Abstract
Purpose and methods
A retrospective study was conducted to identify and assess potential clinical and molecularbiological risk factors for development and recurrence of sinonasal papillomas (i.e. inverted (IP), fungiform (FP), and oncocytic papillomas (OCP)). Investigated risk factors included age, gender, tumor size and localization, tobacco smoking, regular alcohol consumption, essential hypertension, anticoagulant medication, allergies, surgical approach, and HPV infection. Risk factors were evaluated by regression analysis.
Results
Apart from age and incomplete tumor resection, the recurrence of Schneiderian papillomas is independent of conventional risk factors. Patients in this study displayed higher HPV infections rates in IP (38.8%) and in FP (100%) than in healthy mucosa, which is reported 0–5.8% in Germany and central Europe. The proportion of HPV-positive IP decreased with advanced tumor stages: 100% HPV positivity of T1 IP (2/2), 40.9% of T2 IP (9/22), and 35.7% of T3 IP (20/56). Most commonly detected HPV types were HPV 6, 11, and 16; however, patients in this study also displayed HPV types that have rarely or not at all been described in sinonasal papillomas before, such as HPV 58, 42, 83, and 91. Recurrent sinonasal papillomas displayed higher rates of HPV infections than non-recurrent tumors.
Conclusions
Young age at initial diagnosis and incomplete tumor resection are risk factors for recurrence of sinonasal papillomas. Our data suggest that HPV infection supports development and/or perpetuation of sinonasal papillomas. Additionally, sinonasal papillomas seem to display a unique subset of HPV genotypes, including genotypes that have not often been described before.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sinonasal papillomas are rare, usually benign tumors arising from the Schneiderian membrane. They are divided into three groups: (1) the largest group are inverted papillomas (IP), followed by (2) fungiform (exophytic) (FP), and (3) oncocytic papillomas (OCP) [1] (Fig. 1). IP usually arise from the lateral nasal wall (the same is true for OCP), especially from the maxillo-ethmoidal recess. From here, they spread into the paranasal sinuses, foremost into the maxillary sinus and into the ethmoid cells, and more rarely also into sphenoid and frontal sinuses. Papillomas display three main characteristics: (1) a locally destructive growth pattern, (2) development of frequent tumor recurrences, and (3) the potential for malignant progression [2]. Recurrences usually occur within 2–3 years after initial treatment but can also occur delayed after many years. The size of inverted papillomas is described using the Krouse staging system (Table 1) [3]. FP characteristically originate from the nasal septum or, more rarely, the lateral nasal wall without involvement of the paranasal sinuses. They do recur, but usually they do not display malignant transformation [1].
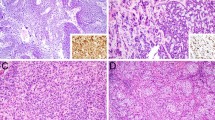
Exemplary growth patterns of sinonasal papillomas. Sinonasal papillomas are divided into three groups according to their growth pattern. This figure provides examples of the three different growth patterns in hematoxylin and eosin staining. a This image exemplifies an exophytic growth pattern, where the multi-layered epithelium covers thin cores of connective tissue (exophytic papilloma, HE staining, ×0.5 magnification). b This picture illustrates (1) an inverted growth pattern, where the epithelium grows endophytically into the connective tissue underneath (*), and (2) an oncocytic growth pattern with columnar cells that resemble oncocytes and small epithelial cysts (→)
The search for possible etiological factors of Schneiderian papillomas is still ongoing. It is widely accepted that IP and FP favour the male gender, while OCP do not [1]. However, many possible risk factors for development of IP (“classical risk factors”), i.e. tobacco smoking, alcohol consumption, allergic rhinitis, essential hypertension, or anticoagulant medication have been discarded over time [1, 4,5,6,7]. Exposition to environmental noxious agents or occupation at a work place with exposition to such a noxious environment may play an etiologic role during onset of inverted papillomas [4, 6, 8].
Human papillomaviruses (HPV) are double-stranded DNA viruses. They infect differentiating skin and mucous membrane cells and can cause uncontrolled growth patterns. The variety of HPV-induced abnormal lesions is high, ranging from benign warts to invasive cancer. HPV infections occur frequently, but most of them are erased spontaneously with time [9]. Human papilloma viruses are classified according to their cancerogenic potential in lesions of the uterine cervix from low to high risk types (a detailed table is given in online resource 1) [10,11,12]. In recent literature, HPV infection has been discussed controversially as a possible etiological factor of sinonasal papillomas [13,14,15,16,17,18,19,20].
The purpose of the present study was to investigate potential clinical and molecularbiological risk factors for development, perpetuation, and recurrence rate of sinonasal papillomas in a large patient cohort and in a long-term follow up.
Methods
Study design
101 patients were examined who were admitted with papilloma of the nasal cavity or of the paranasal sinuses to the Department of Otorhinolaryngology, Head and Neck Surgery (of our tertiary referral teaching hospital) from January 2000 through December 2010. If patients presented with recurrent papillomas within this time (even if initial diagnosis was prior to January 2000), or if patients suffered from recurrence after 2010, they were also enclosed in this study. Thus, the earliest initial diagnosis was recorded in 1996 and the last recurrent tumor in May 2017. To find the patients retrospectively, the electronic hospital information system was searched for code D14.0 or C31.0–31.9, respectively, of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). Only patients with histologically confirmed sinonasal papilloma were included in this study.
In some cases, patients initially presented with non-suspicious sinus or nasal polyps (Fig. 2). They underwent functional endonasal sinus surgery, and the diagnosis of a papilloma was then made afterwards through histopathologic examination. In these cases, a CT scan was performed 3 months after tumor resection, when the wound healing was completed. If suspicion of tumor masses occurred in CT-scans or endoscopically, patients underwent revision surgery under oncologic conditions. These cases were not counted as recurrent papillomas but as persisting tumors.
Exemplary common sinunasal polyp (a) versus inverted papilloma (b). Macroscopically, papillomas presented mostly as pink, gray, or tan polypoid tumor masses, and in contrast to ordinary sinunasal polyps they were often less to non-translucent. On touch, papillomas usually display a firmer texture than common polyps. However, in many cases papillomas are so similar to common nasal polyps that they cannot be distinguished macroscopically. In these cases, papillomas are diagnosed only after resection and histopathological examination
‘Under oncologic conditions’ in this context means, that papillomas were resected through an endonasal approach (microscopically and endoscopically), whenever possible. If complete resection was not possible through this minimal invasive approach, combined surgery was performed: e.g. lateral rhinotomy for papillomas of the nasal cavity, external frontoethmoidectomy incision for papillomas of the frontal/ethmoidal sinuses, maxillary antrostomy and inferior nasoantral window for small/medial lesions of the maxillary sinus, midface degloving/Caldwell Luc surgery for the larger tumors of the maxillary sinus/ethmoid. Tumor-free resection margins were confirmed histopathologically during surgery.
The tissue samples obtained during surgery were fixed in formalin and embedded in paraffin. Patients were divided into three groups according to histopathological criteria: patients with inverted, fungiform, or oncocytic papilloma. The clinical follow up was 3.1 years in average in our hospital.
DNA extraction and quality
DNA was extracted from formalin-fixed paraffin-embedded tissues using the spin column-based QIAamp® DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). After paraffin blocks had been sectioned (5 µm), tumor tissue was identified in five sections after hematoxylin and eosin staining and harvested microscopically for further use in HPV-PCR. Paraffin was removed by Xylol, tissue was dissolved by Proteinase K, and after several washing steps purified DNA was eluted. DNA concentration was measured using the NanoDrop ND-1000 spectrophotometer (Peqlab, Erlangen, Germany), and DNA purity was confirmed by 230/260 and 260/280 wavelength ratio. DNA quality was ensured by GAPDH amplification: four different regions of the GAPDH gene were amplified in a multiplex PCR (for detailed primer information see online resource 2) and detected by gel electrophoresis (4% agarose gel).
PCR
10-20 ng DNA was used as template for PCR. Each reaction additionally contained 2.5 µl 10 × PCR buffer, 0.75 µl (quality PCR) to 1 µl (HPV-PCR with MY09/MY11 and 125) 10 mM dNTP mix (Biozym Scientific, Hessisch Oldendorf, Germany), 0.3 µl (HPV-PCR with MY09/MY11 and 125) to 0.35 µl (quality PCR) HotStar Taq® polymerase (Qiagen, Hilden, Germany), and an appropriate amount of primers (as described in online resource 2). High purity water was added up to a final volume of 25 µl. Homogeneity and identity of PCR products were verified using 4% agarose gels.
DNA microarrays
The HPV Type 3.5 LCD-array kit (Chipron GmbH, Berlin, Germany) was used for HPV genotyping. HPV genes were amplified using biotinylated L1 consensus primer pairs MY09/MY11 and 125 provided by the kit (more detailed information on primers is given in online resource 2). Quality of PCR products was confirmed by gel electrophoresis. Amplicons were mixed with hybridization buffer (provided by the kit) and distributed onto the LCD-chip. After reverse hybridization to genotype-specific capture probes and after several washing steps, the biotinylated primers were labeled by streptavidin coupled with horseradish peroxidase. Substrate oxidation by the horseradish peroxidase formed blue precipitates and the subsequent color change was read on a spectrophotometer at a wavelength of 650 nm. HPV types were analyzed with the Slide Reader Software provided by Chipron. This DNA microarray distinguishes HPV types 6, 11, 16, 18, 31, 33, 35, 39, 42, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 70, 72, 73, 81, 82, 83, 84, 90, and 91.
Statistics
The GraphPad Prism 5 software was used to generate descriptive statistics. To identify significant risk factors for recurrent papilloma, data were compared by uni- and multivariate logistic regression using the R project for statistical computing. Additionally, Chi square test for trend was used to analyze data for trends employing the GraphPad Prism 5 software. Figures were also created with the GraphPad Prism 5 software. Data with p values less than 0.5 were referred to as statistically significant.
Results
Most patients presented with IP most of them already in advanced stages followed by FP and OCP. IP and FP favored the male gender
101 patients with 154 benign papillomas of the nasal cavity or paranasal sinuses were included in this study. Most patients presented with inverted papilloma (IP) (91 patients = 90.1%), followed by fungiform papilloma (FP) (7 patients = 6.9%), and oncocytic papilloma (OCP) (2 patients = 2.0%). For one patient it was not clear whether she suffered from IP or FP, so she was not analyzed as part of these groups. Papillomas were observed in male patients more often than in female patients (men:women ratio = 3.5). Median age for patients with IP was 59 years (range 26–83 years), for FP 46 years (range 26–69 years), and for OCP 77 years (range 73–83 years).
Inverted papillomas appeared equally often on the left and on the right side (ratio roughly 1:1), but very rarely on both sides at the same time (2 of 91 patients, 2.2%). Most IP involved he maxillary sinus (26 of 91 patients, 28.6%) and ethmoid cells (21 patients, 23.1%). As the symptoms of IP may be rather unspecific (such as one-sided rhinorrhoea, chronic rhinitis/sinusitis, or nasal obstruction), many patients presented with advanced disease stages: 19 patients displayed affection of three or even all four paranasal sinuses (20.9%), and 14 cases showed papillomas of the maxillary sinus and ethmoid cells at the same time (15.4%). The tumor extension was graded according to Krouse in stages T1–T4 (Table 1). Most patients presented with Krouse stages T3 (62 patients, 67.4%) and T2 (23 patients, 25.0%).
Fungiform papillomas were diagnosed equally often on the left and on the right side, most of them grew on the nasal septum or in the upper nasal cavity (4 of 7 patients, 57.1%).
OCP is very rare: only two patients displayed an OCP in this study. One OCP arose in the sphenoid sinus on the right side and one spanned three sinuses on the left side.
With regard to tumor recurrences, 20 patients with IP developed one tumor recurrence (22%), five patients developed two recurrences (5.5%), three patients developed three recurrences (3.3%) and one patient more than three recurrences (1.1%). In the FP group, the papilloma returned once in one patient and thrice in a second patient (14.3% each). Patients with OCP did not display tumor recurrences.
Most patients developed recurrences within the first 5 years after initial diagnosis (roughly 74%); 90% of recurrent tumors appeared within six and a half years after first diagnosis.
Young age at initial diagnosis, epithelial dysplasia, and incomplete tumor resection mean increased risk for recurrence of sinonasal papillomas
The etiology of IP is still subject of intense discussion. Patients in this study were interviewed for possible intrinsic and environmental risk factors: i.e. tobacco smoking, essential hypertension, anticoagulant medication, and allergies. When comparing patients suffering from recurrent papilloma with patients that did not develop recurrence, there was no significant difference in any of these possible risk factors. However, patients that presented with recurrent papilloma were significantly younger at the time of initial diagnosis (48.7 years in average) than patients with non-recurrent tumors (60.2 years in average, p = 0.0194) (Fig. 3a).
Younger age and incomplete tumor resection increase the risk for recurrent papillomas. a This graph shows the patients‘ age when they presented with papilloma for the first time. Patients that later on suffered from returning papilloma were significantly younger at the time of initial diagnosis (48.7 ± 13.7 years, n = 70) than patients with non-recurrent tumors (60.2 ± 14.0 years, n = 31) (p = 0.0194). Data is plotted ± SEM (standard error of the mean). b This graph displays the number of patients with inverted papilloma involving the maxillary sinus and the surgical approach that was used for tumor resection. 21 patients underwent maxillary antrostomy, of which ten patients later presented with recurrent papilloma (47.6%). Please note, that patients with tumor recurrence 3 months after initial surgery were counted as patients with persisting rather than recurrent papilloma. The second bar consists of three patients that were treated by maxillary antrostomy as well as inferior nasoantral window. One of them (33.3%) suffered recurrence. The third bar accounts for the 34 patients undergoing combined endonasal and external surgery (Caldwell Luc). Papillomas reappeared in seven of them (20.6%). Therefore, a more radical surgical approach significantly reduced tumor recurrence when inverted papillomas spanned amongst others the maxillary sinus (Chi square test for trend, p = 0.0351)
Patients with regular alcohol consumption (on a daily or nearly daily basis) suffered from significantly fewer recurrent papillomas (p = 0.00993). However, these data have to be discussed carefully, since the sample in this study consisted only of eleven patients who admitted to regular alcohol consumption and none of them presented recurrent papillomas. Therefore, the statistical significance may be biased by the small sample size.
Neither the localization of newly diagnosed sinonasal papillomas nor Krouse stages of IP had a statistically significant impact on the recurrence rates. However, the surgical approach chosen in the initial operation had a significant influence on the recurrence rate, especially in patients with papillomas of the maxillary sinus (Chi square test for trend, p = 0.0351). Recurrence rates decreased in the following order: from patients with maxillary antrostomy alone, to patients with maxillary antrostomy and inferior nasoantral window, to patients with combined endonasal/external approach (midface degloving/Caldwell Luc) (Fig. 3b). This points out the importance of complete tumor resection to achieve long-term tumor control and disease-free conditions.
Epithelial dysplasia (not yet CIS) was seen in two exophytic papillomas. Both of the patients developed tumor recurrence.
IP and even more so FP were often HPV positive; common and rare HPV genotypes were detected. Recurrent sinonasal papillomas displayed higher rates of HPV infection
91 patients with inverted papilloma (IP) were included in this study. 80 tumors could be investigated histopathologically and molecularbiologically at the time of initial diagnosis. The specimens of eleven tumors were not available for closer analysis, mostly because the patient initially presented to a different hospital and were sent to our clinic only when the tumor recurred. 31 of these 80 IP were HPV positive (38.8%). The mean men to women ratio in HPV-positive IP was 3.4 and was therefore similar to that of the overall study population (men:women ratio = 3.5). Most commonly detected HPV types were HPV 6 (in 21 of 80 IP, 26.3%) and HPV 16 (in 18 IP, 22.5%), followed by HPV 11 (in 10 IP, 12.5%), HPV 58 (in 4 IP, 5%), HPV 42 and 83 (each in 1 IP, 1.3%). Most patients displayed an infection with just one HPV type or co-infection with two types (13 of 80 IP each, 16.3%), but there was also one patient with co-infection of four different HPV types at the same time (1.3%). When considering Krouse stages, data showed a decreasing ratio of HPV positivity with advanced tumor stages: 100% HPV positivity of T1 IP (2/2), 40.9% of T2 IP (9/22), and 35.7% of T3 IP (20/56). This trend, however, was statistically not significant (p = 0.2304) (Fig. 4).
FP displayed a higher HPV infection rate than IP. Overall, HPV co-infections were rather common. This graph shows the number of HPV-positive or HPV-negative papillomas at the time of initial diagnosis. Inverted papilloma are grouped by size according to the Krouse staging system. The HPV infection rate for FP was higher than the overall infection rate for IP (100% vs. 38.8%). This figure also illustrates that HPV co-infections with more than one HPV type were rather common. In regard to HPV positivity of inverted papillomas, it is noteworthy that tumors in advanced stages were less often infected by HPV than smaller IP (please be aware of the two-segmented y-axis): 100% HPV positivity of T1 IP (2/2), 40.9% of T2 IP (9/22), and 35.7% of T3 IP (20/56). This trend, however, was statistically not significant (p = 0.2304)
Seven patients with fungiform papilloma (FP) were included in this study, of which four could be analyzed for HPV at first diagnosis. All four tumor samples were derived from men and all of them were HPV positive with either one HPV type (2 of 4 FP, 50%) or two HPV types (2 of 4 FP, 50%) (Fig. 4). HPV 6 and 11 were the most common HPV types in FP (HPV 6 was observed in 3 of 4 FP = 75%, HPV 11 in 2 of 4 FP = 50%), followed by HPV 91 (in 1 FP, 25%).
When analyzing the two OCP, the smaller one, which was localized only in the ethmoid cells, was HPV negative, while the larger one, which spanned three paranasal sinuses, was HPV positive (1 of 2 OCP, 50%) (Fig. 4). The HPV-positive OCP was derived from a male patient. It displayed co-infection with HPV 6, 16, and 42.
Recurrent papillomas displayed higher rates of HPV infection than non-recurrent papillomas: grouping all histopathologically investigated sinonasal papillomas at initial diagnosis (including IP, FP, and OCP), 36 of 86 tumors were HPV positive (41.9%). Grouping all recurrent sinonasal papillomas, 18 of 28 histopathologically investigated tumors were HPV positive (64.3%). Not all primary tumors in this study could be investigated histopathologically, because some patients came to our clinic only when the papilloma recurred or they went to another clinic for treatment of the recurrent tumor (therefore the initial tissue sample or tissue samples from recurrent tumors, respectively, could not be investigated).
Discussion
Sinonasal papillomas are usually benign, but they can display a locally destructive growth pattern and frequent recurrences, and they can transform to malignant conditions. In the present study we focussed on the identification of possible risk factors for initialization and recurrence of sinonasal papillomas, investigating 101 patients with 154 tumors. Furthermore, possible risk factors were weighted according their significance using regression analysis. Patients in our study matched known demographic traits of Schneiderian papillomas, such as prevalence of papilloma subtypes, age at initial diagnosis, gender distribution, and tumor localization [1]: Most patients suffered from inverted papillomas (IP), followed by fungiform (exophytic) (FP), and OCP. IP occur mainly in patients between 40 and 70 years of age (median age in this study was 59 years), FP appear a bit earlier in life (20–50 years; median age in this study 46 years), whereas OCP arise mainly in patients elder than 50 years (median age in this study 77 years). Both inverted and fungiform papillomas favour the male gender, whereas OCP are distributed equally amongst male and female patients, which was also observed in this study. IP usually arise from the lateral nasal wall (likewise does OCP), especially the maxillo-ethmoidal recess, from where they spread into the paranasal sinuses, foremost into the maxillary sinus and into the ethmoid cells. In this study, 61 of the 91 patients with IP presented with tumors of the maxillary sinus and/or ethmoid cells (67.0%). Recurrent papillomas usually develop within 3 years after initial treatment but may sometimes also be delayed for several years. Most patients with recurrent papillomas in this study developed tumor recurrence within the first 5 years after initial diagnosis (roughly 74%); 90% of recurrent tumors appeared within six and a half years after first diagnosis.
The search for possible etiological factors of Schneiderian papillomas and risk factors for tumor recurrence is still ongoing. Many possible environmental factors for development of IP, such as tobacco smoking, alcohol consumption, or allergic rhinitis have been discarded over time [1, 4, 5]. When looking at recurrent Schneiderian papillomas, it has been reported that smoking may promote the recurrence of IP [5, 7, 21]. However, there are data from Germany that contradict this observation: Khonsari et al. examined 47 patients; nine of them were smokers (19.1%). Two tobacco smokers (22.2%) developed a recurrent inverted papilloma, while ten non-smokers (26.3%) developed recurrence [22]. Our study supports these findings: 33.3% of male IP patients and 38.5% of female IP patients with non-recurrent tumors smoked or had a history of tobacco smoking, while 40% of male recurrent IP patients and 16.7% of female recurrent IP patients smoked or had a history of tobacco smoking. These differences were statistically not significant (p = 0.5944 for male and p = 0.6047 for female patients). Thus, tobacco smoking could not be identified as a risk factor for recurrence of IP, at least in these two German subpopulations. When looking at all papillomas (IP, FP, and OCP), there was also no significant impact of tobacco smoking on tumor recurrence (p = 0.7298). Other authors found essential hypertension to promote formation of IP, while anticoagulant medication prevented papillomas [6, 7]. These findings could not be confirmed in the present study (p = 0.7012 for essential hypertension; p = 0.6137 for anticoagulant medication).
To date, it is widely accepted by most authors that complete tumor resection, which includes endonasal endoscopic techniques as well as (if necessary) radical surgical techniques, e.g. lateral rhinotomy or midfacial degloving, is the most important factor to prevent recurrence of sinonasal papillomas [8, 23,24,25,26]. The data from our patients with inverted papillomas of the maxillary sinus (which is especially difficult to resect completely due to its localization) support this observation. Recurrence rates decreased significantly when more radical surgical approaches were performed: from patients with maxillary antrostomy alone, to patients with maxillary antrostomy and inferior nasoantral window, to patients with combined endonasal/external approach (Chi square test for trend, p = 0.0351). One problem is that the diagnosis of an inverted papilloma can only be made histologically and not clinically. This means that many patients are operated on under the idea of nasal or sinus polyps. Thus, an endonasal minimal invasive approach is chosen and surgery is not performed under oncologic conditions. The diagnosis of an inverted papilloma then comes several days later with the histopathological report. In these cases, it is reasonable to wait e.g. 3 months until the wound healing is completed and to re-stage the patient by endoscopic examination and CT or cone beam scans. If there is a suspicion of persistent tumor, a revision operation under oncologic conditions, probably using a more extensive approach, should be performed. For the statistics in this study, patients that had to undergo revision surgery after 3 months were not counted as cases with tumor recurrence but as cases with tumor persistence.
Patients that presented with recurrent papilloma were significantly younger at the time of initial diagnosis (48.7 years in average) than patients with non-recurrent tumors (60.2 years in average) (p = 0.0194). Multivariate logistic regression revealed younger age at initial diagnosis to be the strongest risk factor for tumor recurrence. Several authors described epithelial dysplasia and carcinoma in situ within Schneiderian papillomas as risk factors for tumor recurrence [16, 21, 27]. In the study presented here, two patients showed epithelial dysplasia in FP (not yet CIS). Both suffered from recurrent tumors. Therefore, epithelial dysplasia should alert the clinician to look thoroughly for tumor recurrence.
Human papillomaviruses (HPV) infect differentiating skin and mucosal cells and can induce uncontrolled growth patterns. The main mode of infection is through direct skin and mucosal contact during intercourse. Through oral intercourse HPV can also infect the mucosa of mouth, pharynx, nose, and larynx. The variety of HPV-associated lesions is high, ranging from benign warts to invasive cancer. Human papilloma viruses are classified according to their cancerogenic potential in lesions of the uterine cervix: there are (1) low risk (LR), (2) unknown/undetermined risk (UR), and (3) high risk types (HR) [10,11,12]. In this study we did not investigate HPV infection rates in sinonasal mucosa in an own healthy control group. However, HPV colonization is different depending on geographic localization. In recent literature, HPV infection rates of healthy sinonasal/oropharyngeal mucosa varied between 5.8% in Germany [28] and 0–2.1% in central Europe [29,30,31]. The HPV rates obtained in sinonasal papilloma in this study were compared to the HPV colonization rate obtained by Knoer et al. in a German healthy population (33). Amongst other authors, we have observed higher HPV infection rates in inverted papilloma (up to 79% in the literature [14] and 38.8% in this study), and even higher rates in fungiform papilloma (up to 100% in the literature [15, 19, 32] and in this study). It has therefore been postulated, that HPV infection is a risk factor for development of sinonasal papillomas, particularly IP and FP. Oncocytic papillomas are usually not infected by HPV [14, 19, 32]. However, one of two OCP patients included in this study was HPV positive. Overall, HPV detection rates in Schneiderian papillomas vary significantly between different studies and are usually not as high as e.g. in HPV-associated lesions of the genital tract. Therefore, several theories have been developed to explain the considerable divergence in HPV detection rates in sinonasal papillomas [14, 33, 34]. For example, Lawson et al. hypothesize that HPV is necessary for the initiation of papillomas, but not for their maintenance [14]. This theory is based on the idea, that HPV infects the sinonasal mucosa and causes cellular changes that ultimately lead to the development of papillomas. Then, these papillomas will keep growing even if the HPV infection is erased by the immune system. This ‘hit and run’ theory is supported by our findings: the data showed a decreasing ratio of HPV-positive IP with advanced tumor stages: 100% HPV positivity of T1 IP (2/2), 40.9% of T2 IP (9/22), and 35.7% of T3 IP (20/56). This trend, however, was statistically not significant (p = 0.2304). In contrast, Zhang et al. detected the opposite trend in a Chinese subpopulation: an increasing rate of HPV-positive IP with increasing Krouse stages (45.5% HPV-positive T1 IP and up to 100% HPV positivity for T4 papillomas) [35]. This does not necessarily render the hit and run theory invalid, but it points out that more studies are necessary to discover the point in time at which the hit occurs. HPV could be important for both initiation as well as progression of sinonasal papillomas.
To explain the varying HPV infection rates across different studies, one can also assume that a different subset of HPV genotypes is involved in sinonasal papillomas: when HPV research began, most information on HPV infections and the pathomechanisms behind the infections was gathered in anogenital lesions, particularly in lesions of the uterine cervix. In these lesions, the most common HPV genotypes are low risk types 6 and 11 and high risk types 16 and 18. Therefore, many of the early studies about HPV in sinonasal papillomas focused on these subtypes [14]. However, with advanced molecularbiological techniques and assays that detect multiple HPV genotypes at the same time, more HPV subtypes were found in Schneiderian papillomas as well. Most recently, Zhang et al. found HPV 58 to be the second most frequent HPV genotype in a Chinese population [35]. In the patients investigated in this study, HPV 58 was the fourth most frequent subtype. Additionally, we found HPV types 42, 83, and 91, which have rarely been described in Schneiderian papillomas before. Therefore, one can expect that a partly different subset of HPV genotypes is involved in sinonasal papillomas compared to anogenital lesions. However, the different detection rates of certain HPV types may also reflect regionally varying prevalences of certain HPV genotypes in different countries.
Recurrent papillomas displayed higher rates of HPV infection than non-recurrent papillomas in this study: 41.9% of initial tumors were HPV positive and 64.3% of recurrent tumors were HPV positive. These data suggest that HPV promotes recurrence of sinonasal papillomas (p = 0.0503). Further studies will be performed to determine which HPV genotypes are particularly important for tumor recurrence or even for malignant progression and to analyze recurrent papillomas and the role of HPV in regard to papilloma entities (i.e. IP, EP, OCP).
In conclusion, apart from young age and incomplete tumor resection, recurrences in Schneiderian papillomas seem to be independent of conventional risk factors. The HPV infection rate of exophytic papillomas is higher than that of inverted papillomas, and both rates are higher than the HPV detection rate in healthy sinonasal mucosa. Therefore, HPV infection might be an important factor for formation and/or perpetuation of sinonasal papillomas. However, the subset of HPV genotypes involved in Schneiderian papillomas appears to differ from the subset in anogenital lesions. According to the findings in this study, HPV infections seem to promote development and recurrence of sinonasal papillomas; therefore, all Schneiderian papillomas should be tested routinely for HPV infection. This might have some impact on contemplation of surgical technique and on post-therapeutic tumor monitoring.
Abbreviations
- CIS:
-
Carcinoma in situ
- CT:
-
Computed tomography
- DNA:
-
Deoxyribonucleic acid
- FP:
-
Fungiform = exophytic papilloma
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HPV:
-
Human papillomavirus
- HR:
-
High risk
- ICD-10:
-
International statistical classification of diseases and related health problems
- IP:
-
Inverted papilloma
- LCD:
-
Low cost density
- LR:
-
Low risk
- OCP:
-
Oncocytic papilloma
- PCR:
-
Polymerase chain reaction
- UR:
-
Unknown/undetermined risk
References
Barnes L (2002) Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Modern Pathology 15:279–297 (An official journal of the United States and Canadian Academy)
Vorasubin N, Vira D, Suh JD, Bhuta S, Wang MB (2013) Schneiderian papillomas: comparative review of exophytic, oncocytic, and inverted types. Am J Rhinol Allergy 27:287–292
Krouse JH (2000) Development of a staging system for inverted papilloma. Laryngoscope 110:965–968
Sham CL, Lee DLY, van Hasselt CA, Tong MCF (2010) A case-control study of the risk factors associated with sinonasal inverted papilloma. Am J Rhinol Allergy 24:e37–e40
Wang MJ, Noel JE (2017) Etiology of sinonasal inverted papilloma: a narrative review. World J Otorhinolaryngol Head Neck Surg 3:54–58
Tisch M, Ruf M, Maier H (2008) Risikofaktoren für invertierte Papillome der Nase. Creative Commons. http://www.egms.de/de/meetings/hnod2008/08hnod451.shtml
Moon IJ, Lee DY, Suh MW, Han DH, Kim ST, Min YG, Lee CH, Rhee CS (2010) Cigarette smoking increases risk of recurrence for sinonasal inverted papilloma. Am J Rhinol Allergy 24:325–329
Behringer S (2006) Das invertierte Papillom der Nase und Nasennebenhöhlen. Dissertation, Albert-Ludwigs-University Freiburg im Breisgau
D’Abramo CM, Archambault J (2011) Small molecule inhibitors of human papillomavirus protein protein interactions. Open Virol J 5:80–95
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJF, Meijer CJLM (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348:518–527
Varnai AD, Bollmann M, Bankfalvi A, Griefingholt H, Pfening N, Schmitt C, Pajor L, Bollmann R (2007) The spectrum of cervical diseases induced by low-risk and undefined-risk HPVs: implications for patient management. Anticancer Res 27:563–570
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens part B: biological agents. Lancet Oncol 10:321–322
Jenko K, Kocjan B, Zidar N, Poljak M, Strojan P, Zargi M, Blatnik O, Gale N (2011) In inverted papillomas HPV more likely represents incidental colonization than an etiological factor. Virchows Archiv 459:529–538 (An international journal of pathology)
Lawson W, Schlecht NF, Brandwein-Gensler M (2008) The role of the human papillomavirus in the pathogenesis of Schneiderian inverted. Head Neck Pathol 2:49–59
Ogura H, Fukushima K, Watanabe S (1996) A high prevalence of human papillomavirus DNA in recurrent nasal papillomas. J Med Microbiol 45:162–166
Hwang CS, Yang HS, Hong MK (1998) Detection of human papillomavirus (HPV) in sinonasal inverted papillomas using polymerase chain reaction (PCR). Am J Rhinol 12:363–366
Scheel A, Lin GC, McHugh JB, Komarck CM, Walline HM, Prince ME, Zacharek MA, Carey TE (2015) Human papillomavirus infection and biomarkers in sinonasal inverted papillomas: clinical significance and molecular mechanisms. Int Forum Allergy Rhinol 5:701–707
Lin H, Lin D, Xiong XS (2016) Roles of human papillomavirus infection and stathmin in the pathogenesis of sinonasal inverted papilloma. Head Neck 38:220–224
Kraft M, Simmen D, Casas R, Pfaltz M (2001) Significance of human papillomavirus in sinonasal papillomas. J Laryngol Otol 115:709–714
Justice JM, Davis KM, Saenz DA, Lanza DC (2014) Evidence that human papillomavirus causes inverted papilloma is sparse. Int Forum Allergy Rhinol 4:995–1001
Roh HJ, Mun SJ, Cho KS, Hong SL (2016) Smoking, not human papilloma virus infection, is a risk factor for recurrence of sinonasal inverted papilloma. Am J Rhinol Allergy 30:79–82
Khonsari B (2015) Invertierte Papillome der Nasenhaupt- und Nasennebenhöhlen-eine retrospektive Analyse. Dissertation, Philipps-University Marburg
Kristensen S, Vorre P, Elbrond O, Sogaard H (1985) Nasal Schneiderian papillomas: a study of 83 cases. Clin Otolaryngol Allied Sci 10:125–134
Nachtigal D, Yoskovitch A, Frenkiel S, Braverman I, Rochon L (1999) Unique characteristics of malignant Schneiderian papilloma. Otolaryngology Head Neck Surg 121:766–771
Kuester K (2014) Aktuelle Therapiekonzepte zur Behandlung des invertierten Papilloms der Nasennebenhöhlen. Dissertation, Ludwig-Maximilians-University Munich
Lisan Q, Laccourreye O, Bonfils P (2017) Sinonasal inverted papilloma: risk factors for local recurrence after surgical resection. Ann Otol Rhinol Laryngol 126:498–504
Suh JD, Ramakrishnan VR, Thompson CF, Woodworth BA, Adappa ND, Nayak J, Lee JM, Lee JT, Chiu AG, Palmer JN (2015) Inverted papilloma of the sphenoid sinus: risk factors for disease recurrence. Laryngoscope 125:544–548
Knoer M, Tziridis K, Agaimy A, Zenk J, Wendler O (2015) Human papillomavirus (HPV) prevalence in nasal and antrochoanal polyps and association with clinical data. PLoS One 10:e0141722
Eike A, Buchwald C, Rolighed J, Lindeberg H (1995) Human papillomavirus (HPV) is rarely present in normal oral and nasal mucosa. Clin Otolaryngol 20:171–173
Buchwald C, Franzmann MB, Jacobsen GK, Lindeberg H (1994) Human papiollomavirus and normal nasal mucosa: detection of human papillomavirus dna in normal nasal mucosa biopsies by polymerase chain reaction and in situ hybridization. Laryngoscope 104:755–757
Migaldi M, Pecorari M, Forbicini G, Nanni N, Grottola A, Grandi T, Delle Donne G, Leocata P, Trovato D, Sgambato A (2012) Low prevalence of human papillomavirus infection in the healthy oral mucosa of a Northern Italian population. J Oral Pathol Med 41:16–20
Weiner JS, Sherris D, Kasperbauer J, Lewis J, Li H, Persing D (1999) Relationship of human papillomavirus to Schneiderian papillomas. Laryngoscope 109:21–26
Syrjanen KJ (2003) HPV infections in benign and malignant sinonasal lesions. J Clin Pathol 56:174–181
Zhao RW, Guo ZQ, Zhang RX (2016) Human papillomavirus infection and the malignant transformation of sinonasal inverted papilloma: a meta-analysis. J Clin Virol 79:36–43 (The official publication of the Pan American)
Zhang Y, Sun P, Chen X, Pei F, Zhou E, Xue X, Chen X, Zhu J (2017) The prevalence and distribution of HPV types in nasal inverted papilloma in Chinese Han population. Int J Clin Exp Med 10:12447–12453
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. We would like to inform you, that we are planning to publish a second paper in relation to the methods section of this paper. In this second paper we will focus on histopathological traits of the three different types of sinonasal papillomas and on how we developed the molecular biological methods for HPV detection and genotyping. Additionally, one follow-up study will be performed with this patient cohort, focussing on the role of HPV for recurrence and malignant progression of sinonasal papillomas. Parts of this work have been presented in poster form on international and national ENT congresses.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pähler vor der Holte, A., Fangk, I., Glombitza, S. et al. Prognostic factors and risk factors for development and recurrence of sinonasal papillomas: potential role of different HPV subtypes. Eur Arch Otorhinolaryngol 277, 767–775 (2020). https://doi.org/10.1007/s00405-019-05747-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05747-4