species [16]. We observed that only 2 degrees of difference are enough to modify the developmental time of the C. megacephala of the same population. Many studies on the same species have reported similar results. For instance, Ismail et al. [60] suggested that raising the temperature by 3 °C from 27 °C to 30 °C reduced the total developmental time of C. megacephala from 8.5 to 5 days. Similarly, Barthi et al. [52] found that the total developmental time of C. megacephala was 8.5 days at 28 °C and 5 days at 30 °C. Our meta-analysis results coincide with previous observations that suggest that the duration of the life cycle of C. megacephala is negatively influenced by the temperature [24, 39]. Certainly, our results demonstrate that temperature plays a major role in influencing C. megacephala developmental rate, ratifying the importance of obtaining the most accurate temperature information at the death scene to estimate a more precise PMI.
Frequently, forensic entomology uses developmental time data generated from controlled assays. However, since ambient temperatures in nature fluctuate over 24-h days, the growth data obtained in these controlled assays do not necessarily reflect the natural developmental rate. In Germany, by using a climatic chamber with temperatures ranging between 5 and 29 °C, Niederegger et al. [61] studied the effect of fluctuating temperatures on the development of different forensically relevant flies and found faster development for Sarcophaga argyrostoma and Lucilia illustris but slower development for Calliphora vicina and C. vomitoria. In contrast, our results showed no significant differences between the total life cycle duration at a controlled temperature of 27 °C and that at the field condition temperature of ~ 27.5 ± 3.2 °C. The discrepancies between the study by Niederegger et al. [61] and the present study are likely due to the thermal amplitude evaluated by them in contrast to the low variability of our thermal records. Likewise, research conducted in Australia by Dadour et al. [62] on the forensically relevant fly Calliphora dubia indicated no significant differences in the developmental rate of larvae of this fly at fluctuating temperatures between 19 and 30 °C, when compared with a mean constant temperature of 24 °C. Nevertheless, the effect of temperature fluctuations on the developmental time of flies could also be influenced by the geographic location of the populations. This could generate local adaptations and genetic differences in response to different environmental conditions.
The estimation of the PMI is directly related to the age of the oldest species collected from the corpse [9, 10]. While maggots are feeding, their length is considered the best estimator of larval age [63,64,65,66]. In this study, we constructed length curves for C. megacephala, which contribute as an easy-to-use forensic tool to estimate a more precise PMI in practice. Many studies have already investigated the relationship between the larval body length of C. megacephala and developmental duration at constant [50, 57, 67] and fluctuating [68] temperatures. For example, in China (31.3N, 120.8E), Zhang et al. [57] studied larval length changes at seven constant temperatures, which allowed these authors to build an isomegalen diagram, whereas, similarly, Bambaradeniya et al. [58] carried out the first C. megacephala developmental studies in Sri Lanka (7.2N, 80.6E), at four constant temperatures. However, the results of these studies show some differences with our present results: while our results in Mexico (14.9N, 92.3W) showed that, at 25 °C, the largest size reached by a third instar larva was 14.8 mm, Zhang et al. [57] measured a mean maximum length of 16.3 mm and Bambaradeniya et al. [58] recorded a mean maximum length of 13.5 mm, at the same temperature. This pattern of increasing larval body size with increasing latitudes has been documented in a variety of studies in insects [69, 70]. Blanckenhorn et al. [71] suggested that this is related to developmental, physiological, and behavioral plasticity.
Blowfly development varied between some studies, despite studying the same variables [24, 57, 73]. According to our meta-analysis results, there is a positive significant interaction between the latitude and the developmental time of C. megacephala populations. Although several authors have previously studied the growth rate of C. megacephala, results differ from one another, including the present research [24]. While in Punjab, India (31.2N, 74.3E), Bharti et al. [52] showed that the duration of the total life cycle of C. megacephala at a constant temperature of 25 °C is 299 h, in Chongqing, China (29.5N, 106.5E), Yang et al. [39] concluded that this species requires 254 h at the same temperature. In line with this, our results about the developmental time of C. megacephala in Tapachula, Mexico (14.9N, 92.3W), indicate that colonies develop faster than the populations of the aforementioned localities (231 total h). Similarly, Richards et al. [74] compared the development of various Chrysomya albiceps populations and found that developmental zero (D0; temperature below which development ceases) is directly proportional to the geographic latitude. Trudgill and Perry [75] and Trudgill [76] explained this relationship by proposing that cold-adapted species of high geographic latitude would possess a lower D0 and would take a longer time to develop than a warm-adapted species of lower geographic latitude and higher D0. In line with this, Gallagher et al. [73] studied the developmental times of three L. sericata populations of the USA and found that populations from cooler climate developed slower than those from warmer climate at the same temperature and suggested the existence of regional variation. They mentioned, for example, that, at 26 °C, populations from San Diego (32.71N, − 117.12W) presented a developmental time 74% slower than those from Massachusetts (42.04N, − 71.07W). Since PMI predictions are associated with blowfly development, understanding its variation should aid our ability to improve PMI predictions.
This variability across latitudes could be a result of phenotypic plasticity or a consequence of genetic adaptation to different thermal environments [77]. Although the mechanisms underlying these phenomena are still unknown, some studies in insects have suggested a positive covariation between genotypes and environments across a latitudinal gradient [79,80,81]. For example, Hu et al. [82] compared the developmental time of six populations of C. megacephala collected from different geographic localities in China, and, to eliminate nongenetic parental thermal environment effects, before the experimental assays, they allowed the population to pass through two generations of identical rearing conditions. Their results indicated genetic differences among populations and variations among populations in thermal reaction norms for developmental time. Similarly, Tarone [72] assessed the genetic effects on blowfly age by evaluating gene expression among three regional strains of L. sericata. The author showed that gene expression varies depending on temperature and strain effects, supporting the idea that differences among recorded developmental times could be explained by genetic differences among populations. These backgrounds could be related to the significance of the variables regarding geographic location (latitude, longitude, and their interaction) studied in the present research, and suggest that genetic differences may be the consequence of geographic distance. In line with this, Salem et al. [83] investigated the genetic diversity of C. megacephala and found a high intraspecific divergence between populations of four localities from Egypt. In agreement, Chong et al. [84] also observed intraspecific divergence within C. megacephala between two Malaysian populations and concluded that the presence of the Titiwangsa mountain range between both localities acts as a natural barrier between the East and West Coast of Peninsular Malaysia. However, before we can fully understand how adaptive plasticity in development and body size can evolve and be maintained, the underlying genetic mechanisms require further study.
Environmental conditions near the equator, with warmer and less fluctuating temperatures, are different from those at higher latitudes. Thus, phenotypic plasticity and genetic adaptation between ectothermic animals may be associated with gradual changes in seasonality and temperatures across latitudes [85]. There are many reports on the developmental time and life history traits of geographically different insect populations. For example, in Australia, located in the southern hemisphere and where the climate ranges from temperate in the south to subtropical in the north, populations of the common brown butterfly, Heteronympha merope, from low latitudes have a faster development rate than those from higher latitudes [85]. Similarly, the developmental time of the bee Exoneura robusta in Australia has been found to be directly related to the latitude, with more rapid development in northern populations [86], whereas the larval developmental time of the beetle Colaphellus bowringi has also been found to be directly associated with increasing latitudes [87]. Regarding Diptera, Khan et al. [88] studied different populations of Musca domestica and showed that populations from localities of lower latitudes completed their development faster than those from localities of higher latitudes. However, whether gradual changes in seasonality along the latitudinal gradient were the driving factor of the cogradient variation here observed in C. megacephala remains to be corroborated. Since temperature and seasonality co-vary with latitude, it is difficult to isolate their respective impact on adaptive physiological traits. Furthermore, because the studies evaluated through our meta-analysis reared larvae under constant temperatures and photoperiod, quantifying the relative impacts of season length and temperature on physiology is beyond the scope of this study. Further research on the life history of C. megacephala should compare the effects of fluctuating temperatures and photoperiod.
Relative humidity is known to affect diverse physiological parameters in insects [89]. In low RH environments, water loss through the egg and pupal membranes can be detrimental to the survivorship of holometabolous insects, resulting in desiccation [90]. In this regard, Fatchurochim et al. [91] evaluated six forensically important dipteran species (M. domestica, Muscina stabulans, Ophyra aenescens, Fannia femoralis, F. canicularis, and Hemetia illucens) and concluded that their development was successful only when the RH ranged between 40 and 70%. This is in accordance with the studies by Nielsen and Nielsen [92], who demonstrated that, in C. vicina, diapause can occur during larval development if the RH is below 40%. In contrast, our findings suggest no effect of RH on C. megacephala development. This might be due to the fact that most of the studies using meta-analysis set optimal RH raising conditions. Another explanation could be that the effect of RH is a species-specific relation. In this regard, Fatchurochim et al. [91] found that, within the 40–70% optimal range of RH, the level of humidity did not affect the developmental time of M. domestica, F. femoralis, F. canicularis, or O. aenescens but that, in M. stabulans and H. illucens, developmental time was longer when they were reared at 50% RH than at other humidity levels. Therefore, further studies should be conducted to investigate the duration of the life cycle of Calliphoridae species at constant temperatures but varying RH.
In conclusion, this study provides data on the developmental time of C. megacephala and contributes with length curves and ADH tables that can be used to estimate the insect age and can be applied for PMI estimation in criminal investigations. Considering that temperature exposure conditions could affect insect development, we suggest estimating the insect age by using the accumulated degree days/ADH methods, especially when hourly data are obtained, because the accumulated heat required to complete development remains unchanged [78]. Additionally, this is the first work that generates information regarding the development rate of C. megacephala populations from Mexico. This contribution is very important considering that the developmental time of C. megacephala is affected not only by the temperature but also by the geographic location of the population studied. Currently, estimates of maggot age often depend on reference developmental data from nonlocal populations. However, according to our findings, it is vital to emphasize the importance of advancing the knowledge of the biology of the development of local populations for PMI estimates [5]. This increases the range of possibilities to determine an accurate and reliable PMI in a greater number of forensic cases. Otherwise, if this information is not available, the most appropriate would be to use the developmental time of populations located at similar latitudes and minimal distances.
References
Pittner S, Bugelli V, Weitgasser K et al (2020) A field study to evaluate PMI estimation methods for advanced decomposition stages. Int J Leg Med 134:1361–1373. https://doi.org/10.1007/s00414-020-02278-0
Wells JD, LaMotte LR (2010) Estimating the postmortem interval. In: Byrd JH, Tomberlin J (eds) Forensic entomology: the utility of arthropods in legal investigations, 3rd edn. CRC Press, Boca Raton, FL, pp 213–224
Shrestha R, Kanchan T, Krishan K (2022) Methods of estimation of time since death. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023
Campobasso CP, Vella GD, Introna F (2001) Factors affecting decomposition and Diptera colonization. Forensic Sci Int 120:18–27. https://doi.org/10.1016/S0379-0738(01)00411-X
Amendt J, Richards CS, Campobasso CP et al (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol 7:379–392. https://doi.org/10.1007/s12024-010-9209-2
Sharma R, Garg RK, Gaur JR (2015) Various methods for the estimation of the post mortem interval from Calliphoridae: a review. Egypt J Forensic Sci 5:1–12. https://doi.org/10.1016/j.ejfs.2013.04.002
Lei G, Liu F, Liu P et al (2019) A bibliometric analysis of forensic entomology trends and perspectives worldwide over the last two decades (1998–2017). Forensic Sci Int 295:72–82. https://doi.org/10.1016/j.forsciint.2018.12.002
Lutz L, Zehner R, Verhoff MA et al (2021) It is all about the insects: a retrospective on 20 years of forensic entomology highlights the importance of insects in legal investigations. Int J Legal Med 135:2637–2651. https://doi.org/10.1007/s00414-021-02628-6
Matuszewski S (2021) Post-mortem interval estimation based on insect evidence: current challenges. Insects 12:314–334. https://doi.org/10.3390/insects12040314
Catts EP, Goff ML (1992) Forensic entomology in criminal investigations. Annu Rev Entomol 37:253–272
Archer MS (2004) Annual variation in arrival and departure times of carrion insects at carcasses: implications for succession studies in forensic entomology. Aust J Zool 51:569–576. https://doi.org/10.1071/ZO03053
Anderson GS (2000) Insect succession on carrion and its relationship to determining time of death. In: Byrd JH, Tomberlin J (eds) Forensic entomology: the utility of arthropods in legal investigations, 3rd edn. CRC Press, Boca Raton, FL, pp 143–175
Acosta X, González-Reyes AX, Corronca JA et al (2021) Estimation of the postmortem interval through the use of development time of two South American Species of forensic importance of the genus Lucilia (Diptera: Calliphoridae). J Med Entomol 58:1064–1073. https://doi.org/10.1093/jme/tjab001
Wang M, Wang Y, Hu G et al (2020) Development of Lucilia sericata (Diptera: Calliphoridae) under constant temperatures and its significance for the estimation of time of death. J Med Entomol 57:1373–1381. https://doi.org/10.1093/jme/tjaa046
Catts PE, Haskell NH (1990) Entomology and death: a procedurals guide, 3rd edn. Joyce’s Print Shop, Clemson, pp 52–97
Speight MR, Hunter MD, Watt AD (2008) Insects and climate. Ecology of insects: concepts and applications. 2nd edn, Wiley-Blackwell, Hoboken, NJ, pp 33–60
Bai Y, Dong JJ, Guan DL et al (2016) Geographic variation in wing size and shape of the grasshopper Trilophidia annulata (Orthoptera: Oedipodidae): morphological trait variations follow an ecogeographical rule. Sci Rep 6:1–15. https://doi.org/10.1038/srep32680
Owings CG, Spiegelman C, Tarone AM et al (2014) Developmental variation among Cochliomyia macellaria Fabricius (Diptera: Calliphoridae) populations from three ecoregions of Texas, USA. Int J Legal Med 128:709–717. https://doi.org/10.1007/s00414-014-1014-0
Valladares F, Matesanz S, Guilhaumon F et al (2014) The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol Lett 17:1351–1364. https://doi.org/10.1111/ele.12348
Grassberger M, Reiter C (2001) Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen-and isomorphen-diagram. Forensic Sci Int 120:32–36
Roe A, Higley LG (2015) Development modeling of Lucilia sericata (Diptera: Calliphoridae). PeerJ 3:e803
Pruna W, Guarderas P, Donoso DA et al (2019) Life cycle of Lucilia sericata (Meigen 1826) collected from Andean mountains. Neotrop Biodivers 5:3–9
Wells JD (1991) Chrysomya megacephala (Diptera: Calliphoridae) has reached the continental United States: review of its biology, pest status, and spread around the world. J Med Entomol 28:471–473. https://doi.org/10.1093/jmedent/28.3.471
Badenhorst R, Villet MH (2018) The uses of Chrysomya megacephala (Fabricius, 1794)(Diptera: Calliphoridae) in forensic entomology. Forensic Sci Res 3:2–15. https://doi.org/10.1080/20961790.2018.1426136
Sukontason KL, Narongchai P, Sripakdee D et al (2005) First report of human myiasis caused by Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae) in Thailand, and its implication in forensic entomology. J Med Entomol 42:702–704. https://doi.org/10.1093/jmedent/42.4.702
Olsen AR, Sidebottom TH, Benett SG (1993) The Oriental latrine fly, Chrysomya megacephala (Fabricius 1794)(Diptera: Calliphoridae), as an invading blowfly of public health importance. Bull Soc Vector Ecol 18:133–146
Kurahashi H (1978) The oriental latrine fly: Chrysomya megacephala (Fabricius) newly recorded from Ghana and Senegal, West Africa. Kontyû 46:432
Williams KA, Villet MH (2006) A new and earlier record of Chrysomya megacephala in South Africa, with notes on another exotic species, Calliphora vicina (Diptera: Calliphoridae). Afr Invert 47:347–350
Baumgartner DL, Greenberg B (1984) The genus Chrysomya (Diptera: Calliphoridae) in the new world. J Med Entomol 21:105–113. https://doi.org/10.1093/jmedent/21.1.105
Kurahashi H, Wells JD, Ogino K (1994) The oriental latrine fly, Chrysomya megacephala (Fabricus)(Diptera), newly recorded from Honduras, Central America. Japan J Entomol 62:860
Guimarães J, do Prado A, Linhares AX (1978) Three newly introduced blowfly species in southern Brazil (Diptera, Calliphoridae). Rev Bras Entomol 22:53–60
Barrios BB, Peris SV (1984) Chrysomya megacephala (FABR., 1784) en Paraguay. Eos 59:17
Olsen AR, Angold SC, Gross DF et al (1992) New record of the blowfly, Chrysomya megacephala (Fabr.), from Ecuador (Diptera: Calliphoridae). Pan-Pac Entomol 68:280–281
Schnack JA, Mariluis JC (1995) Status of Chrysomya blow flies (Diptera: Calliphoridae) in Argentina. J Vector Ecol 20:189–194
Yusseff-Vanegas SZ, Agnarsson I (2017) DNA-barcoding of forensically important blow flies (Diptera: Calliphoridae) in the Caribbean Region. PeerJ 5. https://doi.org/10.7717/peerj.3516
Megna YSM, Mayet YL, Abreu YJ (2021) Primer reporte del género Chrysomya Robineau-Desvoidy (Diptera: Calliphoridae) en Cuba: su importancia criminalística. Bol SEA 68:194–198
Pai CY, Jien MC, Li L et al (2007) Application of forensic entomology to postmortem interval determination of a burned human corpse: a homicide case report from southern Taiwan. J Formos Med Assoc 106:792–798. https://doi.org/10.1016/S0929-6646(08)60043-1
Thevan K, Ahmad AH, Md Rawi CS et al (2010) Growth of Chrysomya megacephala (Fabricius) maggots in a morgue cooler. J Forensic Sci 55:1656–1658. https://doi.org/10.1111/j.1556-4029.2010.01485.x
Yang YQ, Li XB, Shao RY et al (2016) Developmental times of Chrysomya megacephala (Fabricius)(Diptera: Calliphoridae) at constant temperatures and applications in forensic entomology. J Forensic Sci 61:1278–1284. https://doi.org/10.1111/1556-4029.13159
Whitworth T (2006) Keys to the genera and species of blow flies (Diptera: Calliphoridae) of America, North of Mexico. Proc Entomol Soc Wash 108:689–725
Schneider C, Rasband W, Eliceiri K (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Rodríguez-Estrella R (2007) Land use changes affect distributional patterns of desert birds in the Baja California peninsula, México. Divers Distrib 13:877–889. https://doi.org/10.1111/j.1472-4642.2007.00387.x
Jácome-Flores ME, Blazquez MC, Sosa VJ et al (2015) Type of soil and temperature range explain the preferred habitat and current distribution of the endemic lizard Aspidoscelis hyperythra in southern Baja California peninsula. J Arid Environ 113:126–133
Tellería JL, Fernández-López J, Fandos G (2016) Effect of climate change on mediterranean winter ranges of two migratory passerines. PLoS ONE 11:e0146958. https://doi.org/10.1371/journal.pone.0146958
Rcore Team (2020) R: a language and environment for statistical computing: Vienna, Austria, R Foundation for Statistical Computing, http://www.R-project.org
Wilson L, Barnett W (1983) Degree-days: an aid in crop and pest management. Calif Agric 37:4–7
Ames C, Turner B (2003) Low temperature episodes in development of blowflies: implications for postmortem interval estimation. Med Vet Entomol 17:178–186. https://doi.org/10.1046/j.1365-2915.2003.00421.x
Subramanian H, Mohan KR (1980) Biology of the blowflies Chrysomyia megacephala, Chrysomyia rufifacies and Lucilia cuprina. Kerala J Vet Sci 11:252–261
O’Flynn MA (1983) The succession and rate of development of blowflies in carrion in southern Queensland and the application of these data to forensic entomology. J Aust Entomol Soc 22:137–147. https://doi.org/10.1111/j.1440-6055.1983.tb01860.x
Wells JD, Kurahashi H (1994) Chrysomya megacephala (Fabricius)(Diptera: Calliphoridae) development: rate, variation and the implications for forensic entomology. Med Entomol Zool 45:303–309. https://doi.org/10.7601/mez.45.303_1
Gabre RM, Adham FK, Chi H (2005) Life table of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae). Acta Oecol 27:179–183. https://doi.org/10.1016/j.actao.2004.12.002
Bharti M, Singh D, Sharma YP (2007) Effect of temperature on the development of forensically important blowfly, Chrysomya megacephala (Fabricius)(Diptera: Calliphoridae). Entomon 32:149–151
Rabêlo KCN, Thyssen P, Salgado RL et al (2011) Bionomics of two forensically important blowfly species Chrysomya megacephala and Chrysomya putoria (Diptera: Calliphoridae) reared on four types of diet. Forensic Sci Int 210:257–262. https://doi.org/10.1016/j.forsciint.2011.03.022
Arias-Di Donato L, Liria J (2016) Vital statistics of Chrysomya megacephala (Fabricius, 1794)(Diptera: Calliphoridae) under different diets from Venezuela. J Entomol Zool Stud 4:247–251
Abd Algalil FM, Zambare SP (2015) Effects of temperature on the development of Calliphorid fly of forensic importance Chrysomya megacephala (Fabricius, 1794). Indian J Appl Res 5:767–769
Gruner SV, Slone DH, Capinera JL et al (2017) Development of the Oriental latrine fly, Chrysomya megacephala (Diptera: Calliphoridae), at five constant temperatures. J Med Entomol 54:290–298. https://doi.org/10.1093/jme/tjw169
Zhang Y, Wang Y, Yang L et al (2018) Development of Chrysomya megacephala at constant temperatures within its colony range in Yangtze River Delta region of China. Forensic Sci Res 3:74–82. https://doi.org/10.1080/20961790.2017.1403007
Bambaradeniya YTB, Karunaratne I, Tomberlin JK et al (2019) Effect of temperature and tissue type on the development of the forensic fly Chrysomya megacephala (Diptera: Calliphoridae). J Med Entomol 56:1571–1581. https://doi.org/10.1093/jme/tjz097
Salleh AFM, Talib A, Marwi MA et al (2009) Effects of temperatures on larval development of Chrysomya megacephala (Fabricius) and Chrysomya rufifacies (Macquart)(Diptera: Calliphoridae): application in forensic science. Malay J Health Sci 7:89–96
Ismail MI, Osman K, King O et al (2007) Accelerating Chrysomya megacephala maggot growth for forensic entomology cases. Jurnal Sains Kesihatan Malays 5:17–26
Niederegger S, Pastuschek J, Mall G (2010) Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci Int 199:72–78. https://doi.org/10.1016/j.forsciint.2010.03.015
Dadour IR, Cook DF, Fissioli JN et al (2010) Forensic entomology: application, education and research in Western Australia. Forensic Sci Int 120:48–52. https://doi.org/10.1016/S0379-0738(01)00420-0
Tarone AM, Foran DR (2008) Generalized additive models and Lucilia sericata growth: assessing confidence intervals and error rates in forensic entomology. J Forensic Sci 53:942–948. https://doi.org/10.1111/j.1556-4029.2008.00744.x
Anderson GS (2000) Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J Forensic Sci 45:824–832. https://doi.org/10.1520/JFS14778J
Núñez-Váquez C, Tomberlin JK, Cantú-Sifuentes M et al (2013) Laboratory development and field validation of Phormia regina (Diptera: Calliphoridae). J Med Entomol 50:252–260. https://doi.org/10.1603/ME12114
Yang YQ, Lyu Z, Li XB et al (2015) Development of Hemipyrellia ligurriens (Wiedemann) (Diptera: Calliphoridae) at constant temperatures: applications in estimating postmortem interval. Forensic Sci Int 253:48–54. https://doi.org/10.1016/j.forsciint.2015.05.006
Wang J, Hu Z, Chen Y et al (2002) Effects of temperature on the larval body length changes of Chrysomya megacepala (Fabricius). Acta Parasitol Med Entomol Sin 9:100–105
Sukontason K, Piangjai S, Siriwattanarungsee S et al (2008) Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology. Parasitol Res 102:1207–1216. https://doi.org/10.1007/s00436-008-0895-6
Armbruster P, Conn JE (2006) Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann Entomol Soc Am 99:1234–1243. https://doi.org/10.1603/0013-8746(2006)99[1234:GVOLGI]2.0.CO;2
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Blanckenhorn WU, Whitman DW, Ananthakrishnan TN (2009) Causes and consequences of phenotypic plasticity in body size: the case of the yellow dung fly Scathophaga stercoraria (Diptera: Scathophagidae). In: Whitman DW, Ananthakrishnan TN. Phenotypic plasticity of insects: mechanisms and consequences. Science Publishers, Enfield, NH, pp 369–422, NH: CRC Press
Tarone AM (2007) Lucilia sericata development: plasticity, population differences and gene expression. East Lansing: Michigan State University. Thesis Dissertation pp 248
Gallagher MB, Sandhu S, Kimsey R (2010) Variation in developmental time for geographically distinct populations of the common green bottle fly, Lucilia sericata (Meigen). J Forensic Sci 55:438–442. https://doi.org/10.1111/j.1556-4029.2009.01285.x
Richards CS, Paterson ID, Villet MH (2008) Estimating the age of immature Chrysomya albiceps (Diptera: Calliphoridae), correcting for temperature and geographical latitude. Int J Leg Med 122:271–279. https://doi.org/10.1007/s00414-007-0201-7
Trudgill DL, Perry JN (1994) Thermal time and ecological strategies-a unifying hypothesis. Ann Appl Biol 125:521–532. https://doi.org/10.1111/j.1744-7348.1994.tb04989.x
Trudgill DL (1995) Why do tropical poikilothermic organisms tend to have higher threshold temperature for development than temperate ones. Funct Ecol 9:136–137
Stillwell RC, Fox CW (2005) Complex patterns of phenotypic plasticity: interactive effects of temperature during rearing and oviposition. Ecology 86:924–934
Greenberg B, Kunich JC (2002) Entomology and the law: flies as forensic indicators. Cambridge Univ Press, Cambridge, UK
Norry FM, Bubliy OA, Loeschcke V (2001) Developmental time, body size and wing loading in Drosophila buzzatii from lowland and highland populations in Argentina. Hereditas 135:35–40
Conner JK, Hartl DL (2004) A primer of ecological genetics. Sinauer Inc, Sunderland, MA
Tarone AM, Picard CJ, Spiegelman et al (2011) Population and temperature effects on Lucilia sericata (Diptera: Calliphoridae) body size and minimum development time. J Med Entomol 48:1062-1068
Hu Y, Yuan X, Zhu F et al (2010) Development time and size-related traits in the oriental blowfly, Chrysomya megacephala along a latitudinal gradient from China. J Therm Biol 35:366–371
Salem AM, Adham FK, Picard CJ (2015) Survey of the genetic diversity of forensically important Chrysomya (Diptera: Calliphoridae) from Egypt. J Med Entomol 52:320–328. https://doi.org/10.1093/jme/tjv013
Chong YV, Chua TH, Song BK (2014) Genetic variations of Chrysomya megacephala populations in Malaysia (Diptera: Calliphoridae). Adv Entomol 2:49–56. https://doi.org/10.4236/ae.2014.21009
Barton M, Sunnucks P, Norgate M et al (2014) Co-gradient variation in growth rate and development time of a broadly distributed butterfly. PLoS ONE 9:e95258. https://doi.org/10.1371/journal.pone.0095258
Cronin AL, Schwarz MP (1999) Latitudinal variation in the life cycle of allodapine bees (Hymenoptera; Apidae). Can J Zool 77:857–864. https://doi.org/10.1139/cjz-77-6-857
Tang J, He H, Chen C et al (2017) Latitudinal cogradient variation of development time and growth rate and a negative latitudinal body weight cline in a widely distributed cabbage beetle. PLoS ONE 12:e0181030. https://doi.org/10.1371/journal.pone.0181030
Khan HAA, Khan MU, Nasiba A (2019) Geographical variations in life histories of house flies, Musca domestica (Diptera: Muscidae), in Punjab, Pakistan. J Med Entomol 56:1225–1230. https://doi.org/10.1093/jme/tjz069
Jaworski T, Hilszczański J (2013) The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the context of expected climate change. For Res Pap 74:345–355. https://doi.org/10.2478/frp-2013-0033
Wigglesworth VB (2012) The principles of insect physiology. Cambridge University Press, New York, NY
Fatchurochim S, Geden CJ, Axtell RC (1989) Filth fly (Diptera) oviposition and larval development in poultry manure of various moisture levels. J Entomol Sci 24:224–231. https://doi.org/10.18474/0749-8004-24.2.224
Nielsen BO, Nielsen SA (1976) Schmeissfliegen (Calliphoridae) und vakuumverpackter Schinken. Anz Schädlingskd Pfl Umwelt 49:113–115. https://doi.org/10.1007/BF01985066
Acknowledgements
The authors thank the Centro Regional de Investigación en Salud Pública of the Instituto Nacional de Salud Pública (CRISP/INSP) of Tapachula, Chiapas, Mexico, for providing their facilities for the development of this research. The main author, Ana Julia Pereira from Argentina, would like to thank the Mexican government for its support through the program “Becas de Excelencia para Extranjeros” to carry out a short research stay in Mexico, where this research was developed under the direction of Dr. Nuñez.
Funding
This study was partially funded by the “Secretaría de Relaciones Exteriores” from Mexico in the program “Becas de Excelencia para Extranjeros” call 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, A.J., Centeno, N.D. & Nuñez-Vázquez, C. Effects of population variations and temperature on Chrysomya megacephala (Diptera: Calliphoridae) development: implications for estimating the postmortem interval. Int J Legal Med 138, 165–175 (2024). https://doi.org/10.1007/s00414-023-03020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-023-03020-2