Abstract
Vestibular schwannoma (VS) growth in neurofibromatosis type 2 (NF2) can be responsible for brainstem compression and hearing loss. Surgical removal remains the standard therapy despite potential morbidity. Previous studies suggested that the inhibition of the VEGF-pathway with bevacizumab could result in hearing improvement, reduction of the tumor volume or both in adults. We retrospectively describe the French experience of bevacizumab treatment delivered for progressive VS in pediatric NF2 patients. Patients received Bevacizumab 5 or 10 mg/kg every 2 weeks according to the physician’s choice. Follow-up included clinical assessment, audiometry and volumetric MRI every 3–6 months. Seven patients harboring 11 VS were included. The median age at inclusion was 15 years (11.4–18.8), and the median treatment duration was 11.3 months (3.2–55.6). At baseline, the median tumor volume was 1.2 cm3 (0.52–13.5) and the median word recognition score was 90 % (0–100). We observed one major response, two minor responses and a decrease in the rate of tumor growth for the 4 other patients. The median annual growth rate before treatment was significantly higher than after 1 year of treatment (138 vs. 36 %, n = 5, p = 0.043). We noted one hearing improvement over the course of 1 year under treatment (hearing response rate was 14 %). Overall, the treatment was well tolerated. Our study supports that bevacizumab is an attractive therapeutic option for pediatric NF2 patients with growing VS. Thorough multidisciplinary evaluation is necessary to identify the best candidates prior to treatment. It is likely that a better functional outcome would be expected if targeted therapies were discussed early in the management of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type 2 (NF2) is a dominantly inherited genetic condition caused by heterozygous mutations in the NF2 tumor suppressor gene located on chromosome 22q12, with a birth incidence of 1 in 25,000 [1].
The hallmark of NF2 is bilateral vestibular schwannomas (also known as acoustic neuromas) and a higher risk of multiple nervous system tumors, including schwannomas, meningiomas and ependymomas.
The mortality risk increases with young age at diagnosis and the functional prognosis remains poor [2]. Untreated tumor growth can be responsible for inevitable spontaneous hearing loss, facial nerve paralysis and progressive brain-stem compression.
Currently, despite frequent functional impairment, surgical removal remains the standard therapy for unilateral vestibular schwannoma (VS). Indeed, patients often experience iatrogenic hearing loss in the treated ear requiring rehabilitation through the use of a cochlear implant or an auditory brainstem implant. As an alternative or in addition to tumor removal, stereotactic irradiation can be used to delay tumor progression, but it is thought to increase the risk for secondary malignancies in these patients [3]. Moreover, radiation therapy frequently accelerates loss of hearing.
Effective and safe treatments, able to control tumor growth without impairing hearing potential, are urgently needed for NF2 patients.
Vascular endothelial growth factor (VEGF) is a critical mediator of tumor angiogenesis and vessel permeability. A previous study showed that VEGF is produced by schwannoma tumor cells and suggested that VEGF may participate in tumor growth [4–6]. Other studies suggested that inhibition of VEGF with the VEGF- neutralizing monoclonal antibody bevacizumab could result in hearing improvement, reduction in tumor volume or both [4, 7, 8]. Moreover, the imaging findings indicated that the mean apparent diffusion coefficient (ADC) at baseline could be a potential predictive marker for a volumetric response to anti-VEGF therapy [4, 8].
In the present study, we investigated the hearing function and VS size in a pediatric population with NF2 treated with bevacizumab.
To the best of our knowledge, this study is the first to examine the effectiveness of bevacizumab in a specific pediatric population of NF2.
Materials and methods
Patients and treatment
For this retrospective study, all French pediatric oncology departments have been contacted. We included all consecutive patients fulfilling the Manchester criteria for the clinical diagnosis of NF2, presenting with evidence of progressive VS (i.e. growing VS), less than 20 years of age at baseline and who received at least 3 months of bevacizumab treatment from October 14, 2009 to June 27, 2013. All children treated by avastin for progressive schwannoma were enrolled, regardless of the progress. Tumor growth was evaluated on the last 3, 6 or 12 months (depending on the center) and calculated as a percentage of linear growth. The minimal percentages of linear growth were +15 % et 3 months, +38 % at 6 months and +100 % at 12 months.
Each patient received intravenous bevacizumab 5 mg/kg (4 patients) or 10 mg/kg (three patients) every 2 weeks, according to the choice of each physician, without any dose modifications. The seven NF2 patients for whom no other treatment options were available opted for treatment with bevacizumab after extensive consultation, discussions with patients and their families, and informed consent.
Data from patients were retrospectively collected. In this context, and according to our institutional guidelines, no institutional review board was mandatory.
Clinical, radiological and audiological evaluations
Before starting treatment, baseline Magnetic Resonance Imaging (MRI) and audiology (pure tone average (PTA) for each patient, speech recognition threshold (SRT) for six patients and speech discrimination score (SDS) for four patients) were performed.
Radiological tumor responses were determined by using serial MRI conducted every 3–4 months. We collected MRI exams throughout France from different pediatric oncology departments.
Tracking of VS growth was based on three-dimensional volumetrics because linear diameter measures underestimate the volumetric growth of VS [9]. Volumetric measurements were blinded and assessed using Osirix software (OsiriX v.5.8.1, Pixmeo Co., Switzerland) on contrast-enhanced T1-weighted axial images, with the delineation of the tumor borders performed by a radiologist. For tumors with indistinct limits, non-enhanced T1-weighted, three-dimensional high-resolution T2-weigthed (3D HR T2) and multiplanar reconstruction contrast enhanced T1-weighted images were reviewed. Volumetric measurements were only validated on a maximum 2.5-mm thickness series with no interslice gap.
The median tumor growth rate was calculated when available 3 months prior to treatment, then 3, 5 and 12 months after starting bevacizumab.
We also evaluated the mean ADC before treatment because of the correlation with tumor shrinkage [4, 8]. ADC was calculated when available with the same region of interest. B values were not the same between the different scanners. ADC correlation with tumor shrinkage was only validated when realized on the same scanner with the same B value (i.e., for four patients).
A tumor radiologic response was defined as minor when the tumor volume decreased between 5 and 19 %, and major when the decrease was greater than 20 %, as accepted criteria for NF2 VS [10].
Hearing response was determined by an audiology test every 3–6 months. Compliance with the American Academy of Otolaryngology-Head and Neck Surgery [AAO-HNS] grading system was not effective in all centers because the usual audiology testing and scoring differs in France compared to North America [11] and was only applicable in the reporting center (Lille).
The reporting center used a combination based on PTA, SDS and SRT. One center used a combination based on PTA and SDS. The third one used only PTA.
Toxicity assessment
Patient evaluation, including a physical examination, complete blood count, chemistry panel, and urinalysis, was performed at baseline, then every 2–4 weeks during treatment. Echocardiography and coagulation tests were performed before and periodically during treatment. Toxicity data were scored according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
Statistical evaluations were performed using the statistical program IBM IPSS statistics 22. We used Spearman coefficient correlation for analyses of mean ADC and tumor shrinkage. Tumor growth rates before and during bevacizumab treatment were compared for tumor volume using a Wilcoxon paired test. A p value of less than 0.05 was considered as statistically significant.
Results
Patient cohort
Seven patients from three pediatric oncology departments were included (three boys and four girls) harboring 11 VS. Two of them had a family history of NF2 (28.6 %), and the median age of symptoms onset was 7 years (range, 3.5–14 years).
None of the patients had previously been treated by medical treatment. Three of them had undergone surgical resection of a controlateral VS before starting bevacizumab (43 %) but presented no VS recurrence after surgery.
At inclusion, the median age was 15 years (range, 11.4–18.8 years). At baseline, the median PTA was 16 (range, 8–58) and the median tumor volume was 1.2 cm3 (range, 0.5–13.5 cm3).
The median treatment duration was 11.3 months (range, 3.2–55.6 months).
Two patients were still being treated with bevacizumab at the end of the study period. (Table 1). Two patients discontinued treatment for severe adverse events and four due to the lack of volumetric response.
Tumor growth rate
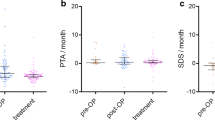
The median annual growth rate in tumor volume before treatment was 138 % (range, 100–254 %).
The evolution of the tumor growth rate was available for 8 VS but response rate was performed on 7 VS because one patient had only one MRI before starting bevacizumab. Comparisons between tumor growth before and during bevacizumab were achievable at 3 months for 4 VS, at 6 months for 3 VS and at 1 year for 5 VS. All of the 8 VS had a lower tumor growth rate.
The largest decrease in growth rate was achieved after 1 year of bevacizumab (Figure 1).
The median annual growth rate before treatment was significantly higher than after 1 year of treatment (138 % (range, 100–254) vs 36 % (range, −18–81), p = 0.043).
The median tumor growth rate during the 3 months prior to initiation of Bevacizumab was higher than the median tumor growth rate observed after 3 months of treatment (24 % (13–33 %) vs −2 % (−12–23 %), p = 0.068), as observed at 6 months (40 % (38–40 %) vs −3 % (−3–12 %), p = 0.11). However, these modifications in tumor growth rate were not really significant.
Volumetric response
Volumetric response was assessable for seven patients harboring 11 VS. Objective tumor shrinkage was observed in three patients (3/7), with one major and two minor responses.
The maximal tumor reduction was 22 % at 9 months of treatment for patient no 3 with stabilization until the last follow up (13 months).
Patient no1 presented a 17 % tumor reduction in size after 3 months of treatment with bevacizumab but treatment was stopped after occurrence of a severe adverse event (osteomyelitis). Patient no 6 had a tumor shrinkage of 6 % at 6 months (Fig. 2). Meanwhile, we observed a slight regression of facial schwannoma during the treatment with bevacizumab for patient 1.
Mean apparent diffusion coefficient and tumor shrinkage
Baseline ADC values were available for 4 VS. We observed no significant correlation, but a trend towards a relationship, between the mean ADC value for the tumor at baseline and tumor shrinkage at 3 months (Spearman’s correlation, −0.80; r2 = 0.72; p = 0.20). However, it is likely that this correlation is underpowered.
Hearing response
Four of seven patients were assessable for hearing response according to AAO-HNS guidelines (due to ceiling effect i.e. the inability to show a hearing improvement in individuals with excellent hearing at baseline).
We noted one hearing benefit with continuous improvement over the course of 1 year in a patient with rapidly growing VS (no 4). One of four eligible patients for hearing response by SDS responded. SDS increased from 60 % at baseline on the left ear to 90 % at 3 months and 100 % at 6 months. On the right ear SDS increased from 90 to 100 % at 3 months. The result was durable at the end of the follow-up.
The three others patients remained stable for SDS, Speech Reception Threshold, PTA and presented no changes relative to the AAO-HNS hearing class classifications.
Adverse events
Overall, the treatment was relatively well tolerated. A total of 7 adverse events in five children were reported.
Two patients discontinued treatment for severe adverse events: grade 3 hypertension and a grade 3 infectious event (osteitis). Osteomyelitis was noted in a bedsore context. The patient already had a prior lesion before bevacizumab. The child was suffering from intraspinal Ependymoma responsible for neuropathic pain and had a small wound at the pressure point on the right foot. The wound was dug under bevacizumab to become an ulcer and then osteomyelitis. Osteomyelitis was probably not related to treatment, but exacerbated by bevacizumab through wound healing impairment.
One patient needed dose reduction because of grade 2 epistaxis and grade 1 non-infectious wound complication. Another patient presented grade 2 inter-mensual bleeding. The last patient had a single episode of faint grade 1. Biologically, we noted one event of grade 2 proteinuria (Table 2).
Discussion
Bilateral VS are distinctive features of NF2. Even if VS are slow-growing benign tumors, they develop on nerves and can be responsible for hearing loss, tinnitus, imbalance, facial paralysis, brainstem compression, and death despite aggressive management. Unfortunately, the tumor growth is unpredictable. Regular clinical and MRI follow-ups are recommended to track the tumor growth rate. In a large cohort of patients with NF2 VS, Slattery et al. reported continuous tumor growth both short and long-term suggesting that slowing the growth rate over extended periods of time should presumably delay morbidity. The changes in size were not related to age at diagnosis, family history of NF2, initial size of the VS and were not related to the potential change in the other schwannoma [12].
Complete surgical resection is curative, but the timing for tumor removal is controversial, with a complex risk–benefit ratio, between risks of surgery and tumor’s natural history.
Recent consensus proposed surgical resection of the largest tumors (>3 cm in diameter) permitting facial nerve preservation and brainstem protection [13]. There is no consensus for the management of VS less than 3 cm in diameter [13]. Unresectable tumors and hearing impairment following surgery justify the introduction of new effective and safe therapies.
VEGF is a protein growth factor involved in tumor angiogenesis. Previous reports have shown that VEGF was expressed in 100 % of VS and VEGFR-2 in 32 % of VS vessels [4]. VEGFR-1 is a decoy receptor that does not signal or promote angiogenesis. Expression levels of VEGF and VEGFR-1 in VS correlate with the tumor growth rate [6]. These preclinical findings resulted in clinical studies of the anti-VEGF monoclonal antibody bevacizumab in adults with VS [4, 7, 8], but data concerning children are rare.
In our pediatric study we found a lower hearing improvement rate compared with previously published study in adults (14 vs 57 %) [4]. Objective tumor shrinkage was observed for three patients (3/7), with only one major response (22 % volume reduction after 9 months) and two minor responses. This response rate seemed to be lower than in adults, and irrespective for baseline tumor volume. In one study, Plotkin et al. showed that the median best response to treatment was a volumetric reduction of 26 % and that 6 of the 10 patients had an imaging response [4]. In another study, treatment with bevacizumab resulted in a 43 and 41 % volume loss in two patients [7]. In a retrospective review including 31 patients, Plotkin et al. reported a radiographic response in 55 % of target VS [8]. However, the median annual growth rate before treatment was two-fold higher in our NF2 pediatric population in contrast to two previously published VS NF2 adult population series (138 %/year vs 62 % [4] and 64 % [8], respectively). These findings could suggest more aggressive tumor behavior in pediatric patients, possibly related to a different biological pattern.
As previously described [4, 8], we observed a relationship between the mean ADC value at baseline and the percent decrease in volume at 3 months. The lack of significance of the correlation could be explained by the variety of MRI scanners (1.5T, 3T) and diffusion protocols (B800, 1000, multi B…) used in the different centers.
In our study, bevacizumab was well tolerated and the risk–benefit balance was favorable. The rare adverse events were satisfactorily manageable and the two severe adverse events (hypertension and delayed recovery of osteitis) were totally reversible with complete recovery. As already described [14], the most commonly associated events seem to be hypertension and proteinuria. In a previous cohort of 33 NF2 patients treated with bevacizumab, 58 % of subjects developed hypertension [15]. However, the median delay in the development of hypertension was 12.8 months, which exceed our median treatment duration and the patients were significantly older. Likewise, proteinuria was less frequent than described in adults in which freedom from proteinuria at 1 year was only 68 % [15]. The best responses were in patients receiving a lower dose of bevacizumab suggesting that this lower dose might be recommended in case of long term treatment.
In addition to the adverse effects, bevacizumab also has other limitations. First, as for three of our patients (No 1, 3 and 5), it seems that treatment effect was temporary and that the benefits could be reversed at discontinuation. Second, the common wound healing impairment may interfere with the necessary surgery for one of the multiple NF2 tumors and could lead to the temporary discontinuation of bevacizumab.
Because of a the crucial need for effective therapies, a few studies investigated other targeted agents [13].
Lapatinib and erlotinib target the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinase (RTK). In 2010, a pilot study with erlotinib on 11 patients with VS, showed three minor responses [16]. In 2012, lapatinib lead to a major response in 1 out of 17 patients and minor responses for 9 out of 17 patients [17]. In vitro, a previous study of 2 drugs targeting platelet-derived growth factor receptor (PDGFR) demonstrated that imatinib and nilotinib could be attractive candidates for VS treatment [18]. Moreover, two preclinical reports showed that histone desacetylase inhibitor (HDAC) AR42 inhibits schwannoma growth at doses leading to AKT pathway inhibition [19, 20].
Furthermore, in vitro, inhibition of the mammalian target of rapamycin complex 1 (mTORC1) delays growth of NF schwannoma [21], but in a recent phase II study, mTORC1 signaling pathway inhibition by everolimus was ineffective in ten NF2 patients [22].
Combination therapies could be a major management tool. The HDAC inhibitor valproic acid was used in combination with sorafinib and with erlotinib but studies were stopped early because of adverse events [23]. An mTOR inhibitor in combination with bevacizumab induced a 33 % volumetric reduction in one patient [23].
NF2 patients should be managed in reference centers, with multidisciplinary evaluation and management to identify the best candidates prior to treatment because treatment should not interfere with other tumor management. Bevacizumab could be an attractive option therapy in the management of the disease to delay surgery but, an early initiation of therapy could involve toxicities limiting long-term treatment. A systematic gene profiling analysis of tumor expression could discriminate subgroups of patients, possibly demonstrating a different profile in pediatric patients compared to adult VS and provide some targets for personalized specific therapies.
References
Evans DGR, Moran A, King A et al (2005) Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol 26(1):93–97
Baser ME, Friedman JM, Aeschliman D et al (2002) Predictors of the risk of mortality in neurofibromatosis 2. Am J Hum Genet 71(4):715–723
Mathieu D, Kondziolka D, Flickinger JC et al (2007) Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery 60(3):460–470
Plotkin SR, Stemmer-Rachamimov AO, Barker FG et al (2009) Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 361(4):358–367
Uesaka T, Shono T, Suzuki SO et al (2007) Expression of VEGF and its receptor genes in intracranial schwannomas. J Neurooncol 83(3):259–266
Cayé-Thomasen P, Werther K, Nalla A et al (2005) VEGF and VEGF receptor-1 concentration in vestibular schwannoma homogenates correlates to tumor growth rate. Otol Neurotol 26(1):98–101
Mautner V-F, Nguyen R, Kutta H et al (2010) Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro-Oncol 12(1):14–18
Plotkin SR, Merker VL, Halpin C et al (2012) Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol 33(6):1046–1052
Harris GJ, Plotkin SR, Maccollin M et al (2008) Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery 62(6):1314–1320
Plotkin SR, Halpin C, Blakeley JO et al (2009) Suggested response criteria for phase II antitumor drug studies for neurofibromatosis type 2 related vestibular schwannoma. J Neurooncol 93(1):61–77
Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol–Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. sept 1995;113(3):179-180
Slattery WH, Fisher LM, Iqbal Z et al (2004) Vestibular schwannoma growth rates in neurofibromatosis type 2 natural history consortium subjects. Otol Neurotol 25(5):811–817
Blakeley JO, Evans DG, Adler J et al (2012) Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A 158A(1):24–41
Shord SS, Bressler LR, Tierney LA et al (2009) Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health-Syst Pharm 66(11):999–1013
Slusarz KM, Merker VL, Muzikansky A et al (2014) Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol 73(6):1197–1204
Plotkin SR, Halpin C, McKenna MJ et al (2010) Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol 31(7):1135–1143
Karajannis MA, Legault G, Hagiwara M et al (2012) Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-Oncol 14(9):1163–1170
Ammoun S, Schmid MC, Triner J et al (2011) Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro-Oncol 13(7):759–766
Bush ML, Oblinger J, Brendel V et al (2011) AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro-Oncol 13(9):983–999
Jacob A, Oblinger J, Bush ML et al (2012) Preclinical validation of AR42, a novel histone deacetylase inhibitor, as treatment for vestibular schwannomas. The Laryngoscope 122(1):174–189
Giovannini M, Bonne N-X, Vitte J et al (2014) mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma. Neuro-Oncol 16(4):493–504
Karajannis MA, Legault G, Hagiwara M et al (2014) Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-Oncol 16(2):292–297
Subbiah V, Slopis J, Hong DS et al (2012) Treatment of patients with advanced neurofibromatosis type 2 with novel molecularly targeted therapies: from bench to bedside. J Clin Oncol 30(5):e64–e68
Acknowledgments
This study was presented in part at the 16th International Symposium on Pediatric Neuro-Oncology in Singapore, June 2014. We thank Isabelle Villers, Cecile Girod and Sylvie Abed for their work with data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hochart, A., Gaillard, V., Baroncini, M. et al. Bevacizumab decreases vestibular schwannomas growth rate in children and teenagers with neurofibromatosis type 2. J Neurooncol 124, 229–236 (2015). https://doi.org/10.1007/s11060-015-1828-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1828-8