Abstract
To investigate the potential preventive effect of lycopene in cisplatin-related ototoxicity. Thirty-five healthy 3–3.5-month adult female Sprague–Dawley rats were randomly divided into three groups and treated as follows: Group 1 (n = 10), received no cisplatin or lycopene. Both group 2 (n = 10) and; Group 3 (n = 15) received a single dose of 12 mg/kg cisplatin intraperitoneally. Lycopene was administered via gavage feeding in group 2 for 15 days. Prior to any medication administration, the baseline distortion product emissions were obtained in three groups. The animals were tested again at 15th day. The resulting distortion product otoacoustic emissions (DPOAE) were evaluated at 1.5, 2, 3, 4, 5, 6, 7, 8, 10, and 12 kHz. On day 0, prior to any medications, the initial DPOAEs measurement results gave similar values in the three groups (p > 0.05). In group 2 and 3, statistically significant differences were recorded for all frequencies between day 0 and day 15 values (p < 0.05). Lycopene group demonstrated significantly higher DP-grams except for 1.5 kHz frequency when compared to cisplatin group (p < 0.05). There was a statistically significant difference in basal and mid turn external ciliated cells number (p < 0.05), but there was no statistically significant difference in apical turn between three groups (p > 0.05). Stria vascularis changes were statistically significant between the groups, and the median score for stria vascularis injury was significantly greater in group 3 than in group 2 (p < 0.05). The median scores for spiral ganglion cells changes were significantly greater in group 3 than in group 2 (p < 0.05). The analyses of the results revealed statistically significant differences between two groups (p < 0.05), suggesting lycopene’s possible protective effect against cisplatin ototoxicity. The present study revealed that administration of lycopene may demonstrate a protective role against cisplatin-induced ototoxicity in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin is a widely used antineoplastic agent that inhibits growth of several types of malignant neoplasms. Beside its effectiveness against cancer, cisplatin has also severe side effects that limits its clinical use; mainly including ototoxicity, nephrotoxicity, and neurotoxicity [1].
Cisplatin-induced ototoxicity was first described by Rossof et al., in 1972, and it has been broadly studied since then [2]. The reported incidence of cisplatin toxicity can vary between 5 and 91 % [3, 4], although alterations in the high frequency audiometry may be seen in almost all the cases. The degree of hearing loss depends on the dose and frequency of drug administration [5].
Cisplatin-induced ototoxicity is manifested by bilateral, progressive, and usually irreversible sensorineural hearing loss. The main targets of the ototoxic effects seem to be the outer hair cells in the organ of Corti, the spiral ganglion cells, and the cells of the stria vascularis in the basal part of the cochlea [6].
Several previous reports have noted a variety of protective agents, such as melatonin [7], dexamethasone [8], vitamin E [9], maytenus ilicifolia [10], N-acetylcysteine [11], allopurinol and ebselen [12], d-methionine [13], aminoguanidine [14], tiopronin [15], ginkgo biloba [16], and trolox [17] against cisplatin-induced ototoxicity.
Lycopene is a natural pigment, synthesized by plants and micro-organisms, but not by animals. It is a carotenoid, an acyclic isomer of β-carotene and has no vitamin A activity [18]. Tomatoes and tomato products, watermelon, pink grapefruit, apricots, guava, and papaya are the main dietary sources of lycopene [19]. It has been reported that lycopene possess a strong antioxidant potency being 100 times more efficient in vitro studies of singlet oxygen quenching action than vitamin E, which in turn has 125 times more the quenching action of glutathione [20]. It has many biochemical functions as an antioxidant scavenger, antihyper-lipidemic agent, and inhibitor of proinflamatory and pro-thrombotic factors [21].
Protective effects of lycopene have been shown against cisplatin-induced nephrotoxicity and oxidative stress in rats [22] but the effects against ototoxicity is currently unknown. To our best of knowledge, the protective effect of lycopene in cisplatin ototoxicity has not been previously reported. In the present study, the effect of lycopene on cisplatin-induced ototoxicity in rats was evaluated.
Materials and methods
Experimental design
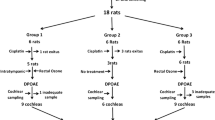
In this investigation, 35 healthy 3–3.5-month-old adult female Sprague–Dawley rats weighting between 215 and 275 g were used. The animals were housed under standard laboratory conditions (12 h light and 12 h dark) in a room with controlled temperature (24 ± 3 °C) during the entire experimental period. The animals had free access to water and were fed a standard rodent diet ad libitum. The study approval was obtained from the Erciyes University Animal Care and Use Committee. Animal care in this study was based on the criteria of the Ethics Review Committee for Animal Experimentation and NIH Guidelines for the Care and Use of Laboratory Animals.
Animals that showed signs of ear disease were excluded. Other exclusion criteria were as follows: otoscopically detectable external ear abnormalities, signs of middle-ear disease, and absence of distortion product-evoked otoacoustic emissions at any of the frequency ranges tested.
Rats were divided randomly into three groups as control, lycopene and cisplatin groups with each group caged individually. Group 1 (n = 10) received single intraperitoneal injection of 1 ml of saline and served as a control group. Both the cisplatin group (n = 10) and lycopene group (n = 15) received a single dose of 12 mg/kg cisplatin intraperitoneally (i.p.); In lycopene group, lycopene [20 mg/kg bw/day in olive oil (1 ml/kg bw/day); Redivivo lycopene 10 %; CWS/-TG, Basel, Switzerland; supplied by DSM Nutritional Products, Istanbul, Turkey] was administered via gavage for 15 days following cisplatin injection. The doses and administration routes of lycopene and cisplatin were selected based on previously published data [15, 20].
Anesthesia
Rats were anesthetized with intramuscular injection of ketamine (40 mg/kg) and xylazine (5 mg/kg) cocktail during DPOAE measurement. The depth of anesthesia was determined with the pedal reflex, and in order to maintain anesthesia, a half dose of this initial cocktail was administered as required.
Distortion product-evoked otoacoustic emission testing
After the rats were anesthetized as described previously, measurements were made on day 0 (before any medication) and day 15. DPOAE testing was carried out in a quiet environment with an Otodynamics Echoport USB cochlear emissions analyzer and the Otodynamics ILO software (MAICO MI 34, Berlin, Germany). The primary tones were introduced into the animals’ outer ear canal through an insert earphone, using a plastic adapter that sealed the probe in the outer ear canal. The stimulus consisted of two pure tones (F1 and F2; F1/F2 ratio = 1.22) at 70 dB SPL. In total, 1,000 acquisitions were analyzed. DPOAEs were determined as DP-grams. The resulting otoacoustic emissions were evaluated at 1.5, 2, 3, 4, 5, 6, 7, 8, 10, and 12 kHz. DPOAE testing was considered positive for signal-to-noise ratios of 6 dB SPL, as specified by the manufacturer.
Histopathological evaluation
Immediately following the post-DPOAE measurement, the animals were euthanized and the cochleae were dissected. The right cochleae of all groups were processed for light microscopic examination. A tiny opening was made in the apical turn of the cochleae by a curved stapes pick. The proper fixative was gently forced through the preformed apical opening by a fine needle fitted onto a tuberculin syringe allowing for good fixation. The cochleae were fixed in 10 % neutral buffered formalin for 48 h at room temperature. Subsequently, decalcification was achieved by submerging the samples in 10 % EDTA at room temperature for 7 days with daily change of the solution. The specimens were then processed for an hour in 10 % neutral buffered formalin, next in 50 % alcohol and were maintained in 70 % alcohol until preparation for paraffin embedding. Specimens were processed to form paraffin blocks. Serial longitudinal sections passing parallel to the modiolus were cut at sections of 5 μm thickness and subjected to haematoxylin and eosin stain. Sections were examined using a Zeiss Axiophot light microscope at 400× magnification equipped with a Zeiss AxioCam MRc camera in which digitalized images were obtained by a pathologist (MK) blinded to the treatment groups. Four-point scoring system for cisplatin induced injury used was those described by Freitas et al. [23]. Scores were based on an assessment of external ciliated cell (ECC) number for the organ of Corti. The expected number of hair cells for each section was compared with control animals. The mid-modiolar sections of two basal and middle turns and one apical turn were evaluated and 15 ECCs were counted (basal turns, 6 cells; middle turns, 6 cells; and apical turn, 3 cells). Based on stria vascularis assessment, which was subjective and included marginal cell blebbing, cytoplasmic vacuolization, and atrophy of intermediate cells (shrinkage), tissues were categorized as follows: normal thickness of the stria vascularis and no marginal cell blebbing, cytoplasmic vacuolization, or shrinkage, 0; slight, 1; mild, 2; moderate, 3; and severe, 4. Spiral ganglion cells, vacuolization, and nuclear degeneration were also evaluated, subjectively, and categorized based on the severity of the changes (i.e., no change, 0; mild, 1; moderate, 2; and severe changes, 3).
Statistical analyses
Data of DP-gram amplitudes were expressed as mean ± SD. Data were analyzed with Statistical Package for the Social Science (SPSS) 18.0 statistical software (SPSS Inc., Chicago, IL, USA). The Wilcoxon test was used to compare data with a normal distribution in the study groups, and the Kruskal–Wallis and Mann–Whitney U tests were used to compare differences between three groups. Within group comparisons of parameters were made using the Wilcoxon sign test. All data were presented as mean and standard errors. P values <0.05 were deemed to indicate statistical significance.
Results
Histopathological results
A four-point scoring system described by Freitas et al. [23] was used to grade injury to the ECCs, as indicated by the number of cells absent from the basal turn of the cochlear duct (0: presence of 3 ECCs with intact nuclei, 1: cochlea with injury to one ECC, 2: cochlea with injury to two ECCs, 3: cochlea with injury to three ECCs). ECCs were separated according to basal, mid, and apical turns (basal turn ECC, BECC; mid turn ECC, MECC; and apical turn, AECC, respectively), counted, and compared between three groups (Fig. 1).
Differences in basal turn ECCs numbers were statistically significant (p < 0.05) between three groups. The BECC numbers in the control group were significantly higher (p < 0.05) than the lycopene and cisplatin group (p < 0.05). BECC numbers in the lycopene group was significantly higher than cisplatin group (p < 0.05). Differences in MECC numbers were statistically significant between three groups (p < 0.05). MECC numbers of the control group were significantly higher compared with the lycopene and cisplatin group (p < 0.05). BECC numbers in the lycopene group was significantly higher than cisplatin group (p < 0.05). There was no statistically significant difference in AECC numbers between three groups (p > 0.05) (Fig. 2).
Injury in stria vascularis
A four-score system was used to grade stria vascularis injury. Scores indicated the degree of blebbing in marginal cells, cytoplasmic vacuolization, and atrophy of intermediate cells (shrinkage; 0: normal thickness of the stria vascularis and no marginal cell blebbing, cytoplasmic vacuolization, or shrinkage; 1: slight; 2: mild; 3: moderate; and 4: severe) (Fig. 3). Changes in stria vascularis were statistically significant between three groups (p < 0.05). The median score for stria vascularis injury was significantly highest in cisplatin group (p < 0.05).
Stria vascularis cross-sectional area in a control group, b cis-Pt + lycopene group showing stria vascularis slightly thickened, marginal cell blebbing and cytoplasmic vacuolization, c; cis-Pt group showing highly fragmented irregular cytoplasm and nuclei-degenerating cells (Hematoxylin & eosin, ×400) (cis-Pt: Cisplatin)
Injury in spiral ganglion cells
Vacuolization, and nuclear degeneration in spiral ganglion cells were evaluated subjectively according to the severity of the changes (0: no change; 1: mild; 2: moderate; 3: severe changes) (Fig. 4). Differences in spiral ganglion cells changes were statistically significant between three groups (p < 0.05). Differences in spiral ganglion cells of the control group were significantly lower compared with the lycopene and cisplatin group (p < 0.05). The median change scores for spiral ganglion cells were significantly higher greater in cisplatin group than in lycopene group (p < 0.05).
a Showing the normal appearance of spiral ganglion cells in control group, b vacuolization and nuclear degeneration are seen in cis-Pt + lycopene group, c showing eosinophilic cytoplasm of spiral ganglion cells; spiral ganglion cells nuclei are not seen even in areas that can be viewed with the severe degeneration in cis-Pt group (Hematoxylin and eosin, ×400) (cis-Pt: Cisplatin)
DPOAE measurement results
The DP-gram results of the three groups corresponding to days 0 and 15 are presented in (Fig. 1). On day 0, the initial baseline DPOAE measurement results presented comparable values in all groups prior to drug administration (p > 0.05) (Fig. 5). In cisplatin group statistically significant differences were recorded for all frequencies between administration before and at day 15 (p < 0.05). Lycopene group had a significantly higher DP-grams except for 1.5 kHz frequency when compared with cisplatin group (p < 0.05). The analyses of the results as the median amplitudes of DP-grams revealed statistically significant differences between three groups (p < 0.05), which may suggest that lycopene had a protective effect against cisplatin induced ototoxicity (Table 1).
Discussion
Cisplatin has a strong and well-known antitumor activity against several tumors. The main side effects are ototoxicity, nephrotoxicity, bone marrow suppression, and gastrointestinal toxicity. The ototoxicity was observed in as high as 36 % of patients receiving cisplatin [1, 5].
Morphologically, cisplatin targets three important tissues in the cochlea, including organ of Corti, spiral ganglion, and stria vascularis. Cisplatin causes the formation of reactive oxygen radicals, such as superoxide anion in cochlear tissues [6]. The mechanism of cisplatin ototoxicity is based on the generation of the reactive oxygen radicals, which interfere with the antioxidant protection of the organ of Corti. Reactive oxygen radicals react with cell membrane lipids to produce toxic aldehydes such as 4-hydroxynonenal. These are implicated in apoptosis and cell death in the organ of Corti explants and spiral ganglion cell cultures, which consequently give rise to inner ear hairy cell degeneration [14–16].
We monitored cisplatin ototoxicity by using DPOAEs, which is a highly selective tool for the cochlea. The most important benefits of DPOAEs are their non-invasive capacity and objectivity to determine the early stages of sound processing and evaluate the biomechanical activity of the outer hair cells [24]. DPOAE measurement is a well-described method for detecting the effects of cisplatin on the cochlea before changes are detected by pure-tone audiometry [25].
In recent years, naturally occurring antioxidant compounds that are consumed in the diet have gained attention in ototoxicity. The administration of antioxidants such as vitamin E, N-acetylcysteine, Ginkgo Biloba extract, pomegranate extract, resveratrol and erdosteine, before or after treatment with cisplatin has been used to protect or ameliorate against experimental ototoxicity models in animals [7–17]. To date, there is no FDA-approved product that has demonstrated efficacy in preventing or reducing cisplatin ototoxicity.
A diet rich in carotenoid-containing food is associated with a number of health benefits. Interest in effect of tomatoes and tomato-based products has increased as a consequence of many epidemiological studies showed the protective action of carotenoids, in particular lycopene, on cancer and cardiovascular diseases [19–21]. Lycopene a naturally occurring carotenoid has attracted considerable attention as a potential chemopreventive agent. Tomatoes and tomato products, watermelon, pink grapefruit, apricots, guava, and papaya are the main dietary sources of lycopene [19, 20]. Recently, lycopene has received particular attention as a result of studies that have reported its highly efficient antioxidant property and singlet-oxygen and free radical scavenging capacity. Experimental rodent models suggest that lycopene plays an important role in the modulation of organ injury evoked by inflammatory process [26, 27]. Boileau et al. demonstrated that rats could be used as a model to study biological actions of lycopene isomers. Male F344 rats fed lycopene-containing diets for 8 weeks achieved lycopene tissue concentrations and isomer patterns similar to those observed for humans [28]. Lycopene treatment, either before or after cisplatin administration, provided a significant protection against cisplatin induced nephrotoxicity. This study was the first demonstration of lycopene’s attenuating effects against cisplatin induced ototoxicity.
In our study, statistically significant reductions in DP-gram amplitudes were noted at frequencies of 1.5, 2, 3, 4, 5, 6, 7, 8, 10, and 12 kHz in the cisplatin group, strongly suggesting cisplatin-related ototoxicity. In the cisplatin and lycopene combination group, a statistically significant difference in all frequencies except 1.5 kHz was found between days 0 and 15.
The results of our current experiment suggest that lycopene may be a potential candidate drug to eliminate cisplatin induced ototoxicity. Further studies may reveal optimal dosing and potential clinical usefulness of lycopene as a chemopreventive agent against cisplatin induced ototoxicity.
References
Rabik CA, Dolan ME (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33:9–23
Borges GC, Borges RHM, Baraúna GN, Lopes Filho O (2001) Ototoxicidade causada pela cisplatina em crianças. Estudo retrospectivo. Rev Bras Otorrinolaringol 67:292–295
Merrin C (1978) Treatment of advanced bladder cancer with cisplatinum. J Urol 119:493–495
Helson L, Okonkwo E, Anton L, Critkovic E (1978) Cisplatinum ototoxicity. Clin Toxicol 13:493–495
Kopelman J, Budnick AS, Kramer MB, Sessions RB, Wong GYB (1998) Ototoxicity in high-dose cisplatin by bolus administration in patients with advanced cancers and normal hearing. Laryngoscope 98:858–864
Sockalingam R, Freeman S, Cherny TL, Sohmer H (2000) Effect of high-dose cisplatin on auditory brainstem responses and otoacoustic emissions in laboratory animals. Am J Otol 21:521–527
Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F (2000) Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res 28:73–80
Daldal A, Odabasi O, Serbetcioglu B (2007) The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck Surg 137:747–752
Kalkanis JG, Whitworth C, Rybak LP (2004) Vitamin E reduces cisplatin ototoxicity. Laryngoscope 114:538–542
Kasse CA, Cruz OL, Iha LC, Costa HO, Lopes EC, Coelho F (2008) The use of Maytenus ilicifolia to prevent cisplatin-induced ototoxicity. Braz J Otorhinolaryngol 74:712–717
Feghali JG, Liu W, Van De Water TR (2001) l-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope 111:1147–1155
Lynch ED, Gu R, Pierce C, Kil J (2005) Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res 201:81–89
Campbell KC, Meech RP, Rybak LP, Hughes LF (1999) d-Methionine protects against cisplatin damage to the stria vascularis. Hear Res 138:13–28
Kelly TC, Whitworth CA, Husain K, Rybak LP (2003) Aminoguanidine reduces cisplatin ototoxicity. Hear Res 186:10–16
Fetoni AR, Quaranta N, Marchese R, Cadoni G, Paludetti G, Sergi B (2004) The protective role of tiopronin in cisplatin ototoxicity in Wistar rats. Int J Audiol 43:465–470
Huang X, Whitworth CA, Rybak LP (2007) Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol Neurotol 28:828–833
Teranishi MA, Nakashima T (2003) Effects of trolox, locally applied on round windows, on cisplatin-induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol 67:133–139
Jonker D, Kuper CF, Estrella A, Rodrigues Otero C (2003) Ninety day oral toxicity study of lycopene from Blakeslea trispora in rats. Regul Toxicol Phamacol 37:396–406
Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP (2007) Lycopene as a natural protector against γ- radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochim Biophys Acta 1770:659–665
Kumar P, Kumar A (2009) Effect of lycopene and epigallocatechin- 3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: a novel nitric oxide mechanism. Food Chem Toxicol 47:2522–2530
Mordente A, Guantario B, Meucci E, Silvestrini A, Lombardi E, Martorana GE, Giardina B, Böhm V (2011) Lycopene and cardiovascular diseases: an update. Curr Med Chem 18:1146–1163
Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A (2005) Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 212:116–123
de Freitas MR, de Castro Brito GA, de Carvalho JV Jr, Gomes RM Jr, Barreto Martins MJ, de Albuquerque Ribeiro R (2009) Light microscopy study of cisplatin-induced ototoxicity in rats. J Laryngol Otol 123:590–597
Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F (2000) Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res 28:73–80
Ozturan O, Jerger J, Lew H, Lynch GR (1996) Monitoring of cisplatin ototoxicity by distortion-product otoacoustic emissions. Auris Nasus Larynx 23:147–151
Bignotto L, Rocha J, Sepodes B, Eduardo-Figueira M, Pinto R, Chaud M, de Carvalho J, Moreno H Jr, Mota-Filipe H (2009) Anti-inflammatory effect of lycopene on carrageenan–induced paw edema and hepatic ischemia-reperfusion in the rat. Br J Nutr 102:126–133
Reifen R, Nissenkorn A, Matas Z, Bujanover Y (2004) 5-ASA and lycopene decrease the oxidative stress and inflammation induced by iron in rats with colitis. J Gastroenterol 39:514–519
Boileau TW, Boileau AC, Erdman JW Jr (2002) Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med (Maywood) 227:914–919
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özkırış, M., Kapusuz, Z., Karaçavuş, S. et al. The effects of lycopene on cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol 270, 3027–3033 (2013). https://doi.org/10.1007/s00405-013-2352-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-013-2352-0