Abstract
The hallmark of neurofibromatosis type 2 (NF2) is bilateral vestibular schwannomas (VS) and severe hearing loss is common in NF2 patients. Vascular endothelial growth factor (VEGF) expression level in NF2 correlates with tumour growth rate and bevacizumab, a VEGF-binding antibody, has previously been shown to induce tumour shrinkage and improve hearing. We retrospectively reviewed the effect of bevacizumab on hearing and VS tumour size in 12 consecutive NF2 patients. Bevacizumab 10 mg/kg was administered intravenously every second week for 6 months; hereafter, bevacizumab 15 mg/kg was administered every third week. Patients were evaluated with repeated audiometries, MR scans and clinical evaluations. Radiological response was defined as a 20 % or greater reduction in VS volume. A total of 398 treatments (median 36) were administered and the median duration on therapy was 22 months (range 7–34). We observed a radiological response (≥20 % tumour shrinkage) in seven out of 18 tumours (39 %) in six out of 12 patients (50 %). Sustained radiological responses were maintained in six tumours (33 %) for more than 2 months. Three patients had objectively improved hearing and five patients reported subjective benefit in neurological symptoms, including improved hearing. Toxicity was in general manageable; however, one patient died from cerebral haemorrhage which was possibly related to therapy. In conclusion, bevacizumab improved hearing and reduced the size of VS in some patients with progressive NF2 which corroborates previous findings; however, the risk of severe side effects should be carefully considered and discussed with the patients prior to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type 2 (NF2) is an autosomal dominant inherited disease characterized by the development of multiple benign tumours in the central nervous system. As the hallmark of NF2 is bilateral vestibular schwannomas (VS), severe hearing loss is common in patients with this disease. The benign VS arises from the Schwann cells surrounding the vestibular part of the eighth cranial nerve and progressive tumour growth may cause hearing loss because of compression of the cochlear nerve. However, other mechanisms may also influence hearing, since a correlation between tumour growth and hearing decline has not been unequivocally demonstrated in all studies [1]. Iatrogenic hearing loss may also occur as a result of surgical intervention or radiation therapy performed to achieve tumour control. In addition to bilateral VS, NF2 is characterized by meningiomas, ependymomas, cranial nerve sheath tumours and eye abnormalities. Common signs and symptoms are decreased hearing, tinnitus and imbalance, cranial nerve palsies and loss of vision, depending on the anatomic location of the lesions.
Angiogenesis, the formation of new vessels, is a prerequisite for growth of any solid tumour. The pro-angiogenic factor vascular endothelial growth factor (VEGF) is expressed in both sporadic and NF2-related VS and the tumour expression of VEGF correlates with the tumour growth rate [2, 3]. These findings led to the assumption that treatment with the VEGF-A binding antibody bevacizumab, which inhibits angiogenesis, may inhibit VS growth and improve hearing. This was subsequently demonstrated by Plotkin et al. [4] in a small series and an extended series of NF2 patients [5]. Tumour regression or improved hearing has also been reported in case studies [6–8]. Based on these encouraging data, we offered treatment with bevacizumab to patients with progressive NF2 outside the setting of a clinical trial. Here, we report our results from the first 12 treated patients.
Materials and methods
Hearing and radiological responses were retrospectively reviewed in consecutively referred NF2 patients with progressive VS treated with bevacizumab from January 2010 through April 2013. Cut-off for data analysis was May 2013. The diagnosis of NF2 had to comply with established NIH criteria: Bilateral VS or family history of NF2 plus unilateral VS or any two of: Meningioma, glioma, neurofibroma, schwannoma, posterior subcapsular lenticular opacities. Additional criteria were: Unilateral VS plus any two of the following: Meningioma, glioma, neurofibroma, schwannoma, and posterior subcapsular opacities or multiple meningioma (two or more) plus unilateral VS or any two of: glioma, neurofibroma, schwannoma, and cataract. [9, 10]. Prior to referral, all patients were evaluated by specialist neurosurgical team and considered not suitable for radiotherapy or surgery. The clinical indication for bevacizumab therapy was progressive VS and brainstem compression, imbalance or progressive hearing loss. Bevacizumab was administered intravenously at 10 mg/kg every second week. After 6 months of therapy, bevacizumab 15 mg/kg was administered every third week in a maintenance phase.
Hearing
The evaluation of hearing included pure tone thresholds (PTT), word recognition scores (WRS) and patient reported subjective effects. WRS measurement consisted of recognition of 25 standardized words presented at most comfortable hearing level and the percentage correct answers were reported. The pure tone average (PTA) was defined as the average of the PTT for 500, 1,000, 2,000, 4,000 and 8,000 Hz, respectively, and values reported are in decibel (dB HL). The American academy of otolaryngology-head and neck surgery (AAO-HNS) classifies hearing loss in patients with VS in four grades: A (good hearing), B (moderate hearing loss), C (severe hearing loss) and D (profound hearing loss) and changes during treatment are reported as unchanged, improved or worsened [11].
Radiology
Patients had cranial MRIs prior to bevacizumab and every 3 months during treatment. The MRI protocol consisted of T1-weighted pre-contrast, T2-weighted fast spin-echo, FLAIR diffusion and T1 MPR 3D images after contrast media. Slice thickness was 1 mm. Some patients had Fiesta or 3D T2 fast spin-echo images available. The tumour contours were outlined manually in AGFA ImPacs system (Markup Freeform) by an experienced neuroradiologist (KC) blinded for treatment status (pre- or post-bevacizumab) during a central review specifically for this study. Total tumour volume was defined as the sum of the transversal area measurements done on each slice on a post-contrast transversal T1 3D-MPR with distance factor taken into account.
Response criteria
Radiological response was determined according to the criteria suggested by Ploktin et al. [12]. Response was defined as a 20 % or greater reduction in VS volume based on post-contrast T1-weighted MRI. Sustained response was defined as confirmed response for 3 months or longer. Progressive disease was defined as a 20 % or more increase in tumour volume relative to the lowest tumour volume measured at any time following the start of treatment. Stable disease was declared in case of neither response nor progression. Time to progression was defined as the time from start of therapy to first documented radiological progression. Best response was defined as the smallest volume measured any time after start of therapy. We calculated the VS growth rate prior to start of treatment, normalized to 1 year for comparison, as: Annual growth rate prior to bevacizumab = (VS volume at start of bevacizumab minus VS volume on prior MRI x-months before treatment) divided by (x months) multiplied by 12. If patients stopped treatment and had a follow-up MRI before May 2013, these were also included in the analysis.
A hearing response was defined as: (1) at least 10 dB decrease in PTA and stable WRS according to what was suggested by Plotkin et al. [13] as a minor hearing response or (2) as an increase in WRS ≥8 percentage points. Patients were only eligible for a hearing response if the WRS allowed for improvement, i.e. they had a baseline word recognition score of 92 % or less. Patient self-reported hearing responses are reported as subjective hearing response.
Clinical evaluations
At every clinical visit, patients had a physical examination, blood sample and urine analysis and toxicity was recorded according to Common Terminology Criteria for Adverse Events, Version 3.0 at every treatment visit.
Ethics
Data collection was approved by the Danish Data Protection Agency.
Results
A total of 12 patients (7 women) with a median age of 34 years (range 23–78) were included in this retrospective analysis. Eight patients had prior unilateral surgery. Three patients had prior unilateral radiotherapy (2, 3 and 15 years before bevacizumab) and one patient had prior bilateral radiotherapy (16 years before bevacizumab).
A total of 398 treatments (median 36, range 13–60) were administered and the median duration on therapy was 22 months (range 7–34) (Table 1).
Hearing
In total, 35 audiological tests were performed (median 4, range 2–5) in 9 patients. Two patients were bilaterally deaf (Pt4 and Pt8) and one patient (Pt5) had no measurable hearing (Table 2).
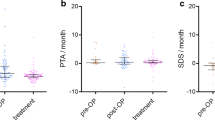
Five patients were unilaterally deaf and thus 13 ears in 9 patients were evaluable. At baseline, four ears had normal PTA and seven ears had normal WRS and these ears were, therefore, not able to improve. PTA: Of 13 evaluable ears, one ear improved by 12 decibel (dB) (8 %), two ears worsened by, respectively, 11 and 21 dB (15 %) and 10 (77 %) remained stable.
WRS: Three of 13 ears (23 %) improved 10–22 percentage points and five ears (38 %) worsened by 13–40 percentage points. Five ears remained stable (38 %). Notably, 5 of 10 patients (50 %) reported subjective hearing improvement during treatment.
If only improvable ears are considered, one of nine ears (11 %) improved, two ears (22 %) deteriorated and seven (79 %) remained stable with regard to PTA. Similarly, three of six ears (50 %) improved in WRS. According to the AAO-HNS classification, one ear improved, two ears worsened and 10 ears were unchanged (Table 2). In summary, an improvement in hearing was measured in 3 of 13 evaluable ears (23 %) in three of nine patients (33 %) and 5 of 10 patients (50 %) reported subjective hearing improvement.
Radiological response
A total of 87 MRIs (median 8, range 3–11) were reviewed. Six patients had prior unilateral tumour resection, leaving 18 VS in 12 patients evaluable for a radiological response. The growth rate prior to treatment was evaluable in 16 tumours and 13 of these (81 %) had documented tumour growth with a median annual growth of 21 % (range 2–282) (Table 3). We observed a volume reduction in 15 of 18 tumours (83 %) in 10 of 12 patients (83 %) following bevacizumab treatment. The median reduction in volume was 19 %, ranging from a 38 % reduction in volume to an increase of 16 % and seven tumours (in six patients) met the formal criteria for radiological response. The median time to radiographic response was 7.5 months (range 3–37). Some tumours continued to decrease in size over a longer period of time and the median time to the lowest volume measured was 11.5 months (range 3–45), while the median duration of response was 24 months (range 8–31). The median time to progression was 7.5 months (range 3–26) (See Supplemental Fig. 1 for details on tumour volumes for each patient separately). At the cut-off date of analysis, five of seven tumours in four patients (patient 2, 3, 5 and 7) with radiographic response were still non-progressing and seven patients were still on treatment. Only three patients had follow-up MRIs three to 9 months after end of treatment: Patient 1 had stable disease and patient 5, a 78-year-old female who had improved clinically during treatment and had a sustained radiographic response stopped after nearly 9 months of treatment because of fatigue and a feeling of exhaustion from the need to travel back and forth for treatment. Patient 9 progressed during treatment and had a novel lesion opposite to the primary lesion. Patient 4 had no MRI performed during follow-up and patient 2 died during treatment (see below).
Subjective benefit
Five patients reported subjective hearing improvement during treatment. One reported improved ability to hear her television set without her usual hearing aids and another reported recovered ability to hear nearby church bells. Furthermore, two patients reported meaningful improvement of balance and one patient experienced an improvement in tinnitus (Table 2).
Adverse events
A 23-year-old female (patient 2) died following an intracerebral haemorrhage (subarachnoidal hematoma) while on therapy. Two years prior to the start of bevacizumab, she had radiotherapy to her right VS and previously had a resection of an occipital meningioma. She also had an intracerebral shunt because of hydrocephalus and was diagnosed with epilepsy but was not on antiepileptic medications. She suffered from severe imbalance and experienced many minor falls and traumas. After one month on treatment, she fell on her bicycle and had a 4 cm large subcutaneous hematoma in the forehead and had a single episode of seizure in relation to the head trauma; a cerebral CT scan ruled out haemorrhage. She continued bevacizumab and had no subsequent head traumas prior to the fatal event. After 20 months while still on therapy, she was admitted with repeated epileptic seizures. An acute CT scan showed a large intracerebral haemorrhage and acute neurosurgical evacuation of the haemorrhage and closure of a ruptured aneurism was performed; however, the procedure was complicated by a large cerebral apoplexia that led to fatal increased intracranial pressure.
Besides this fatal event, toxicity was manageable and as expected for bevacizumab: CTC grade I–II oligomenorrhea, fatigue, hypertension and epistaxis were observed in 5, 11, 4 and 2 patients, respectively. Proteinuria ≤+3 was found in 8 of 12 (67 %) patients during treatment using a urine test strip. Renal function was normal in 11 out of 12 patients throughout the study period. One patient had a minor transient decrease in kidney function that recovered while still on full-dose bevacizumab.
Discussion
Tumour control
Our data in patients with progressive NF2 treated with bevacizumab outside a clinical trial corroborate previous reports that bevacizumab reduces the size of VS and may improve hearing and neurological symptoms [4–6, 8]. Radiological responses (≥20 % tumour shrinkage) were achieved in seven tumours (39 %) in six patients (50 %). Sustained radiological responses were maintained in six tumours (33 %) for more than 2 months. Importantly, all but one patient had progressive tumour growth prior to bevacizumab. In comparison, Plotkin et al. [4] found higher sustained tumour response rates than we did (6 out of 10 tumours in one study and in 17 out of 31 tumours in another [5]). In addition, both Mautner et al. [7] and Eminowicz et al. [6] have reported clinical cases with significant bevacizumab-induced tumour regression of VS.
The bevacizumab-induced response may be useful before surgery, even though the response may be transient since the risk of surgical complications may be dependent on tumour size [14]. It should be noted that we were not able to assess a possible accelerated tumour re-growth after bevacizumab was stopped and because of the long plasma half-life, a safety interval from last dosing to surgery should be respected.
Complications
One patient died from a cerebral haemorrhage and this was considered possibly related to bevacizumab since tumour-related haemorrhage is an infrequent complication to treatment with bevacizumab in colorectal cancer, lung cancer and in glioblastoma multiforme. Tumour-related haemorrhages were observed in only 0.24 % of patients and cerebrovascular events were even rarer consisting of 7 out of 13.656 bevacizumab-treated patients included in a large meta-analysis [15]. In the case we report, a ruptured mycotic meningeal media aneurism was detected and the event was considered cerebrovascular. Thus, causality may be difficult to establish with certainty. There is only one previously published report of a meningeal media aneurism in a patient with NF2 [16]; thus, aneurysmal bleeding is not established as a risk factor in NF2. We observed proteinuria in 67 % of patients which is in accordance with the literature [17].
Treatment dosage
The optimal dosing of bevacizumab is a debated issue in the NF2 medical community. Cerebral haemorrhage is of great concern and how this risk may possibly be related to bevacizumab dosing is unclear. A recent paper did not find any clear association between the dose or the duration of bevacizumab treatment and toxicity [18]; however, according to a recent publication [17], future studies should investigate optimal dosing schedules to minimize long-term toxicity. Based on our own experience with the use of bevacizumab in the treatment of malignant brain tumours as well as the vast amount of published data in this population with intracranial disease [19], we applied the same dosing of 10 mg/kg every 2 weeks in NF2 patients, though this was different from what was published by Plotkin et al. [4].
Change of hearing
NF2 patients often become severely disabled due to progressive hearing loss, which was the indication for bevacizumab in 67 % of the patients in our series. An improvement of hearing or just slowing of the progressive hearing loss may be clinically important, even if this is only temporary, as this provides an opportunity for the patient to learn sign language and take other necessary measures. WRS improved in 3 ears (50 %), but the improvement was relatively small. PTA improved in one ear (11 %). It is important to note, though, that 50 % of patients reported subjective neurological improvements during treatment.
In addition, 11 of 13 evaluable ears (85 %) remained stable or improved in PTA. In total, 7 of 13 ears (54 %) remained stable or improved with regard to WRS and only 2 ears worsened in hearing grade. The natural history of hearing decline with regard to WRS in NF2 patients is reported as 5 % at one year, 13 % at two years and 16 % at three years in newly diagnosed NF2 patients [20]. In comparison, Plotkin et al. [5] found a hearing response in 57 % in a retrospective review of 31 NF2 patients treated with bevacizumab and among six NF2 patients treated with bevacizumab Hawasli et al. [8] reported stable hearing in four and improved hearing in two patients.
We found a significant effect of bevacizumab on tumour volume reduction, whereas the effect on hearing was not as impressive as has previously been shown. Thus, our data may indicate dissociation between volume reduction and the effect on hearing. This we believe is important to discuss with the patient before initiating bevacizumab treatment.
Similarly, though toxicity is in general manageable, the risk of possibly fatal side effects should be considered and must be discussed with the patient.
References
Peyre M, Goutagny S, Bah A, Bernardeschi D, Larroque B, Sterkers O, Kalamarides M (2013) Conservative management of bilateral vestibular schwannomas in neurofibromatosis type 2 patients: hearing and tumor growth results. Neurosurgery 72:907–913
Caye-Thomasen P, Werther K, Nalla A, Bog-Hansen TC, Nielsen HJ, Stangerup SE, Thomsen J (2005) VEGF and VEGF receptor-1 concentration in vestibular schwannoma homogenates correlates to tumor growth rate. Otol Neurotol 26:98–101
Caye-Thomasen P, Baandrup L, Jacobsen GK, Thomsen J, Stangerup SE (2003) Immunohistochemical demonstration of vascular endothelial growth factor in vestibular schwannomas correlates to tumor growth rate. Laryngoscope 113:2129–2134
Plotkin SR, Stemmer-Rachamimov AO, Barker FG, Halpin C, Padera TP, Tyrrell A, Sorensen AG, Jain RK, di Tomaso TE (2009) Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 361:358–367
Plotkin SR, Merker VL, Halpin C, Jennings D, McKenna MJ, Harris GJ, Barker FG (2012) Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol 33:1046–1052
Eminowicz GK, Raman R, Conibear J, Plowman PN (2012) Bevacizumab treatment for vestibular schwannomas in neurofibromatosis type two: report of two cases, including responses after prior gamma knife and vascular endothelial growth factor inhibition therapy. J Laryngol Otol 126:79–82
Mautner VF, Nguyen R, Kutta H, Fuensterer C, Bokemeyer C, Hagel C, Friedrich RE, Panse J (2010) Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol 12:14–18
Hawasli AH, Rubin JB, Tran DD, Adkins DR, Waheed S, Hullar TE, Gutmann DH, Evans J, Leonard JR, Zipfel GJ, Chicoine MR (2013) Antiangiogenic agents for nonmalignant brain tumors. J Neurol Surg B Skull Base 74:136–141
Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V, Harris R (1992) A clinical study of type 2 neurofibromatosis. Q J Med 84:603–618
(1994) National Institutes of Health Consensus Development Conference Statement on Acoustic Neuroma, December 11–13, 1991. The Consensus Development Panel. Arch Neurol 51:201–207
(1995) Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg 113:179–180
Plotkin SR, Halpin C, Blakeley JO, Slattery WH III, Welling DB, Chang SM, Loeffler JS, Harris GJ, Sorensen AG, McKenna MJ, Barker FG (2009) Suggested response criteria for phase II antitumor drug studies for neurofibromatosis type 2 related vestibular schwannoma. J Neurooncol 93:61–77
Plotkin SR, Halpin C, McKenna MJ, Loeffler JS, Batchelor TT, Barker FG (2010) Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol 31:1135–1143
Springborg JB, Fugleholm K, Poulsgaard L, Caye-Thomasen P, Thomsen J, Stangerup SE (2012) Outcome after translabyrinthine surgery for vestibular schwannomas: report on 1244 patients. J Neurol Surg B Skull Base 73:168–174
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Lesley WS, Thomas MR, Abdulrauf SI (2004) N-butylcyanoacrylate embolization of a middle meningeal artery aneurysm in a patient with neurofibromatosis type 2. Am J Neuroradiol 25:1414–1416
Slusarz KM, Merker VL, Muzikansky A, Francis SA, Plotkin SR (2014) Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol 73:1197–1204
Huang H, Zheng Y, Zhu J, Zhang J, Chen H, Chen X (2014) An updated meta-analysis of fatal adverse events caused by bevacizumab therapy in cancer patients. PLoS One 9:e89960
Reardon DA, Turner S, Peters KB, Desjardins A, Gururangan S, Sampson JH, McLendon RE, Herndon JE, Jones LW, Kirkpatrick JP, Friedman AH, Vredenburgh JJ, Bigner DD, Friedman HS (2011) A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw 9:414–427
Plotkin SR, Merker VL, Muzikansky A, Barker FG, Slattery W III (2014) Natural history of vestibular schwannoma growth and hearing decline in newly diagnosed neurofibromatosis type 2 patients. Otol Neurotol 35:e50–e56
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alanin, M.C., Klausen, C., Caye-Thomasen, P. et al. The effect of bevacizumab on vestibular schwannoma tumour size and hearing in patients with neurofibromatosis type 2. Eur Arch Otorhinolaryngol 272, 3627–3633 (2015). https://doi.org/10.1007/s00405-014-3398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-3398-3