Abstract
In the present study, the radioactivity levels to which terrestrial non-human biota were exposed are examined. Organisms (grass and herbivore mammals) and abiotic components (soil) were collected during the period of 2010 to 2014 from grasslands where sheep and goats were free-range grazing. Natural background radionuclides (226Ra, 228Ra, 228Th) and artificial radionuclides (137Cs, 134Cs, 131I) were detected in the collected samples using gamma spectrometry. The actual measured activity concentrations and site-specific data of the studied organisms were imported in ERICA Assessment Tool (version 1.2.0) in order to provide an insight of the radiological dose rates. The highest activity concentrations were detected in samples collected from Lesvos island and the lowest in samples collected from Attiki and Etoloakarnania prefectures. The highest contribution to the total dose rate was clearly derived from the internal exposure and is closely related to the exposure to alpha emitters of natural background (226Ra and 228Th). The Fukushima-derived traces of 137Cs, 134Cs, and 131I, along with the residual 137Cs, resulted in quite low contribution to the total dose rate. The obtained results may strengthen the adaptation of software tools to a wider range of ecosystems and may be proved useful in further research regarding the possible impact of protracted low level ionizing radiation on non-human biota. This kind of studies may contribute to the effective incorporation of dosimetry tools in the development of integrated environmental and radiological impact assessment policies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, more attention has been given to the assessment of radiological impact on non-human biota taking into account that human protection may be inadequate to ensure environmental protection (ICRP 2003, 2007). Significant consideration has been given to the estimation of non-human biota radiological dose rates, independently from dose rates calculations for humans. The quantification of non-human biota exposure to ionizing radiation may be performed applying specific methodologies and software that were developed over the last years (Beresford et al. 2007, 2008; Brown et al. 2008; IAEA 2010, 2014b; ICRP 2008, 2009; USDoE 2002, 2004; Wood et al. 2009).
Within the 6th Framework Program of the European Commission, the ERICA Integrated Approach (ERICA I.A.) and the ERICA Assessment Tool were developed for calculation of radiological dose rates, risk characterization and prediction of eventual effects of ionizing radiation to non-human biota (Beresford et al. 2007; Brown et al. 2008; Larsson 2008; ERICA 2014). The ERICA I.A. and ERICA Tool may provide a valuable methodology for the integrated radiological impact assessment of a region on the ecosystem level. Thus, over the last years, several research works have studied its application on specific populations and ecosystems using actual radioactivity measurements (e.g., Karimullina et al. 2013; Nedveckaite et al. 2011; Vetikko and Saxén 2010; Wood et al. 2008, 2009).
Natural radioactivity levels in the terrestrial environment of Greece are generally known (mainly referring to members of 238U and 232Th natural series) (Florou et al. 2007; Kioupi et al. 2015; Probonas and Kritidis 1993). However, there is no information concerning the levels of internal and external exposure to which natural terrestrial organisms are subjected taking into consideration that terrestrial natural radioactivity may be a significant source (Probonas and Kritidis 1993).
Artificial radionuclides have been introduced in the terrestrial environment of the country mainly from the Chernobyl nuclear power plant accident and the global fallout with 137Cs still detectable in the environment (Kritidis and Florou 1995, 2001). Additionally, in 2011 traces of artificial radionuclides were also introduced by the radioactive materials released from Fukushima Dai-Ichi nuclear power plant accident. Radioactive traces were also detected in air over Greece (Athens, Thessaloniki, and Ioannina) (Kritidis et al. 2012; Manolopoulou et al. 2011; Potiriadis et al. 2012) and it can be assumed that traces of 137Cs that resulted from the Fukushima accident were added to the residual ones. Concerning 134Cs and 131I, they were detected again in 2011 and it is clearly assumed that they are originated from Fukushima, since there was a long time intervened from their last presence at the early period after the Chernobyl accident.
The aim of the present work was to characterize the baseline levels of natural and artificial radioactivity in semi-natural areas of the Greek rural territory. Actual radioactivity measurements were performed in organisms and abiotic components in regions of free-range grazing, since there is a general lack of information concerning the radiological exposure of natural organisms in the country. The objective was to identify the process that is related to the calculation of radiological dose rates to non-human biota, in order to contribute to the development of the radiological impact assessment procedure. The application of ERICA Tool towards this objective may reveal insights related to the extents or limitations of its application in similar environments.
Materials and methods
Sampling locations
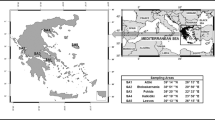
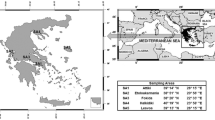
During the period from 2010 to 2014, a total sum of 52 soil, grass, and mammals’ tissue samples were collected from five randomly chosen sampling areas (SA) in Greece (Fig. 1). The specific case study sites were chosen because they are representative of the semi-natural environment of the country where uncultivated grasslands are used for free-range grazing of herbivore mammals. Furthermore, at these locations, we could gain accessibility to the remote grasslands and to the shepherds and slaughter houses of the regions. From each case study site, samples of soil, grass, and mammals were collected.
The studied regions are mainly covered by phryganic ecosystem (similar to the Garrigue vegetation) which is one of the typical vegetation of the Mediterranean type ecosystem developed at the most arid and warm margins of the region. The vegetation at these regions consists mainly of woody dwarf shrubs (shorter than 1 m height) and from grasses of the Poaceae family (Margaris 1976, 1981; Margaris and Vokou 1982). A significant part of the rural territory in Greece is also covered by grasslands (Merou and Papanastasis 2009; Papanastasis 1981). The phryganic communities are important grazing lands for livestock, mainly sheep in the autumn–winter time and are extensively used for free range grazing of livestock, mainly sheep and goats (Arianoutsou-Faraggitaki 1985; Giourga et al. 1998). At the studied regions loam and silt clay loam soils are widespread (JRC 2001).
The studied plants were grasses of the Poaceae family (formerly Gramineae, order of Monocotyledons of the Angiosperms class), with great abundance at the phrygana vegetation sites and at the grasslands, which are the main pasture of the grazing animals at these regions. The collected mammals (sheep and goats) were primary herbivore mammals, ruminants of the Bovidae family, mainly used for human consumption. The classification used in this study is at the taxonomic order of family, in accordance to the taxonomic class used in the Reference Organism (RO) module of the ERICA Tool (ERICA 2014) and to the Reference Animals and Plants (RAP) of ICRP Publication 108 (ICRP 2008). The choice of sheep and goats for the conduction of this work was due to the fact that the chosen organisms are representative species of the terrestrial semi-natural Mediterranean ecosystem and significant part of human food chain. Furthermore, the access to sufficient number of samples could be obtained through official slaughter houses.
Sampling and treatment procedures
Soil samples were collected in a polythene bag using a split-blade corer, covering an area of 1 m2 where 4–6 sampling points resulted to a composite sample representative for each site. Samples were collected from 0 to 10 cm soil depth which is the extent of the rooting system of most species of the Poaceae family, and is also considered the standardized soil zone for the definition of soil-to-plant transfer factors (IAEA 2009). Extraneous materials like roots, leaves, pieces of gravel and stones were removed from the samples and samples were weighted to wet mass, dried at 100 °C for 14 h and weighted again to dry mass. Samples were homogenized, sieved through 2-mm sieve, separated to subsamples, and transferred to plastic pots of 70 mm diameter and 20 mm height to be weighted again.
Soil and grass samples were collected from the areas where herbivores were grazing, using the same coverage. The above ground plants’ body was kept for measuring and roots were removed from the samples, as they are not taken into consideration in the dosimetric calculations. The collected grass samples were washed with distilled water, weighted to fresh mass, and transferred to porcelain dishes where they were dried at 200 °C for 5 h and at 250 °C for 7 h. Afterwards, the samples were weighted again to dry mass, homogenized, and transferred to the above-mentioned plastic pots.
The studied mammals (sheep, Ovis aries, and goats, Capra aegagrus) were collected from accredited slaughtering houses located close at the grazing areas (Fig. 1). Specific tissues were sampled (muscle, bones, and organs) as they are the major target tissues for specific radionuclides (e.g., muscle for 137Cs, bone for 226Ra, etc.). In order to ensure sufficient material for analysis, muscle and bone samples comprised of about 2 kg of tissue, while organ samples were composed from lungs, thyroid, kidneys, heart, spleen, and liver (gastrointestinal tract was excluded). Tissue samples were immediately frozen after sampling, when needed, in order to prevent tissues decomposition. It has to be noted that samples of grass and mammals’ tissues that were collected during the first month of the Fukushima impact to Greece were sliced into small pieces, in order to fit into Marinelli bakers of 1 L, and measured immediately after sampling for the detection of the short-lived 131I. Afterwards, tissue samples were dried in order to obtain the best counting geometry for gamma spectrometry. Sliced samples were weighted to fresh mass, dried to 300 ° C to remove organic matter, weighed to dry mass, grounded, and homogenized. Subsamples were transferred to the appropriate aforementioned plastic pots.
The Environmental Radioactivity Laboratory (ERL) of the National Centre for Scientific Research ‘Demokritos’ (Greece) has adopted protocols and methods (briefly described above) from the relevant international literature (IAEA 1989; Klement 1982; USDoE 1997).
Gamma spectrometry measurements
The determination of activity concentration of natural and artificial gamma emitting radionuclides, in soil, grass, and mammals’ tissues was performed with gamma spectrometry. All samples were measured for natural and artificial gamma-emitting radionuclides using two high-resolution gamma ray spectrometry systems. The first system consists of a high-purity Germanium (hpGe) coaxial detector of 91.5 % relative efficiency and 1.99-keV resolution at the 1.33-MeV photopeak of 60Co. The system is connected to an 8-k multi-channel analyzer (MCA) with an energy calibration of 0.25 keV/channel operated with Canberra Gennie 2000 software used for the spectrum analysis. The second system consists of a 20 % relative efficiency hpGe coaxial detector with an energy resolution of 1.9 keV at 1.33 MeV (of 60Co) connected to an 4 k MCA with an energy calibration of 0.5 keV/channel operated with Ortec Maestro II software. Both systems are carefully shielded, in order to reduce the influence of background radiation.
For the determination of 226Ra the photopeaks of 214Pb (295.2 and 352.0 keV) and 214Bi (609.4 keV) were used, for 228Ra the photopeaks of 228Ac (338.4 and 911.1 keV) and for 228Th the photopeaks of 212Pb (238.6 keV) and 208Tl (583.1 keV). For the artificial radionuclides 137Cs, 134Cs, and 131I, the photopeaks used were 661.6, 604.7, and 364.5 keV, respectively. All samples were kept sealed before measuring for at least 24 days in order to ensure radioactive equilibrium between 226Ra and its daughter nuclides. The measuring duration was around 2 · 105 seconds. The activity concentrations are reported with 2σ confidence level propagated uncertainty and the relative statistical error is up to 30 %. Samples whose activity concentration was below the minimum detectable activity (MDA) were excluded from subsequent statistical analysis and further consideration.
The energy and efficiency calibration of both hpGe systems in ERL performed using standard sources of the same geometry and density as the samples. The standard sources used were appropriate to cover the full energy range of up to 2000 keV; a source of 226Ra, prepared from an original standard solution of 3.7 kBq L−1 and a multi-nuclide source of 241Am, 137Cs, and 60Co of 584 Bq total activity (produced by Institute for Nuclear Research and Nuclear Energy, Sofia, Bulgaria). Quality control of the results is constantly performed by participating in national and international interlaboratory comparison and calibration exercises and in proficiency tests (e.g., IRMM 426, IAEA 375, IAEA CRP1471-01, IAEA-ALMERA-TEL-2014-04, etc.).
Application of ERICA Assessment Tool
The model that is currently used is the ERICA Assessment Tool (version 1.2.0), which is the supporting software of the ERICA Integrated Approach (ERICA I.A.). The ERICA I.A. and the ERICA Tool have been developed under the 6th Framework Program of the EC for the assessment and management of environmental risks from ionizing radiation (Beresford et al. 2007; Brown et al. 2008; Larsson 2008; ERICA 2014).
At the present study, the second Tier of the ERICA Tool was implemented because it is site-specific dependent and user-defined data can be introduced. The site-specific data refer to the radioactivity measurements of soil and biota and to the dimensions and mass of the mammals that were sampled. Tier 1 was not used due to the fact that it is a screening Tier in which only the default parameter datasets can be used. Due to the absence of any interaction of animals with humans (i.e., animals were not domesticated or breed) it is assumed that the ERICA Tool may be applied at a semi-natural environment. As mentioned above, the studied radionuclides were 226Ra of 238U natural series and 228Ra and 228Th of 232Th natural series detected in the case study sites. The studied artificial radionuclides were 137Cs, which is from the Chernobyl and Fukushima accidents and from global fallout, and 134Cs and 131I that are Fukushima-derived.
Within ERICA Assessment Tool dose rates are calculated on the basis of external (D ext), internal (D int), and total (D tot) absorbed dose rate (in μGy h-1). The method of dose conversion coefficients (DCC in μGy h-1 per Bq kg-1) is applied, which is based on the calculation of dose to an organism, based on the activity concentration in which the organism is exposed (Brown et al. 2003, 2008; ERICA 2014; Prohl et al. 2003; Ulanovsky et al. 2008). The calculation of D ext is performed using the activity concentration in soil (Cmedia), assuming that organism is exposed in uniformly distributed radionuclides in soil and using the typical source–target relation of external exposure, multiplied by the respective dose conversion coefficient for external exposure (DCCext). The calculation of D int is performed using organism activity concentration (Corganism) multiplied by dose conversion coefficient of internal exposure (DCCint), assuming homogenous distribution of radionuclides within organism’s body and that all relevant pathways of internal exposure have been taken into consideration (ingestion, inhalation, and water uptake for mammals and root and foliar uptake for grass). Additionally, the radiation weighting factors (wf) had to be defined, whereas in this study the default values of ERICA Tool were applied (10 for α, 3 for β, and 1 for γ radiation) (ERICA 2014; Prohl et al. 2003). The weighted total dose rate (D tot) for each radionuclide considered (i) is the sum of D int and D ext, as it is shown in the following equation, while the ∑D tot is the total dose rate from all radionuclides considered.
For the representation of grass samples, the default reference organism (RO) “Grasses and Herbs” was used which is based on “Wild Grass” of the ICRP list of Reference Animals and Plants (RAP) (ICRP 2008). The DCCext and DCCint that correspond to each organism–radionuclide combination were used in the calculation of D ext and D int, multiplied by the mean activity concentrations of soil and grass samples from each sampling area.
The introduction of sampled mammals within the Tool was performed through the “Add Organism Wizard”. Once all necessary information about the new organism (Sheep5) was imported in the Tool (taxonomic characteristics, dimensions, mass, etc.) the ellipsoid phantom was formed and the relevant DCCext and DCCint were calculated (Table 1). The DCCs of any new organism are calculated based on the absorbed energy fraction which is estimated after the Monte Carlo calculations performed within the Tool. The DCCs include the contribution of the daughter radionuclides if their half-lives are shorter than 10 days, assuming that they are in secular equilibrium with the parent radionuclide (Taranenko et al. 2004; Ulanovsky et al. 2008).
For the calculation of D ext, the DCCext was multiplied by the arithmetic mean of the activity concentration measured in soil samples of each sampling area. In order to calculate mammals internal exposure, the measured activity concentration in the sampled body parts (muscle, bones, and organs) was converted to whole-body activity concentration applying the appropriate conversion factors derived from the literature (IAEA 2014a; Yankovich et al. 2010). This is due to the fact that in the biota dose assessment models the parameters and benchmark values usually refer on whole-body basis (CR, DCC, etc.) and the available dose-effect data for radionuclides are mostly based on a whole-body exposure. The D int was calculated through the DCCint multiplied by the mean activity concentration in organism for each sampling area on whole-body basis. For both grass and mammals, the time that the organism spent at a specific location in its habitat (which is represented by the occupancy factor (OF) within the tool) was set to unity on the ground.
Results and discussion
Radioactivity measurements in the terrestrial environment
The measured activity concentrations of natural radionuclides in soil (Table 2) exhibited a good agreement with measurements performed earlier at the country. The mean value of 226Ra activity concentration in soil samples calculated equal to 32 ± 18 Bq kg−1 (n = 16) which is within the range of 7–310 Bq kg−1 (mean value equal to 40 ± 17 Bq kg−1) reported in previous studies performed in the country (Florou et al. 2007; Probonas and Kritidis 1993). The mean value of 228Ra in soil was equal to 48 ± 28 Bq kg−1 (n = 16), which is also within the reported values from Probonas and Kritidis (1993) of 7–190 Bq kg−1, who reported a mean of 47 ± 17 Bq kg−1. They also reported a mean of 33 ± 12 Bq kg−1 for 228Th (3–150 Bq kg−1) which is close to the mean calculated for this study at 42 ± 25 Bq kg−1 (n = 16). As shown in Table 2, the concentrations of naturally occurring radionuclides in soil (226Ra, 228Ra, and 228Th) exhibited some variations which are generally correlated with the chemical composition of soils from region to region (Probonas and Kritidis 1993).
Natural radionuclides detected in plants due to the transfer from soil through root uptake and to foliar absorption of the resuspended radionuclides. The mean activities in grass samples from all sampling areas were equal to 1.9 ± 1.6 Bq kg−1 (n = 11) for 226Ra, to 5.0 ± 7.0 Bq kg−1 (n = 12) for 228Ra and to 1.0 ± 0.7 Bq kg−1 (n = 11) for 228Th (Table 2). It should be noted that the higher activities in soil and grass were detected in samples collected from SA5 (Lesvos island) and the lower activities in samples from SA2 (Etoloakarnania).
In soil samples, the mean activity ratio of 228Th/232Th was 0.93 (n = 16) which implies secular equilibrium among the members of 232Th decay series. The equilibrium is also supported by the activity ratio of 228Th/228Ra that was calculated equal to 0.87 (Fig. 2a). On the other hand, low degree of secular equilibrium was calculated for grass samples. The mean 228Th/232Th activity ratio was 0.49 (n = 10) and the 228Th/228Ra ratio was 0.36, furthermore, 228Th activity concentration in grass was about four times lower than 228Ra activity concentration (Fig. 2b). The low degree (or absence) of equilibrium in organisms can be attributed to radionuclides’ behavior within organisms (transfer, uptake, accumulation, etc.) which may be affected, to a greater or lesser degree, by the biological processes of organisms, soil characteristics and elemental and isotopic properties of radionuclides (e.g., radiogenic profile of 228Th) (Sheppard et al. 2008; Vera Tomé et al. 2002).
During the conduction of the sampling campaigns that had been already planned, the Fukushima Nuclear Power Plant accident occurred on March 2011. Thus, radionuclides released in the atmosphere were also detected in environmental samples (Manolopoulou et al. 2011; Kritidis et al. 2012; Potiriadis et al. 2012). The Fukushima-derived 134Cs and 131I were detected in grass samples with mean activity concentration of 0.2 ± 0.2 Bq kg−1 (n = 6) and 1.3 ± 0.8 Bq kg−1 (n = 3), respectively.
Traces of 137Cs were detected in grass samples that were collected before and after the Fukushima impact in Greece. In 2 grass samples collected prior to the accident 137Cs mean activity concentration was calculated equal to 0.3 ± 0.1 Bq kg−1, while, in 13 samples collected after the arrival of the radioactive cloud 137Cs mean activity concentration was calculated equal to 0.5 ± 0.4 Bq kg−1 (n = 15). According to the measurements, it can be assumed that Fukushima-derived traces were added to the residual ones. This is also supported by the presence of detectable amounts of 134Cs in undisturbed grass. Considering that the measured 137Cs/134Cs ratio in air in Greece equaled to 1.1 ± 0.3 (Kritidis et al. 2012) it can be assumed that this ratio may be applicable for grass samples as well. Thus, a rough estimation of the Fukushima-derived 137Cs in grass is provided and is equaled to 0.2 Bq kg−1, which is in good agreement with the aforementioned calculated values. It has to be noted that according to the measurements, the ratio of 137Cs/134Cs in grass was equal to 3.4 indicating that 137Cs has an additional origin except Fukushima which is the Chernobyl accident and the global fallout.
In soil samples mean activity concentration of 137Cs equaled to 22 ± 19 Bq kg−1 (n = 15) which is within the range that has been previously reported by Theocharopoulos et al. (2000) and Kioupi et al. (2015) of 17 to 572 Bq kg−1. 134Cs and 131I were not detected in soil indicating that the uptake by grass is attributed to the radioactive deposition on foliar surfaces.
For comparison reasons, studies dealing with the Fukushima impact in European region are indicatively mentioned. The Fukushima-derived 134Cs activities concentrations in grass samples that reported by Baeza et al. (2012) exhibited a mean value of 0.2 Bq kg−1, while Perrot et al. (2012) reported a range of 0.2–0.6 Bq kg−1 that were quite close to 0.2 ± 0.2 Bq kg−1 of this study. Activity concentrations reports on 131I are also in very good agreement to the mean value of this study (1.3 ± 0.8 Bq kg−1) where Baeza et al. (2012) exhibited a range of 0.4–9.6 Bq kg−1 and Perrot et al. (2012) calculated a mean of 1.5–15.4 Bq kg−1. Taking into consideration the peculiarities of 137Cs activities where newly imported traces are mixed with residual 137Cs, the values reported by Baeza et al. (2012) (0.4 Bq kg−1) and Perrot et al. (2012) (0.2-0.7 Bq kg−1) are also in good agreement to the mean value calculated within this study (0.5 ± 0.4 Bq kg−1). In a number of studies, Fukushima-derived traces were detected in a bulk of environmental samples such as air, rainwater, plants, animals and dairy products (e.g., Baeza et al. 2012; Kritidis et al. 2012; Manolopoulou et al. 2011; Perrot et al. 2012; etc.), while, results concerning soil measurements are not reported.
Activity concentrations measured in muscle and bone tissue and in organs are shown in Table 3. Natural radionuclides were detected in bone tissue, with mean activities equal to 2.2 ± 3.0 Bq kg−1 (n = 14) for 226Ra, 4.4 ± 7.3 Bq kg−1 (n = 16) for 228Ra, and 0.8 ± 0.9 Bq kg−1 (n = 12) for 228Th. Radium (226Ra and 228Ra) concentrations in soft tissues (muscle and organs) are lower than in bone. This is attributed to the behavior of radium in mammals that in general is similar to the behavior of calcium (IAEA 2014b). The radiogenic profile of 228Th, assuming a possible decay of 228Ra already present in tissues, may have led to ingrowth of 228Th in tissues. This may cause a slight overestimation of its measured activities. These radionuclides were scarce in soft tissues, where 228Th was detected in 3 samples of muscle (with a mean of 0.1 ± 0.01 Bq kg−1), 228Ra in 1 sample (0.2 ± 0.07 Bq kg−1) and 226Ra in 2 samples of muscle (0.1 ± 0.06 Bq kg−1) and in 1 sample of organs (0.1 ± 0.04 Bq kg−1) (Table 3).
The significant difference of 228Th and 228Ra activities in bone tissue (228Ra was five times higher than its progeny 228Th) with a mean activity ratio of 228Th/228Ra at 0.27 and a mean ratio of 228Th/232Th at 0.40 indicated low degree of secular equilibrium in bones (Fig. 2c). The low degree of equilibrium in bones is attributed to the elemental and isotopic properties that affect radionuclides’ transfer from the abiotic components to organism and to the accumulation by different type of tissues. The activity concentration of 228Ra and 228Th in grass and mammals were considered separately due to the low degree of equilibrium. Similarly, dose rates were calculated separately for these radionuclides.
Cesium is mainly accumulated in soft tissues in mammals and particularly in muscle due to their relatively large mass. The mean activity concentration of 137Cs in muscle was calculated equal to 0.4 ± 0.3 Bq kg−1 (n = 13) and of 134Cs equal to 0.1 ± 0.1 Bq kg−1 (n = 8) (Table 3). Since cesium behaves as potassium in mammals it can be uniformly distributed in body; hence, it was also detected in bones and organs. The mean activity concentration of 137Cs in bones was equaled to 0.2 ± 0.2 Bq kg−1 (n = 4) and in organs equaled to 0.7 ± 0.6 Bq kg−1 (n = 3), while 134Cs mean value respectively equaled to 0.1 ± 0.1 Bq kg−1 (n = 4) and to 0.1 ± 0.1 Bq kg−1 (n = 5). It should be noted, that the mean ratio of 137Cs/134Cs in muscle tissue was calculated equal to 3 and equal to 7 in organs indicating the existence of an additional source of 137Cs besides Fukushima accident. It can be assumed that it is the residual 137Cs (even though 137Cs was not detected in the two samples of mammals that had been collected before the accident) since quite a small number of samples were measured.
The Fukushima-derived 131I was detectable in mammals for a finite period after Fukushima accident. 131I is accumulated in soft tissues of mammals and mainly in thyroid. Thus, it was detected in organs (mean value equal to 1.2 ± 0.5 Bq kg−1) and in muscle tissue (0.3 ± 0.1 Bq kg−1) of 2 samples collected from SA1 (Attiki) and SA5 (Lesvos island).
The mean whole-body activity concentrations were calculated equal to 2.5 ± 2.3 Bq kg−1 (n = 14) for 226Ra, to 4.5 ± 7.3 Bq kg−1 (n = 16) for 228Ra and to 0.8 ± 0.9 Bq kg−1 (n = 13) for 228Th. Significantly lower were the activities calculated for 137Cs at 0.4 ± 0.3 Bq kg−1 (n = 13), for 134Cs at 0.2 ± 0.1 Bq kg−1 (n = 8) and for 131I at 0.7 ± 0.2 Bq kg−1 (n = 2). The whole-body activity concentrations were imported in the ERICA Tool for the subsequent calculation of internal dose rates.
Radiological dose rates to biota
The mean total dose rate from all radionuclides considered (∑D tot) for each sampling area is illustrated in Fig. 3a for grass and Fig. 3b for mammals. The lowest ∑D tot was calculated for grass collected from SA2 (Etoloakarnania) (1.8 · 10−2 μGy h−1) and for mammals from SA1 (Attiki) (7.3 · 10−2 μGy h−1). The highest ∑D tot was calculated for samples collected from SA5 (Lesvos island) and equaled to 57 · 10−2 μGy h−1 for grass and to 76 · 10−2 μGy h−1 for mammals.
In Fig. 3a, b the calculated percentage contribution of each radionuclide to the ∑D tot is shown. The dose rates obtained through the application of ERICA Tool indicated that the higher contribution to ∑D tot derived from the exposure to 226Ra and 228Th with a joint contribution of 85 % for grass and 80 % for mammals. The contribution of 228Ra corresponds to 9 % for grass and 3 % for mammals. The artificial radionuclides (137Cs, 134Cs, and 131I) exhibited a relatively low contribution of 5 % for grass and 1 % for mammals with the highest contribution deriving from 137Cs and equaled to 4 % and 0.8 %, respectively.
For the integrated evaluation of each radionuclide’s contribution to the total dose rate the examination of D int and D ext influence was performed. As it can be seen in Fig. 4a for grass and Fig. 4b for mammals the highest contribution derives from the exposure to 226Ra and 228Th with D int higher than D ext for about one order of magnitude. This is due to the fact that the organism is internally exposed by 226Ra and 228Th, which are alpha emitters and cannot reach the organism through external exposure. This is not the case for 228Ra and 137Cs that exhibited higher contribution to the total dose rate through external exposure. Concerning 134Cs and 131I, these two Fukushima-derived radionuclides were not detected in soil and hence, only D int was calculated associated to the incorporated radionuclides. The mean internal dose rate for grass equaled to 20 · 10−2 μGy h−1 and the mean external equaled to 3 · 10−2 μGy h−1. For mammals, the mean internal equaled to 31 · 10−2 μGy h−1 and the mean external to 2 · 10−2 μGy h−1.
The calculated ∑D tot for grass and mammals is indicative of the exposure of non-human biota to natural and artificial radionuclides at the case study sites, since specific radionuclides were considered. The mean ∑D tot for grass samples was calculated equal to 23 · 10−2 μGy h−1 and for mammals samples equal to 34 · 10−2 μGy h−1. In order to estimate the dose rate resulted from Fukushima impact, 134Cs, 131I, and 137Cs were taken into account, whereas the contribution of the residual 137Cs was excluded as it was described previously. In this case, the mean total dose rate was calculated equal to 1.7 · 10−4 μGy h−1 for grass and to 2.7 · 10−4 μGy h−1 for mammals.
The calculated dose rates were clearly below the screening criterion of ERICA Tool at 10 μGy h−1, referred to the ecosystem level (Garnier-Laplace et al. 2008). The report of the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) about the effects of ionizing radiation on non-human biota (UNSCEAR 2008) proposed a chronic dose rate of 100 μGy h−1 for animals and 400 μGy h−1 for plants in order to indicate a threshold below of which it would be unlikely to have significant effects on population level. Furthermore, the ICRP (ICRP 2008) proposed numerical guidance with respect to RAP in the form of dose consideration reference level (DCRL), in order to prevent unnecessary or lack of effort concerning the environmental protection. The dose rates that were calculated within the framework of this study were below the lower band of 0.1 mGy day−1. It should be noted however that the aforementioned comparison of the calculated dose rates values to the proposed benchmarks is indicative, seeking to provide an initial comparison of the current baseline levels of exposure at the studied ecosystems to the international levels of concern.
Conclusions
The aim of the present study was to analyze the recent radioactivity levels at the terrestrial environment in Greece and to estimate the radiological exposure of selected non-human biota. The application and adaptation of ERICA Tool in the specific ecosystems was performed using actual radioactivity measurements of organisms and abiotic components collected from the case study sites.
The results of gamma spectrometry showed that organisms are exposed to natural background radionuclides (226Ra, 228Ra, and 228Th) and to artificial radionuclides of Fukushima origin (137Cs, 134Cs, 131I) and from residual traces of the Chernobyl accident and global fallout (137Cs). The dose rates calculated by ERICA Tool indicated that natural radionuclides exhibited significantly higher contribution to the total dose rate than the artificial radionuclides. Internal dose rate, which was significantly higher than external, is mainly attributed to alpha emitters (226Ra and 228Th) and to the incorporation of their daughter nuclides at the calculation of dose rates. On the other hand, the radiological exposure to Fukushima-derived radionuclides was quite low and owed to internal exposure from the incorporated radionuclides. The studied non-human biota is exposed to low-level ionizing radiation and no significant impact can be estimated. However, further consideration of the exposure levels could be considered taking into account the eventual effects of protracted low level ionizing radiation on the various levels of life organization.
The present work is the first effort in recent years to evaluate the level of exposure of non-human biota to naturally and artificially derived radionuclides. Taking into consideration the lack of relevant studies at the region, the availability of this kind of measurements may be valuable in future research in order to draw more reliable findings about the radiological exposure of terrestrial non-human biota. The measured site-specific data may be proved useful in studies relevant to the assessment of radiological exposure in areas dominated by similar ecosystems. In summary, the results obtained through the application of dose assessment models could contribute to the development of integrated decision making policies respecting the environmental and radiological impact assessment.
References
Arianoutsou-Faraggitaki M (1985) Desertification by overgrazing in Greece: the case of Lesvos island. J Arid Environ 9:237–242
Baeza A, Corbacho JA, Rodriguez A, Galvan J, Garcia-Tenorio R, Manjon G et al (2012) Influence of the Fukushima Dai-Ichi nuclear accident on Spanish environmental radioactivity levels. J Environ Radioact 114:138–145
Beresford NA, Brown J, Copplestone D, Garnier-Laplace J, Howard B, Larsson C-M et al (2007) D ERICA: an integrated approach to the assessment and management of environmental risks from ionising radiation. Description of purpose, methodology and application. A Deliverable Report for the Project “ERICA” (Contract No. FI6R-CT-2004-508847)
Beresford NA, Balonov M, Beaugelin-Seiller K, Brown J, Copplestone D, Hingston JL et al (2008) An international comparison of models and approaches for the estimation of the radiological exposure of non-human biota. Appl Radiat Isotopes 66:1745–1749
Brown J, Strand P, Hosseini A, Borretzen P et al (2003) Handbook for assessment of the exposure of biota to ionising radiation from radionuclides in the environment. Deliverable 5 to the Project “FASSET—Framework for Assessment of Environmental Impact” (Contract No FIGE-CT-2000-00102)
Brown JE, Alfonso B, Avila R, Beresford NA, Copplestone D, Pröhl G, Ulanovsky A (2008) The ERICA tool. J Environ Radioact 99:1371–1383
ERICA (2014) The ERICA assessment tool: environmental risk from ionizing contaminants: assessment and management (version 1.2.0). Help Function Document. Available from: http://www.erica-tool.eu
Florou H, Trabidou G, Nikolaou G (2007) An assessment of the external radiological impact in areas of Greece with elevated natural radioactivity. J Environ Radioact 93:74–83
Garnier-Laplace J, Copplestone D, Gilbin R, Alonzo F, Ciffroy P, Gilek M et al (2008) Issues and practices in the use of effects data from FREDERICA in the ERICA integrated approach. J Environ Radioact 99:1474–1483
Giourga H, Margaris NS, Vokou D (1998) Effects of grazing pressure on succession process and productivity of old fields on Mediterranean islands. Environ Manage 22(4):589–596
IAEA (1989) Measurements of radionuclides in food and the environment: a guidebook. Technical Report Series No. 295. International Atomic Energy Agency, Vienna
IAEA (2009) Quantification of radionuclide transfer in terrestrial and freshwater environments for radiological assessments. Technical Document No. 1616. International Atomic Energy Agency, Vienna
IAEA (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments. Technical Report Series No. 472. International Atomic Energy Agency, Vienna
IAEA (2014a) Handbook of parameter values for the prediction of radionuclide transfer to wildlife. Technical Report Series No. 479. International Atomic Energy Agency, Vienna
IAEA (2014b) The environmental behaviour of radium: revised edition. Technical Report Series No. 476. International Atomic Energy Agency, Vienna
ICRP (2003) A framework for assessing the impact of ionising radiation on non-human species. ICRP Publication 91. Ann ICRP 33(3)
ICRP (2007) The 2007 recommendations of the international commission on radiological protection. ICRP Publication 103. Ann ICRP 37(2–4)
ICRP (2008) Environmental protection: the concept and use of reference animals and plants. ICRP Publication 108. Ann ICRP 38(4–6)
ICRP (2009) Environmental protection: transfer parameters for reference animals and plants. ICRP Publication 114. Ann ICRP 39(6)
Joint Research Center (JRC) (2001) European soil database. TEXT-SRF-DOM: Dominant surface textural class of the STU. Soil Geographical database of Eurasia. Available from: http://eusoils.jrc.ec.europa.eu/
Karimullina E, Antonova E, Pozolotina V (2013) Assessing radiation exposure of herbaceous plant species at the East-Ural Radioactive Trace. J Environ Radioact 124:113–120
Kioupi V, Florou H, Kapsanaki-Gotsi E, Gonou-Zagou Z (2015) Bioaccumulation of the artificial Cs-137 and the natural radionuclides Th-234, Ra-226 and K-40 in the fruit bodies of Basidiomycetes in Greece. Environ Sci Pollut R. doi:10.1007/s11356-015-5298-5
Klement AW (1982) Handbook of environmental radiation. CRC series in radiation measurement and protection. CRC Press Inc., Boca Raton
Kritidis P, Florou H (1995) Environmental study of radioactive caesium in Greek lake fish after the Chernobyl accident. J Environ Radioact 28(3):285–293
Kritidis P, Florou H (2001) Radiological impact in Greece of the Chernobyl accident in Greece—a ten year retrospective synopsis. Health Phys 80(5):440–446
Kritidis P, Florou H, Eleftheriadis K, Evangeliou N, Gini M, Sotiropoulou M et al (2012) Radioactive pollution in Athens, Greece due to the Fukushima nuclear accident. J Environ Radioact 114:100–104
Larsson C-M (2008) An overview of the ERICA Integrated Approach to the assessment and management of environmental risks from ionising contaminants. J Environ Radioact 99:1364–1370
Manolopoulou M, Vagena E, Stoulos S, Ioannidou A, Papastefanou C (2011) Radioiodine and radiocesium in Thessaloniki, Northern Greece due to the Fukushima nuclear accident. J Environ Radioact 102:796–797
Margaris NS (1976) Structure and dynamics in a phryganic (East Mediterranean) ecosystem. J Biogeogr 3:249–259
Margaris NS (1981) Adaptive strategies in plants dominating Mediterranean-type Ecosystems. In: Castri D et al (eds) Mediterranean-type shrublands. Elsevier, Amsterdam, pp 309–316
Margaris NS, Vokou D (1982) Structural and physiological features of woody plants in phryganic ecosystems related to adaptive mechanisms. In: Publication de l’ Université de Droit, d’ Economie et des Sciences d’ Aix Marseille, Ecologia Mediterranea: Definition and localization of terrestrial Mediterranean biota, pp 449–459
Merou TP, Papanastasis VP (2009) Factors affecting the establishment and growth of annual legumes in semi-arid Mediterranean grasslands. Plant Ecol 201:491–500
Nedveckaite T, Filistovic V, Marciulioniene D, Prokoptchuk N, Plukiene R, Gudelis A, Remeikis V et al (2011) Background and anthropogenic radionuclide derived dose rates to freshwater ecosystem—nuclear power plant cooling pond—reference organisms. J Environ Radioact 102:788–795
Papanastasis VP (1981) Species structure and productivity in grasslands of Northern Greece. In: Margaris NS, Mooney HA (eds) Components of productivity of Mediterranean climate regions. Basic and applied aspects. Springer, Netherlands, pp 205–217
Perrot F, Hubert P, Marquet C, Pravikoff MS, Bourquin P, Chiron H et al (2012) Evidence of 131I and 134,137Cs activities in Bordeaux, France due to the Fukushima nuclear accident. J Environ Radioact 114:61–65
Potiriadis C, Kolovou M, Clouvas A, Xanthos S (2012) Environmental radioactivity measurements in Greece following the Fukushima Daichi nuclear accident. Radiat Prot Dosim 150(4):441–447
Probonas M, Kritidis P (1993) Exposure of the Greek population to natural gamma radiation of terrestrial origin. Radiat Prot Dosim 46(2):123–126
Prohl G, Brown J, Gomez-Ros J-M, Jones S, Woodhead D, Vives i Batlle J et al (2003) Dosimetric models and data for assessing radiation exposure to biota. Deliverable 3 to the Project “FASSET—Framework for Assessment of Environmental Impact” (Contract No FIGE-CT-2000-00102)
Sheppard SC, Sheppard MI, Ilin M, Tait J, Sanipelli B (2008) Primordial radionuclides in Canadian background sites: secular equilibrium and isotopic differences. J Environ Radioact 99:933–946
Taranenko V, Pröhl G, Gómez-Ros JM (2004) Absorbed dose rate conversion coefficients for reference terrestrial biota for external photon and internal exposures. J Radiol Prot 24:A35–A62
Theocharopoulos SP, Florou H, Kritidis P, Belis D, Tsouloucha F, Christou M et al (2000) Use of 137Cs isotopic technique in soil erosion studies in Central Greece. Acta Geol Hispanica 3–4(35):301–310
Ulanovsky A, Pröhl G, Gómez-Ros JM (2008) Methods for calculating dose conversion coefficients for terrestrial and aquatic biota. J Environ Radioact 99:1440–1448
UNSCEAR (2008) United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionising Radiation, 2008, Report to the General Assembly, with scientific annexes. Annex E: Effects of ionising radiation on non-human biota. United Nations, New York
USDoE (1997) Environmental measurements laboratory, procedures manual, HASL-EML-300. 28th edn. US Department of Energy, New York
USDoE (2002) A graded approach for evaluating radiation doses to aquatic and terrestrial biota. U.S. Department of Energy, Washington
USDoE (2004) RESRAD family of codes. RESRAD-BIOTA: a tool for implementing a graded approach to biota dose evaluation. U.S. Department of the Environment, Washington D.C. Available from: http://www.ead.anl.gov/resrad
Vera Tomé F, Blanco Rodriguez P, Lozano JC (2002) Distribution and mobilization of U, Th and 226Ra in the plant-soil compartments of a mineralized uranium area in south-west Spain. J Environ Radioact 59:41–60
Vetikko V, Saxén R (2010) Application of the ERICA assessment tool to freshwater biota in Finland. J Environ Radioact 101:82–87
Wood MD, Marshall WA, Beresford NA, Jones SR, Howard BJ, Copplestone D et al (2008) Application of the ERICA Integrated Approach to the Drigg coastal sand dunes. J Environ Radioact 99:1484–1495
Wood MD, Beresford NA, Barnett CL, Copplestone D, Leah RT (2009) Assessing radiation impact at a protected coastal sand dune site: an intercomparison of models for estimating the radiological exposure of non-human biota. J Environ Radioact 100:1034–1052
Yankovich T, Beresford N, Wood M, Aono T, Andersson P, Barnett C et al (2010) Whole-body to tissue concentration ratios for use in biota dose assessments for animals. Radiat Environ Bioph 49:549–565
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
M. Manolopoulou is deceased
Rights and permissions
About this article
Cite this article
Sotiropoulou, M., Florou, H. & Manolopoulou, M. Radioactivity measurements and dose rate calculations using ERICA tool in the terrestrial environment of Greece. Environ Sci Pollut Res 23, 10872–10882 (2016). https://doi.org/10.1007/s11356-016-6240-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6240-1