Abstract
Purpose
Systemic immune-inflammation index (SII) has been demonstrated to be closely associated with prognosis of a series of solid tumors. However, its role in small cell lung cancer (SCLC) remains poorly understood. The present study aims to evaluate the prognostic significance of pretreatment SII in SCLC treated with etoposide and platinum-based chemotherapy.
Methods
Sixty hundred and fifty-three newly diagnosed SCLC patients were enrolled. The optimal cut-off values for SII and LDH (lactate dehydrogenase) were obtained by a receiver operating characteristic (ROC) curve analysis. Overall survival (OS) was assessed by univariate and multivariate analyses.
Results
The optimal cut-off values of pretreatment SII and LDH were 748.51 × 109/L and 188.5 U/L, respectively. High pretreatment SII was significantly associated with advanced tumor stage (limited disease, LD vs. extensive disease, ED; 26.3% vs 46.5%; p < 0.001). On univariate analysis, age < 65 years, female, non-smoker, limited disease, SII < 748.51 × 109/L, LDH < 188.5 U/L, distant metastasis numbers < 2, chemotherapy + radiotherapy, and chemotherapy + surgery were closely correlated with a prolonged OS (p < 0.05). The median OS for patients in high SII group was 12.0 months, compared with that of 17.0 months for patients in low SII group. Multivariate analysis showed smoking history (p = 0.014), tumor stage (p < 0.001), pretreatment SII (p < 0.001), LDH (p = 0.002), distant metastasis numbers (p = 0.006), and chemotherapy + radiotherapy (p < 0.001) were independent prognostic factors of OS. Furthermore, SII remained prognostic significance for SCLC stratified by variable subgroups analysis.
Conclusion
Pretreatment SII represents a powerful prognostic biomarker for SCLC patients treated with etoposide and platinum-based chemotherapy. It is significant for treatment strategy making in clinics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small cell lung cancer (SCLC) represents a highly aggressive neuroendocrine tumor characterized as rapid growth and early metastasis. It accounts for approximately 15% of total lung cancer cases, resulting in 250,000 deaths worldwide yearly [1, 2]. SCLC usually carries a poor prognosis, with a 5-year survival rate of less than 10% [1]. Etoposide and platinum-based chemotherapy plus thoracic radiotherapy remain the standard first line treatment strategy of SCLC. The identification of biomarkers with the potential of prognosis predicting has great impact on treatment strategy making in clinics.
Inflammation and immunity play a key role in tumorigenesis, progression, invasion, metastasis as well as responses to therapies [3]. In peripheral blood, circulating inflammatory and immune cells mainly involve neutrophil, lymphocyte, monocyte and platelet [4]. Systemic immune-inflammation index (SII), which is calculated as platelet (P) × neutrophil (N)/lymphocyte (L) counts, has been demonstrated to be closely associated with the prognosis of solid tumors including lung cancer [4,5,6,7,8,9,10]. High SII is consistently found to be an independent negative prognostic indicator of cancer patients.
At present, the prognostic role of pretreatment SII in SCLC remains poorly understood. In this study, we aim to evaluate the prognostic value of pretreatment SII in SCLC patients treated with uniform baseline etoposide and platinum-based chemotherapy.
Methods and Materials
Patients
A retrospective analysis with 653 pathologically confirmed SCLC between January 2008 and December 2009 was conducted. The inclusion criteria were listed as: 1. pathologically confirmed SCLC; 2. newly diagnosed SCLC; 3. age ≥ 18 years; 4. complete clinical, laboratory, imaging and follow-up information; 5. no prior anti-tumor therapies. The exclusion criteria included: 1. died within perioperative period; 2. evidence of infection, bone marrow, hematological or autoimmune disease. The present study was approved by the Ethics Committee of Linyi People’s Hospital, Harbin Medical University Cancer Hospital and Cancer Hospital & Shenzhen Hospital. Written informed consent was obtained from each patient prior to the present study.
Data Collection
Patient characteristics including age, gender, smoking history, family history of tumor, full blood counts, routine biochemistry test (LDH), distant metastasis numbers and details of treatment strategies were collected by electronic medical records. The full blood counts and routine biochemistry test were obtained within 1 week of the diagnosis of SCLC. Pretreatment SII was calculated as platelet counts × neutrophil counts/lymphocyte counts.
Statistical Analysis
All analyses were performed using SPSS 16.0 statistical software. Mean values ± standard deviation (SD) was calculated for continuous variables. The optimal cut-off values of pretreatment SII and LDH were obtained by receiver operating characteristic (ROC) curves analysis, and the end-point was based on overall survival (OS) status. OS was defined as the time between the diagnosis of SCLC and death or the last follow-up. Χ2 test was performed to assess the potential correlations of SII and clinicopathological characteristics. Survival analysis was obtained by using Kaplan–Meier method and log-rank test. Variables with a p value < 0.05 were enrolled in multivariate Cox hazard regression analysis with corresponding 95% confidence intervals (CI). All p values less than 0.05 were considered statistically significant.
Results
Baseline Patient Characteristics
The baseline characteristics of 653 SCLC patients were listed in Table 1. All patients were treated with etoposide and platinum-based chemotherapy. Chemotherapy plus radiotherapy or surgery was performed in 267 (40.9%) and 22 (3.4%) patients, respectively. The median survival time (MST) for total cases was 14.5 months, with a 1-, 2-, 3- and 5-year survival rate of 57.0%, 30.9%, 20.7% and 16.7%, respectively (Fig. 1a). Median age was 56.0 years (range 23–75) and 64.6% cases were male. Of all 653 patients, 269 (41.2%) patients suffered extensive disease (ED), while the rest 384 (58.8%) patients suffered limited disease (LD).
Definitive Diagnostic Methods
The most common definitive diagnostic methods of SCLC are transbronchial biopsy (n = 554, 84.8%), computed tomography-guided needle biopsy (n = 35, 5.3%), lymph node biopsy (n = 30, 4.6%) and surgical biopsy (n = 22, 3.4%). At the same time, hydrothorax cytology (n = 2, 0.3%), exfoliative cytology of sputum (n = 1, 0.2%) and biopsy of other organs (n = 9, 1.4%) including liver, brain, pleura etc. were also important complementary diagnostic methods of SCLC. Of total 22 surgical samples, 6 cases received transbronchial biopsy before surgical resection.
Selection of the Optimal Cut-off Value for Pretreatment SII and LDH
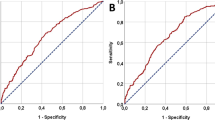
The mean (± SD) SII and LDH were 798.06 (± 739.75) × 109/L and 211.23 (± 139.97) U/L, respectively. The optimal pretreatment SII cut-off value based on ROC analysis was 748.51 × 109/L (p < 0.001, AUC 0.625; 95% CI 0.577–0.673) (Fig. 1b). The number of patients in high (≥ 748.51 × 109/L) and low SII (< 748.51 × 109/L) groups was seen in 226 (34.6%) and 427 (65.4%), respectively. The optimal cut-off value for LDH was 188.5 U/L (p = 0.001, AUC 0.599; 95% CI 0.543–0.655). High (≥ 188.5 U/L, n = 273, 41.8%) and low LDH (< 188.5 U/L, n = 380, 58.2%) groups were then divided based on the optimal LDH cut-off value (Fig. 1c).
The Relationship Between Pretreatment SII and Clinicopathological Characteristics
The relationship between pretreatment SII levels and clinicopathological characteristics was shown in Table 2. Pretreatment SII levels were closely associated with tumor stage, and patients with high SII were more likely to have extensive disease (LD vs. ED, 26.3% vs 46.5%, p < 0.001). There was no significant pretreatment SII counts difference in age, gender, smoking history, family history of cancer, LDH and distant metastasis numbers (p > 0.05). However, high SII tended to have a positive family history of cancer (no vs. yes, 33.2% vs 41.4%, p = 0.097) and more distant metastasis numbers (< 2 vs. ≥ 2, 33.7% vs 43.5%, p = 0.120).
Univariate Analysis
On univariate analysis, age (p = 0.022), gender (p = 0.047), smoking history (p = 0.003), tumor stage (p < 0.001), pretreatment SII (p < 0.001), LDH (p < 0.001), distant metastasis numbers (p < 0.001), chemotherapy + radiotherapy (p < 0.001) and chemotherapy + surgery (p = 0.003) were significantly associated with OS (Table 3). Age < 65 years, female, non-smoker, limited disease, low pretreatment SII counts, LDH < 188.5 U/L, distant metastasis numbers < 2, chemotherapy + radiotherapy and chemotherapy + surgery were correlated with a prolonged OS (Fig. 2). Patients in low SII group were significantly associated with a better OS compared with patients in high SII group (median OS in high SII group vs. low SII, 12.0 vs 17.0 months, p < 0.001). The 1-, 2- and 5-year survival rates for patients in low SII group were 61.6%, 38.2% and 23.0%, compared with that of 48.2%, 17.3% and 4.4% in low SII group. No significant difference in OS was found in family history of cancer (p = 0.408).
Multivariate Analysis
Variables entered in multivariate analysis were age, gender, smoking history, tumor stage, pretreatment SII, LDH, distant metastasis numbers and treatment strategies (p < 0.05 on univariate analysis). Multivariate analysis showed that smoking history (p = 0.014), tumor stage (p < 0.001), pretreatment SII (p < 0.001), LDH (p = 0.002), distant metastasis numbers (p = 0.006) and chemotherapy + radiotherapy (p < 0.001) were independent prognostic factors of OS (Table 3). Patients in high SII group had a 55.3% increase in death risk compared with patients in low SII group [hazard ratio (HR) 1.553; 95% CI 1.299–1.856; p < 0.001]. The death risks of patients in positive smoking history, extensive disease stage, high LDH level and distant metastasis numbers ≥ 2 groups increased 26.2%, 57.6%, 32.1% and 51.7%, compared with patients in negative smoking history, limited disease stage, low LDH level and distant metastasis numbers < 2. The additional of radiotherapy decreased a 27.6% death risk compared with the treatment of single chemotherapy. Smoking history, extensive disease, high SII, LDH ≥ 188.5 U/L, distant metastasis numbers ≥ 2 and patients treated without radiotherapy represented independent negative prognostic factors of SCLC.
Prognostic Value of Pretreatment SII in Variable Subgroups of SCLC Patients
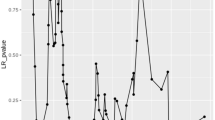
In order to clearly reveal the relationship between pretreatment SII and OS, we assessed the potential prognostic significance of pretreatment SII in variable subgroups of SCLC patients. Total 653 SCLC cases were divided into two subgroups based on variables enrolled in our present study except SII, and 18 subgroups were then divided based on the method of Table 1. Pretreatment SII was closely correlated with OS (p < 0.05) in almost all subgroups (Table 4). Pretreatment SII could better predict the prognosis of SCLC patients regardless of age, gender, smoking history, family history of cancer, tumor stage, LDH levels and treatment strategy (Figs. 3, 4). For example, in subgroup of limited disease, 101 patients with high SII have a shorter median OS and 5-year survival rate than 283 patients with low SII (22.0 vs. 16.5 months; 28.3% vs. 7.9%, p < 0.001). While in subgroup of extensive disease, 125 patients with high SII have a shorter OS and 5-year survival rate than 144 patients with low SII (10.0 vs. 9.5 months; 12.5% vs. 2.4%, p = 0.010). All results indicated pretreatment SII had optimal prognostic significance for SCLC patients.
Prognostic role of pretreatment SII in patients with variable tumor stage (a, b), LDH levels (c, d), chemotherapy ± radiotherapy (e, f), distant metastasis numbers (g) and chemotherapy ± surgery (h). Pretreatment SII was closely associated with OS of SCLC regardless of tumor stage, LDH level and management of chemotherapy ± radiotherapy (p < 0.05). Pretreatment SII levels were closely correlated with prognosis of patients in distant metastasis numbers < 2 and chemotherapy + surgery groups, and patients with high pretreatment SII level were inclined to have a poorer OS (p < 0.05)
Discussion
Our present study shows that pretreatment SII represents a powerful convenient, noninvasive, inexpensive and reproducible prognostic factor for SCLC treated with etoposide and platinum-based chemotherapy. High SII is significantly associated with a poorer OS. It may help clinicians to identify selected patients who will benefit more from the treatment strategies.
At present, only two studies have been performed to assess the potential role of pretreatment SII in SCLC, and pretreatment SII is found to be a negative prognostic factor of SCLC in both studies [7, 10]. However, of the two studies, one study does not distinguish patients treated with variable strategies, and patients treated with best supportive care and any anti-tumor therapies are all enrolled [7]. The other study involves 228 SCLC patients and it mainly focuses on the management of radiotherapy [10]. In addition, some important prognostic factors such as LDH are not analyzed. In our study, pretreatment SII is also demonstrated to be an unfavorable prognostic factor of SCLC with the inclusion of a panel of serum inflammatory markers, which is consistent with previous studies.
SII is also helpful for treatment strategy making, and the intensity of treatment and medication strategy can be adjusted based on variable pretreatment SII counts [10]. For newly diagnosed SCLC, high SII is closely associated with an advanced tumor stage, indicating a heavier tumor burden and immunosuppression status. The management of best supportive care or monotherapy may be more suitable for patients with high tumor burden and poor performance status (PS) scores, while positive anti-tumor therapies can be performed on patients with low pretreatment SII and better PS scores.
The median OS for total cases in our study was 14.5 months, with a 1-, 2-, 3- and 5-year survival rate of 57.0%, 30.9%, 20.7% and 16.7%, respectively. The result is consistent with one previous SCLC study involving 114 combined small cell lung cancer (C-SCLC) [11]. The median OS for these patients is 14.0 months, with a 1, 2, and 5-year survival rate of 51.2, 30.3, and 15.9%, respectively. Another large samples retrospective study with 47,351 SCLC patients enrolled shows the median OS for patients treated with chemotherapy is 9.6 months, and this data in our study is 12.0 months [12]. The median age at diagnosis of the whole population is 71 years, and this is higher than that in our study. The majority patients enrolled in this study are White (87%), stage IV (62%), age ≥ 65 years (84%) and of male gender (52%). Our study only involves Asian patients, and 64.6% cases are male with a median age of 56 years at diagnosis. On one hand, compared with Caucasian populations, more patients in our study show a young age at diagnosis and LD-SCLC (58.8%). On the other hand, our study only involves patients treated with uniform baseline chemotherapy and patients treated with best supportive care or other anti-tumor therapies are excluded, and the above reasons contribute to a prolonged OS.
Inflammation has been demonstrated to be closely correlated with cancer development and progression [3]. SII is calculated by serum platelet (P) × neutrophil (N)/lymphocyte (L) counts, and it is convenient to be access to and assessed. In our study, high SII is found to be an unfavorable prognostic factor of SCLC. High SII represents a status of high platelet and neutrophil counts, or a decreased lymphocyte counts. Previous studies have shown that platelets play an important role in tumor activities. Firstly, platelets can promote tumor invasion, migration and metastasis by the secretion of vascular epidermal growth factor (VEGF), transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF) [13, 14]. PDGF can promote epithelial to mesenchymal transition (EMT) by the activating of Smad and NF-kB pathways, which is critical for tumor metastasis [15]. At the same time, tumor cells can also induce differentiation of megakaryocytes to platelets and proliferation of platelets [16]. Secondly, tumor cells can evade immune surveillance by the induction of platelets aggregation [17]. Neutrophils are also found to play a critical role in cancer progression by the secretion of proangiogenic factor VEGR [18, 19]. All factors mentioned above consistently contribute to cancer progression.
To a certain extent, low SII reveals a high level of lymphocytes. Lymphocytes are main body immune cells, which play a key role in immunosurveillance by the inhibition of tumor cells proliferation, invasion and migration [20]. Tumor invasion can induce inflammatory signals and other changes of tumor micro-environment, leading to the recruitment of natural killer T (NKT), NK and T cells. NK and NKT cells can mediate spontaneous killing of tumor cells through the cytolytic activity and the production of cytokines like interleukin (IL)-2 and IL-12, leading to a prolonged survival [21,22,23]. Previous studies also show that high tumor infiltrating lymphocytes (TILs) level indicates an improved OS in variable solid cancers including lung cancer [24, 25]. Cytotoxic T lymphocytes (CTLs) can not only induce tumor cell apoptosis, but also inhibit tumor growth by the secretion of cytokines and antiangiogenic factors [26, 27]. High lymphocytes can induce a prolonged OS through the cytolytic activity including spontaneous tumor cell killing, apoptosis and cytokines secretion.
Tumor stage, LDH and distant metastasis numbers are also found to be independent prognostic factors of SCLC patients, and this is consistent with previous studies [18, 23, 28]. Extensive disease, high LDH and more distant metastasis numbers reflect a high tumor burden, leading to a negative prognosis. In addition, high LDH partly reflects a high tumor metabolism status, which is critical for cancer progression [29].
Chemotherapy plus radiotherapy is also found to be correlated with an improved OS. Etoposide and platinum-based chemotherapy plus thoracic radiotherapy remain the standard treatment strategy of SCLC for the past 30 years. In recent years, with the development of new treatment strategies including immunotherapy and anti-angiogenesis drugs, SCLC prognosis has been improved. A double-blind, placebo-controlled, phase 3 trial reveals that, compared with the management of carboplatin and etoposide-based chemotherapy alone, first line atezolizumab plus carboplatin and etoposide-based chemotherapy result in significantly longer OS and progression-free survival (PFS) of extensive stage SCLC [30].
There are also some limitations in our present study. Firstly, our study is a retrospective study and only limited institution is enrolled. In addition, there are some limitations present due to its retrospective features. These results need to be validated in further studies with multi-centric and more patients enrolled.
In conclusion, pretreatment SII represents a powerful prognostic biomarker for SCLC patients treated with etoposide and platinum-based chemotherapy. Smoking history, extensive disease, high SII, LDH ≥ 188.5 U/L, distant metastasis numbers ≥ 2 and patients treated without radiotherapy represented independent negative prognostic factors of SCLC.
References
Gazdar AF, Bunn PA, Minna JD (2017) Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 17(12):725–737
Rudin CM, Poirier JT (2017) Small-cell lung cancer in 2016: Shining light on novel targets and therapies. Nat Rev Clin Oncol 14(2):75–76
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899
Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q (2018) Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta 484:272–277
Tong YS, Tan J, Zhou XL, Song YQ, Song YJ (2017) Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med 15(1):221
Chen L, Yan Y, Zhu L, Cong X, Li S, Song S et al (2017) Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manage Res 9:849–867
Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q (2015) Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 236(4):297–304
Tomita M, Ayabe T, Maeda R, Nakamura K (2018) Systemic immune-inflammation index predicts survival of patients after curative resection for non-small cell lung cancer. In Vivo 32(3):663–667
Tomita M, Ayabe T, Maeda R, Nakamura K (2018) Comparison of inflammation-based prognostic scores in patients undergoing curative resection for non-small cell lung cancer. World J Oncol 9(3):85–90
Wang D, Guo D, Shi F, Zhu Y, Li A, Kong L et al (2019) The predictive effect of the systemic immune-inflammation index for patients with small-cell lung cancer. Future Oncol 15(29):3367–3379
Wang X, Jiang R, Li K (2014) Prognostic significance of pretreatment laboratory parameters in combined small-cell lung cancer. Cell Biochem Biophys 69(3):633–640
Behera M, Ragin C, Kim S, Pillai RN, Chen Z, Steuer CE et al (2016) Trends, predictors, and impact of systemic chemotherapy in small cell lung cancer patients between 1985 and 2005. Cancer 122(1):50–60
Bambace NM, Holmes CE (2011) The platelet contribution to cancer progression. J Thromb Haemost 9(2):237–249
Lim JU, Yeo CD, Kang HS, Park CK, Kim JS, Kim JW et al (2018) Prognostic value of platelet count and lymphocyte to monocyte ratio combination in stage IV non-small cell lung cancer with malignant pleural effusion. PLoS ONE 13(7):e0200341
Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5):576–590
Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE (2014) Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol 229(8):1005–1015
Jurasz P, AlonsoEscolano D, Radomski MW (2004) Platelet–cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol 143(7):819–826
Cao S, Jin S, Shen J, Cao J, Zhang H, Meng Q et al (2017) Selected patients can benefit more from the management of etoposide and platinum-based chemotherapy and thoracic irradiation-a retrospective analysis of 707 small cell lung cancer patients. Oncotarget 8(5):8657–8669
Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH (2003) Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 6(4):283–287
Shoji F, Morodomi Y, Akamine T, Takamori S, Katsura M, Takada K et al (2016) Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer 98:15–21
Smyth MJ, Hayakawa Y, Takeda K, Yagita H (2002) New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer 2(11):850–861
Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L (2012) Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 12(4):239–252
Jin S, Cao S, Xu S, Wang C, Meng Q, Yu Y (2018) Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum-based chemotherapy. Clin Respir J 12(9):2433–2440
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 31(7):860–867
Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA et al (2011) Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res 171(1):1–5
Fu Y, Chen SW, Chen SQ, Ou-Yang D, Liu WW, Song M et al (2016) A preoperative nutritional index for predicting cancer-specific and overall survival in chinese patients with laryngeal cancer: a retrospective study. Medicine 95(11):e2962
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H et al (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Can Res 58(16):3491–3494
Bernhardt D, Aufderstrasse S, Konig L, Adeberg S, Bozorgmehr F, Christopoulos P et al (2018) Impact of inflammatory markers on survival in patients with limited disease small-cell lung cancer undergoing chemoradiotherapy. Cancer Manage Res 10:6563–6569
Wei XL, Zhang DS, He MM, Jin Y, Wang DS, Zhou YX et al (2016) The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour Biol 37(2):1879–1887
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ et al (2018) First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 379(23):2220–2229
Acknowledgements
This work was funded by Doctor’s Foundation of Linyi People’s Hospital (No 803) and National Natural Scientific Foundation of China (Grant No. 81673007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, C., Jin, S., Xu, S. et al. High Systemic Immune-Inflammation Index (SII) Represents an Unfavorable Prognostic Factor for Small Cell Lung Cancer Treated with Etoposide and Platinum-Based Chemotherapy. Lung 198, 405–414 (2020). https://doi.org/10.1007/s00408-020-00333-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-020-00333-6