Abstract
The substantial complexity in ecosystem–radionuclide interactions is difficult to be represented in terms of radiological doses. Thus, radiological dose assessment tools use typical exposure situations for generalized organisms and ecosystems. In the present study, site-specific data and radioactivity measurements of terrestrial organisms (grass and herbivore mammals) and abiotic components (soil) are provided. The retrieved data are used in combination with the ERICA Assessment Tool for calculation of radiological parameters. The process of radionuclide transfer within ecosystem components is represented using concentration ratios (CRs), while for the calculation of dose rates the dose conversion coefficient (DCC) methodology is applied. Comparative assessments are performed between the generic and assessment-specific radiological parameters and between the resulting dose rates. Significant differences were observed between CRs calculated in this study and those reported in the literature for cesium and thorium, which can easily be explained. On the other hand, CRs calculated for radium are in very good agreement with those reported in the literature. The DCCs exhibited some small differences between the reference and the assessment-specific organism due to mass differences. The differences were observed for internal and external dose rates, but they were less pronounced for total dose rates which are typically used in the assessment of radiological impact. The results of the current work can serve as a basis for further studies of the radiological parameters in environments that have not been studied yet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On the ecosystem level, the variability between organisms may lead to significant uncertainty in the calculation of radiological doses to non-human biota, after radioactive contamination of the environment. Thus, any software used for the assessment of the radiological impact to non-human biota needs to account for the significant variability between different ecosystems and organisms. Because a significant amount of data is required for detailed individual analyses, however, organisms representative for an ecosystem are required and application of generic radiological parameters may be adequate. Typically, concentration ratios (CRs) are used in most of the available assessment tools for the quantification of radionuclide transfer and distribution within an ecosystem, while dose conversion coefficients (DCCs) are used for the calculation of dose rates.
The CRs are normally preferred to other transfer parameters that describe radionuclide transfer within the human food-chain, due to the fact that they are non-human biota oriented and describe the transfer on a whole-organism basis, as it is required for dose calculations (Copplestone et al. 2013; Howard et al. 2013). Over the past years, significant work has been done towards the collation of data related to the quantification of radionuclide transfer processes and of the main variables affecting them and nowadays soil-to-plant and soil-to-mammal equilibrium CRs have been organized in databases (Beresford et al. 2008; Copplestone et al. 2013; ERICA 2016; IAEA 2010, 2014; ICRP 2009; US-NRC 2003; Yu et al. 2013).
In biota dose assessment tools, such as the ERICA Assessment Tool (Brown et al. 2016; ERICA 2016), dose rates are calculated on the basis of reference organisms (ROs), which are hypothetical entities, represented by a three-dimensional ellipsoidal or cylindrical phantom, representative of a generic ecosystem type or of a contaminated environment. They show well-defined anatomical, physiological and life-history characteristics that can be used to relate exposure to dose rate (ICRP 2008; ERICA 2016; Pentreath and Woodhead 2001; Pentreath 1999, 2009). In the ERICA Tool the ROs are categorized according to generic ecosystems, while in ICRP’s list of Reference Animals and Plants (RAP) (ICRP 2008) they are representative for the taxonomic level of family.
For each RO-radionuclide combination specific DCCs (in μGy h−1 per Bq kg−1) relate the organism or soil activity concentration to absorbed dose (the energy that is deposited in the living tissue). DCCs are not designed to provide exact dose estimates to specific body parts, but they provide a rough estimation of the radiological dose to the whole organism. Thus, the methodology of DCCs provides a comprehensive approach for the calculation of non-human biota dose to a range of target organisms (ICRP 2008; US-DOE 2002).
Over the past years, several studies related to non-human biota dosimetry have been performed. Most of these studies are based on ROs, as it may not be practicable or necessary to develop species-specific assessments for all non-human biota (Aliyu et al. 2015; Marshall et al. 2010; Mazeika et al. 2016; Vetikko and Saxén 2010). Those studies that use specific organism data or both reference and measured organisms are limited, and this is largely because of the demanding sampling and measuring procedures, in particular for wildlife organisms (Biermans et al. 2014; Reinardy et al. 2011; Vetikko and Kostiainen 2013; Wood et al. 2008).
The aim of the present study was to define a site-specific organism representative for the Mediterranean-type ecosystem. The radiological parameters (CR, DCC) used for the description of radionuclides’ distribution within the ecosystem and for the calculation of dose rates are calculated for this organism. In addition, comparative assessments are conducted between the calculated parameters and those existing in the literature. It should be investigated whether there are any differences in calculated dose rates when using default or assessment-specific radiological parameters.
Materials and methods
Sampling and treatment procedures
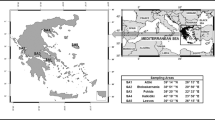
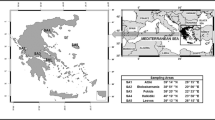
The sampling sites investigated in the present study are grasslands of free-range grazing and they were chosen under a random geographical scheme. Sampling locations (latitude and longitude) were recorded using a handheld geographical positioning system (GPS) (Fig. 1). Sixteen sampling campaigns were conducted between 2010 and 2014 at randomly chosen grasslands in the country. At each campaign one sample of soil and grass and three samples of mammals’ tissues were collected (muscle, bones and organs, without the gastrointestinal tract). Sampling was conducted at regions where animals were free-range grazing. Soil samples were collected in a polythene bag using a split-blade corer, covering an area of 1 m2 where 4–6 sampling points resulted in a composite sample considered representative for each site. Samples were collected from 0 to 10 cm soil depth which is the extent of the rooting system of most species of the Poaceae family and is also considered the standardized soil zone for the definition of soil-to-plant transfer factors. Grass samples, implying taxonomically related plants of the Poaceae family, were also collected from these areas using the same coverage. The above ground plants’ body was kept for measuring and roots were removed from the samples, as they are not taken into consideration in the dosimetric calculations. The studied mammals’ tissues were muscle, bones and organs. Tissues were taken from accredited slaughtering houses located close to the grazing areas. Species chosen (sheep, Ovis aries, and goats, Capra aegagrus) are endemic species of the region and representative of the terrestrial semi-natural environment of Greece and typical of the Mediterranean-type ecosystem (Giourga et al. 1998). The organisms were chosen as introducing a baseline food-chain, which is also part of the human food-chain, typical of these habitats. Furthermore, free-ranged grazing organisms were selected, in order to ensure direct consumption of grass instead of feedstuff.
All samples were transferred to the Environmental Radioactivity Laboratory (ERL) of the National Centre for Scientific Research “Demokritos”, Greece, where they were dried according to the protocols of ERL that are based on IAEA (1989), Klement (1982) and US-DOE (1997). More specifically, soil samples were dried at 100 °C for approximately 14 h, while organism samples (grass and mammal-tissues) were dried sequentially to up to 300 °C, also for 14 h. The resulting dried material was transferred to plastic cylinders (70 mm diameter and 20 mm height) based on which the gamma-spectrometry systems had been calibrated. Samples were kept sealed before measuring for at least 24 days in order to ensure radioactive equilibrium between 226Ra and its daughter nuclides. The samples that were collected in Greece during the Fukushima accident were also measured right after sampling in Marinelli beakers, in order to trace the short-lived 131I (Sotiropoulou et al. 2016).
Determination of radionuclides in the collected samples
In the present study, radionuclides of the natural decay chains (226Ra, 228Ra, 228Th) and artificial radionuclides originating from the Chernobyl and Fukushima N.P.P. accidents (137Cs, 134Cs and 131I) were measured. Following the treatment procedure as described above, the collected samples were measured for radioactivity using two low-background HPGe (high purity germanium) detectors. The first system consisted of an HPGe coaxial detector of 91.5% relative efficiency with 1.99 keV resolution at the 1.33 MeV photopeak of 60Co, while the second system consisted of a 20% relative efficiency HPGe detector with an energy resolution of 1.9 keV at 1.33 MeV. Both systems were calibrated (in terms of energy and efficiency) for the geometries of Marinelli bakers and plastic cylinders using multi-nuclide standard reference sources of same geometry and density as the samples. The duration of the measurements was at least 2 × 105 s. The measured activity concentration of the samples is reported with 2σ confidence intervals including uncertainties from the calibration procedure and statistical uncertainty (Kritidis et al. 2012; Sotiropoulou et al. 2016). The activity concentration of the short-lived radionuclides is decay-corrected to the day of sampling. Quality control of the results was constantly performed through ERL participation in worldwide proficiency tests and inter-laboratory exercises (e.g. IRMM 426, IAEA 375, IAEA CRP1471-01, IAEA-ALMERA-TEL-2014-04, etc.).
Radionuclide transfer within the ecosystem: soil to organism concentration ratio
The concentration ratio (CR) of a nuclide in an organism is the activity of the nuclide per unit mass of the organism [on a fresh mass (f.m.) basis], divided by the activity of the same nuclide per unit mass of the substrate [on a dry mass (d.m.) basis] from which the organism obtains the nuclide, here:
In the present study the soil-to-muscle and soil-to-bone concentration ratios (CRt) are calculated based on the radionuclide content determined in the collected samples. The equilibrium CRt represents the relative transfer of radionuclides from the media (soil) to each tissue (muscle and bones) and is the activity concentration in the tissue (Bq kg−1, f.m.) to the activity concentration in the substrate-soil (Bq kg−1, d.m.). The absorbed dose within the different organs and tissues reflects the variability of CRt. The finally derived whole-body absorbed dose will also be a function of organism geometry and size and of the different types of radiation (ICRP 2009). The estimation of CRt for each tissue may provide significant information concerning the accumulation of radionuclides in the edible parts of the organisms and hence indispensable information for human radiological protection studies.
It is noted that the radiological dose assessment tools that deal with the effects of ionizing radiation to non-human biota are mainly based on the available dose–effect data of whole-body exposures (Copplestone et al. 2013; Howard et al. 2013; ERICA 2016; US-DOE 2002; Yankovich et al. 2010). Additionally, since the CR describes the transfer from soil to the whole organism, it may be applicable within different species with similar characteristics for which there are no data (Howard et al. 2013). Thus, radionuclide transfer and organism exposure to ionizing radiation are calculated here on a whole-body basis.
The whole-organism CR have been calculated based on the assumption that muscle and bones represent the whole body, taking into account that these tissues dominate the radionuclide concentration of the whole body. The skin and gastrointestinal tract were not taken into consideration in the present study, because radioactive traces were below the detection limit. Therefore, they were considered insignificant compared to the whole organism. Similarly, organ samples were also excluded from calculations, because minor traces of radionuclides were found in only two out of sixteen organ samples. Therefore, whole-body CR was calculated as:
where W WB denotes the whole mass of the organism (kg), W m the mass of muscle and W b of bones (kg), CR is the soil to organism concentration ratio on a whole body basis and CRm,b the corresponding soil to tissue concentration ratios for muscle and bone. This procedure was based on the mass-balance approach described by Yankovich et al. (2010) and on the assumptions applied by Beresford et al. (2008) for the calculation of organ–whole body percentages in lack of the necessary information.
Organism exposure to radioactivity: dose rate calculation and dose conversion coefficient
The ERICA Assessment Tool (version 1.2.1, February 2016) is a software that can be used for the calculation of activity concentration and dose rate in non-human biota, as well as, for the quantification of impact to the ecosystem (Brown et al. 2016; ERICA 2016). In the ERICA Tool dose rate is calculated on the basis of the DCC methodology. The nuclide-specific DCCs are dependent on the radiation type, on the geometry of the exposure (the shape, size and mass of the target organism), on the organism’s habitat (soil, air or water), on the occupancy factor and on the exposure scenario (internal or external) (Amiro 1997; ERICA 2016; Gomez-Ros et al. 2008; Pröhl et al. 2003; Taranenko et al. 2004; Ulanovsky et al. 2008).
The contribution of the radiation type to the dose (and the relevant impact) is expressed by the weighting factors (W f) that here have been set at 10 for alpha particles, three for beta and one for gamma emitters. The radioactive decay products with short half-lives were taken into account by assuming that the progeny are in secular equilibrium with the parent radionuclide. Daughter nuclides with half-lives less than 10 days were included within the calculation of DCCs of their parent nuclide while those with half-lives larger than 10 days were considered separately. Here, 228Ra and 228Th (nuclides of the232Th natural decay chain) have been considered separately and DCCs calculated for both of them.
Contaminated soil was assumed as isotropic source of infinite diameter where the radionuclides are distributed uniformly to up to 10 cm depth. Internal and external dose rates (D int and D ext, respectively) were considered, for which DCCint and DCCext have been calculated. The exposure to radionuclides due to ingestion and inhalation was calculated in terms of internal dose rate (D int) using the activity concentration measured in organism (assuming uniformly distributed radionuclides in the body) and the respective DCCint. The external dose rate (D ext) was calculated from the activity concentration measured in soil and the respective DCCext. The total dose rate (D tot) is the sum of the dose rates from internal to external exposure. The mean activity concentrations derived from the measured samples of soil, grass and mammals were used for the dose calculations.
In this study, dose rates were calculated for both a reference and an assessment-defined organism. The reference organism that was studied was the “mammal-large” as this was considered the most relevant organism to the studied organisms (i.e., sheep and goats), in terms of taxonomy. For mammal-large the default DCCs of the ERICA Tool were applied. The assessment-defined organism (MyOrganism) was created based on the characteristics of the sampled organisms (Table 1) and the DCCs were calculated using the embedded dosimetric module of the tool.
Results and discussion
Radionuclides transfer to mammal tissues
Radionuclide transfer from soil to targeted tissues (muscle and bones) was calculated in order to provide information on the radionuclide distribution within the organism’s body. For further statistical manipulation of the data, the Minimum Detectable Activity (MDA) was applied in cases where radionuclide content was lower.
The mean CRm calculated in this study for 226Ra and 228Ra are 0.2 ± 0.2 · 10−2 and 0.2 ± 0.1 · 10−2, respectively, and as it can be seen in Fig. 2, the variation is small for both radionuclides. Interestingly, based on an Australian literature review for free-range grazing ruminants’ (Bovidae family) Johansen and Twining (2010) calculated for 226Ra a CR value of 5.1 ± 8.8 · 10−3 which is only a factor of two larger than the value calculated in the present study. The CRb values are much higher as expected due to the retention of radium in bones. For 228Ra the mean CRb was equaled to 6.6 ± 7.7 · 10−2 and for 226Ra to 5.5 ± 6.5 · 10−2 (Johansen and Twining (2010) reported for 226Ra 4.4 ± 3.8 · 10−2). It should be noted that 228Th measured activities in biological tissues have an associated uncertainty due to the ingrowth from 228Ra, thus, both the presence and the magnitude of 228Th depend on 228Ra behaviour. The mean CRm for 228Th is 0.2 ± 0.2 · 10−2 and CRb to 1.2 ± 1 · 10−2, while there was no significant variation between the samples. The mean CRm for 137Cs is 4.3 ± 6.4 · 10−2 and the CRb is 1.5 ± 4.1 · 10−2, with insignificant variation between samples.
Soil-to-organism concentration ratio
CRt values were used for the calculation of CR; thus, the dose rates were estimated on a whole-body basis. The soil-to-plant and soil-to-mammals CR obtained for each study site are tabulated in Table 2, as mean values (AM: arithmetic mean) with its standard deviation. The geometric mean (GM) and geometric standard deviation (GMSD) are presented as well, for the comprehensive presentation of the central tendency of the dataset, since CRs are ratios and tend to be lognormally distributed.
Concerning the artificial radionuclides (137Cs, 134Cs and 131I) specific manipulation of the data was necessary. In Greece, traces of Fukushima-derived radionuclides were detected during 2011 in air, vegetation and mammals (Kritidis et al. 2012). As a result, the contribution of the Fukushima-derived and residual (Chernobyl impact and weapons-testing fallout) 137Cs in vegetation had to be estimated, in order to be able to calculate the amount of 137Cs that has been transferred from soil to plant through root-uptake. It has to be noted that the transfer of 134Cs and 131I from soil to plants was not calculated since these radionuclides were not detected in soil at that time (Sotiropoulou et al. 2016).
Concerning the natural radionuclides it is usually assumed that 232Th is in equilibrium with its daughter nuclides. This may not be the case for biological samples, due to their considerable differences in biological behavior (intake rates, metabolic ratios, etc.) (Linsalata et al. 1989, 1991). Here this is demonstrated by the high degree of disequilibrium of the daughter/parent ratio of 228Th/228Ra in grass (0.18) and in mammal-bones (0.15), as have been calculated by the corresponding mean activity concentrations.
The mean CR values for soil-to-grass that were calculated in the present study (Table 2) were compared to the values reported in ERICA Tool (Beresford et al. 2008; ERICA 2016), IAEA (2014), and ICRP (2009) publications. This comparison referred to organisms that are taxonomically related (i.e. grass or herbs). The mean soil-to-grass CRs for cesium, radium and thorium obtained in the present study are generally lower compared to those reported in the literature by up to one order of magnitude (Fig. 3).
The observed differences can be attributed to the processes that affect radionuclide transfer in terrestrial ecosystems. For example, root-uptake is highly affected by the physical and chemical properties of the element, the species, the life stage of the organisms, the availability of the radionuclide, etc. Furthermore, the properties of soil (e.g., chemical and granulometric composition, organic matter content, pH-value, etc.) have also a significant influence on the uptake and retention of a radionuclide by plants (IAEA 2010, 2014; ICRP 2009; US-NRC 2003). Note that the studied areas are governed by loam and silt–clay–loam soils (JRC 2001) and as it has been reported in the literature at this type of soil there may be a limited uptake of cesium by plants.
For radium, the values obtained in this study are in a very good agreement with those reported in ERICA (2016) and in reasonable agreement with those reported in IAEA (2014) and ICRP (2009). It should be noted that the isotopic ratio of 226Ra/228Ra was low (i.e., 0.66 for soil, 0.35 for grass and 0.43 for mammals-bones). This low ratio in soil can be explained by the fact that the two isotopes originate from different decay chains. In contrast, the low ratio in organisms is attributed to the fact that the transfer factors of 226Ra and 228Ra largely agreed (for both soil-to-grass and soil-to-mammal). Consequently, the differences in the activities of the two isotopes in soil are transferred to the organisms, taking also into account that the transfer process is mainly affected by the chemical properties of the elements, instead of any isotopic properties.
For soil-to-mammal, in ERICA (2016) CR values refer to large-mammal RO and are derived from the Wildlife Transfer Database (Copplestone et al. 2013). IAEA (2014) refers to herbivorous mammals in general while ICRP (2009) refers to deer. The mean CR of the present study for cesium is almost two orders of magnitude lower than the reported values (Fig. 3). This may be attributed to (1) the wide range of different species included in the literature and (2) the uncertainties caused by the estimation of the whole-body values using data that were measured in certain tissues (muscle and bone). On the other hand, the calculated CR values for radium are in very good agreement with those reported in the literature. The calculated soil-to-mammal CR values for 228Th are higher than the values reported in the literature by almost two orders of magnitude. On the contrary, the calculated soil-to-grass CR values are by one order of magnitude lower than the reported ones. This may be attributed to the fact that the literature values refer to 232Th while the values obtained in the present study refer to 228Th which includes a component arising from the ingrowth of 228Ra (Fig. 3a). Furthermore, it can be attributed to the high uptake of thorium by bone-tissue in contrast to the weak root uptake by plants.
Radiological exposure of terrestrial organisms
The dose rates calculated in the present study for the assessment-specific organism (MyOrganism) were compared to those for mammal-large (deer) which is the most relevant RO of the ERICA Tool. The D ext, calculated for MyOrganism is higher by about 38% (Fig. 4), because the external radiation dose, by definition, is inversely proportional to mass. Note that in the calculation of D ext alpha and low-beta particles were not taken into account, as these are unlikely to penetrate through the external layer (skin) of the organism. The D int is lower for MyOrganism for 228Ra, 137Cs, 134Cs and 131I, by about 26%, while a much smaller difference of about 2% was observed for 226Ra and 228Th. For gamma emitters, the internal exposure is significantly affected by the mass of the organism, due to the energy absorption within the organism. If the mass of the organism increases, then the D int proportionally increases. This is the case for 228Ra, 134Cs, 137Cs and 131I, while the mass difference did not affect the alpha-emitting radionuclides (226Ra and 228Th).
In the applied methodology, the dose rates are calculated from the scaling parameters used for the simulation of the assessment-defined organism. The phantoms used for the investigated organisms are considered homogeneous and three-dimensional, and are defined by the body mass and the lengths of their principal axes. The ratio of the dimensions of the assessment-defined organism (length of the minor to the length of the major axis of the ellipsoids that represent the organisms) influences the calculated DCCs and, accordingly, D ext and D int. Thus, on the basis of spheres and ellipsoids, the differences between shapes are mainly important for beta-emitters due to the self-shielding effect of the organism body.
Since the factors that influence the dose rates (exposure geometry, source geometry, density of the medium, etc.) were not altered at the comparative assessment of the two ellipsoids (reference and assessment-defined organisms), the comparison of dose rates comes down to a comparison of DCCs (Fig. 5). For beta-gamma emitters the assessment-defined DCCext (MyOrganism) was by 39% higher compared to the reference DCCext (mammal-large), while the assessment-defined DCCint was by 28% lower than the reference DCCint. It is important to note that comparing the D tot, the main quantity used in radiological risk characterization, any differences in D int and D ext were reduced at the level of D tot, at a mean of 19% (Fig. 4c).
Conclusions
The aim of the present study was to estimate any differences in dose rate that might be caused when using default instead of assessment-specific radiological parameters.
The findings of the present study obtained with respect to soil-to-muscle and soil-to-bones transfer factors highlight the accumulation of radionuclides in specific tissues of the organisms. Significant differences were obtained between the whole-body CRs calculated in this study and those reported in the literature for cesium in plants and mammals, and for thorium in mammals, while thorium CR values for plants showed reasonable agreement with those reported in the literature. Radium CR values are in very good agreement with literature values for both plants and mammals. The results show that there may be some limitations in the use of the literature data. However, it is important to note that the calculated CR values are consistent with the reported ones (within the reported ranges of values) for both soil-to-grass and soil–to-mammals transfer. Nevertheless, taking into account the substantial variability of CR values, further research on radionuclide transfer processes in the terrestrial environment may be useful, in order to draw more reliable findings.
Comparing the DCCs for the reference and the assessment-specific organism it was observed that the DCCs are only affected by the mass of the investigated organism. Thus, DCCs for internal exposure are higher for the reference organism, while DCCs for external exposure are lower, compared to those for the assessment-specific organism. The differences between the DCCs of the reference and assessment-specific organisms are transferred to differences between the internal and external doses (up to 40%). Importantly, it was observed that the differences in internal and external doses are reduced at the level of total dose (up to 20%), which is the quantity typically used in risk characterization.
The use of reference organisms for dose estimation after exposure to ionizing radiation may be adequate for the purposes of planning and regulatory control, as it provides a sufficient approach for the calculation of radiological doses. The ERICA Tool, and similar assessment methodologies, is useful as an integrated alternative approach for the quantification of radiological risk to the environment through the rough estimation of dose rate to non-human biota. The results of the present study are considered valuable in further research, in order to obtain more reliable results on radiological doses to non-human biota in environments that have not been studied yet. Moreover, the results presented here are also useful for the consideration of radioecological parameters and dose assessment tools, in general, as part of methodologies to be developed for a comprehensive assessment of the environmental impact of radioactive contamination.
References
Aliyu AS, Ramli AT, Garba NN, Saleh MA, Gabdo HT, Liman MS (2015) Fukushima nuclear accident: preliminary assessment of the risks to non-human biota. Radiat Prot Dosim 163(2):238–250
Amiro BD (1997) Radiological dose conversion factors for generic non-human biota used for screening potential ecological impacts. J Environ Radioact 35:37–51
Beresford NA, Barnett CL, Howard BJ, Scott WA, Brown JE, Copplestone D (2008) Derivation of transfer parameters for use within the ERICA Tool and the default concentration ratios for terrestrial biota. J Environ Radioact 99:1393–1407
Biermans G, Horemans N, Vanhoudt N, Vandenhove H, Saenen E, Hees MV, Wannijn J, Vives i Batlle J, Cuypers A (2014) An organ-based approach to dose calculation in the assessment of dose-dependent biological effects of ionising radiation in Arabidopsis thaliana. J Environ Radioact 133:24–30
Brown JE, Alfonso B, Avila R, Beresford NA, Copplestone D, Hosseini A (2016) A new version of the ERICA Tool to facilitate impact assessments of radioactivity on wild plants and animals. J Environ Radioact 153:141–148
Copplestone D, Beresford NA, Brown JE, Yankovich T (2013) An international database of radionuclide concentration ratios for wildlife: development and uses. J Environ Radioact 126:288–298
ERICA (2016) The ERICA Assessment Tool: environmental risk from ionizing contaminants: assessment and management (version 1.2.1, February 2016). Help Function Document. http://www.erica-tool.eu. Accessed 15 Oct 2016
Giourga H, Margaris NS, Vokou D (1998) Effects of grazing pressure on succession process and productivity of old fields on Mediterranean islands. Environ Manag 22(4):589–596
Gomez-Ros JM, Pröhl G, Ulanovsky A, Lis M (2008) Uncertainties of internal dose assessment for animals and plants due to non-homogeneously distributed radionuclides. J Environ Radioact 99:1449–1455
Howard BJ, Beresford NA, Copplestone D, Telleria D, Pröhl G, Fesenko S, Jeffree RA, Yankovich TL, Brown JE, Higley K, Johansen MP, Mulye H, Vandenhove H, Gashchak S, Wood MD, Takata H, Andersson P, Dale P, Ryan J, Bollhöfer A, Doering C, Barnett CL, Wells C (2013) The IAEA handbook of radionuclide transfer to wildlife. J Environ Radioact 121:55–74
IAEA (1989) Measurements of radionuclides in food and the environment: a guidebook. Technical report series no. 295. International Atomic Energy Agency, Vienna
IAEA (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments. Technical report series no. 472. International Atomic Energy Agency, Vienna
IAEA (2014) Handbook of parameter values for the prediction of radionuclide transfer to wildlife. Technical report series no. 479. International Atomic Energy Agency, Vienna
ICRP (2008) Environmental protection: the concept and use of reference animals and plants. ICRP Publication 108. Ann ICRP 38(4–6):1–237
ICRP (2009) Environmental protection: transfer parameters for reference animals and plants. ICRP Publication 114. Ann ICRP 39(6):1–113
Johansen MP, Twining JR (2010) Radionuclide concentration ratios in Australian terrestrial wildlife and livestock: data compilation and analysis. Radiat Environ Biophys 49:603–611
Joint Research Center (JRC) (2001) European Soil Database. TEXT-SRF-DOM: dominant surface textural class of the STU. Soil Geographical Database of Eurasia. http://eusoils.jrc.ec.europa.eu/. Accessed 09 June 2015
Klement AW (1982) Handbook of environmental radiation. CRC series in radiation measurement and protection. CRC Press Inc., Boca Raton
Kritidis P, Florou H, Eleftheriadis K, Evangeliou N, Gini M, Sotiropoulou M, Diapouli E, Vratolis S (2012) Radioactive pollution in Athens, Greece due to the Fukushima nuclear accident. J Environ Radioact 114:100–104
Linsalata P, Morse R, Ford H, Eisenbud M (1989) Transport pathways of Th, U, Ra and La form soil to cattle tissues. J Environ Radioact 10:115–140
Linsalata P, Morse R, Ford H, Eisenbud M (1991) Th, U, Ra and rare earth element distributions in farm animal tissues from an elevated natural radiation background environment. J Environ Radioact 14:233–257
Marshall K, Watson S, McDonald P, Copplestone D, Watts SJ (2010) Exposure of birds to radionuclides and other contaminants in special protection areas (SPAs) in North-West England. Sci Total Environ 408:2567–2575
Mazeika J, Marciulioniene D, Nedveckaite T, Jefanova O (2016) The assessment of ionising radiation impact on the cooling pond freshwater ecosystem non-human biota from the Ingalina NPP operation beginning to shut down and initial decommissioning. J Environ Radioact 151:28–37
Pentreath RJ (1999) A system for radiological protection of the environment: some initial thoughts and ideas. J Radiol Prot 19(2):117–128
Pentreath RJ (2009) Radioecology, radiobiology and radiological protection: frameworks and fractures. J Environ Radioact 100:1019–1026
Pentreath RJ, Woodhead DD (2001) A system for protecting the environment from ionising radiation: selecting reference fauna and flora, and the possible dose models and environmental geometries that could be applied to them. Sci Total Environ 277:33–43
Pröhl G, Brown J, Gomez-Ros JM, Jones S, Taranenko V, Thørring H, Vives i Batlle J, Woodhead D (2003) Dosimetric models and data for assessing radiation exposure to biota. Deliverable 3 to the Project “FASSET—Framework for Assessment of Environmental Impact” (Contract No. FIGE-CT-2000-00102)
Reinardy HC, Teyssie J-L, Jeffree RA, Copplestone D, Henry TB, Jha AN (2011) Uptake, depuration and radiation dose estimation in zebrafish exposed to radionuclides via aqueous or dietary routes. Sci Total Environ 409:3771–3779
Sotiropoulou M, Florou H, Manolopoulou M (2016) Radioactivity measurements and dose rates calculations using ERICA Tool in the terrestrial environment of Greece. Environ Sci Pollut Res 23(11):10872–10882
Taranenko V, Pröhl G, Gómez-Ros JM (2004) Absorbed dose rate conversion coefficients for reference terrestrial biota for external photon and internal exposures. J Environ Radioact 24:35–62
Ulanovsky A, Pröhl G, Gómez-Ros JM (2008) Methods for calculating dose conversion coefficients for terrestrial and aquatic biota. J Environ Radioact 99:1440–1448
US-DOE (1997) Environmental measurements laboratory, procedures manual, HASL-EML-300, 28th edn. U.S. Department of Energy, New York
US-DOE (2002) A graded approach for evaluating radiation doses to aquatic and terrestrial biota. U.S. Department of Energy, Washington, DC
US-NRC (2003) Literature review and assessment of plant and animal transfer factors used in performance assessment modeling. U.S. Nuclear Regulatory Commission, Washington, DC
Vetikko V, Kostiainen E (2013) Assessment of doses to game animals in Finland. J Environ Radioact 125:69–73
Vetikko V, Saxén R (2010) Application of the ERICA Assessment Tool to freshwater biota in Finland. J Environ Radioact 101:82–87
Wood MD, Marshall WA, Beresford NA, Jones SR, Howard BJ, Copplestone D, Leah SR (2008) Application of the ERICA Integrated Approach to the Drigg coastal sand dunes. J Environ Radioact 99:1484–1495
Yankovich T, Beresford N, Wood M, Aono T, Andersson P, Barnett C, Bennett P, Brown J, Fesenko S, Fesenko J, Hosseini A, Howard BJ, Johansen MP, Phaneuf M, Tagami K, Takata H, Twining JR, Uchida S (2010) Whole-body to tissue concentration ratios for use in biota dose assessments for animals. Radiat Environ Biophys 49:549–565
Yu C, Cheng J-J, Kamboj S (2013) Effects of the new wildlife transfer factors on RESRAD-BIOTA’s screening biota concentration guides and previous model comparison studies. J Environ Radioact 126:338–351
Acknowledgements
The authors wish to thank the reviewers for their valuable contribution to the improvement of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sotiropoulou, M., Florou, H. & Kitis, G. Calculating the radiological parameters used in non-human biota dose assessment tools using ERICA Tool and site-specific data. Radiat Environ Biophys 56, 443–451 (2017). https://doi.org/10.1007/s00411-017-0703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-017-0703-8