Abstract
Although it is widely used, there is still no valid treatment for ototoxicity caused by the antineoplastic drug cisplatin. In this study, we aimed to investigate the efficacy of intratympanic resveratrol and intratympanic dexamethasone treatment in cisplatin-induced ototoxicity. We also compared intratympanic atosiban (oxytocin antagonist) and oxytocin in cisplatin ototoxicity. In this study, 30 rats (60 ears) were used by separating into 5 groups. Cisplatin, oxytocin, dexamethasone, atosiban and 0.9% NaCl were administered intraperitoneally to all groups separately. Auditory Brainstem Response and Distortion Product Otoacoustic Emission tests were performed on all groups before and 72 h after the procedure. Pre-treatment values were higher than post-treatment values in all groups (p < 0.001). There was no significant prolongation of the post-treatment Auditory Brainstem Response I-IV interval in the oxytocin and dexamethasone groups (p > 0.05). There was no significant decrease in the frequencies of 2832 and 4004 after treatment in the oxytocin and dexamethasone group compared to pre-treatment in Distortion Product Otoacoustic Emission. As a result, it has been shown that intratympanic oxytocin may be an option that can be used in the treatment, although it is not as effective as dexamethasone in preventing cisplatin ototoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin (cis-diamminedichloroplatinum) is a commonly used antineoplastic agent. Cisplatin is mainly used in the treatment of many malignant diseases [1]. Cisplatin causes ototoxicity that may be even permanent with high toxic damage to the inner ear [2]. Cisplatin reduces glutathione by producing reactive oxygen radicals and inhibits the activity of antioxidant enzymes [3]. Follow-up tests for ototoxicity, audiometry, Distortion Product Otoacoustic Emission (DPOAE) and Auditory Brainstem Response (ABR) may be performed.
Oxytocin is a neurohypophyseal peptide hormone synthesized in the hypothalamus. It is the stimulation of the uterine contractions and myoepithelial contractions in the mammary gland. Many studies have shown the antioxidant and anti-inflammatory effects of oxytocin [4, 5]. Oxytocin prevents apoptosis by reducing consumption of glutathione and superoxide dismutase [6]. Atosiban is a reversible oxytocin receptor and can decrease uterine contractions. Atosiban may reduce the antioxidant activity of oxytocin by binding to oxytocin receptors [7].
Steroids were shown to limit the effect of reactive oxygen species in the inner ear8. Therefore, it is used in cisplatin ototoxicity [8]. Although intratympanic dexamethasone is used in various diseases, they have advantages such as less side effects and higher concentration in the perilymphatic area compared to systemic steroids.
In our study, it was aimed to evaluate the effectiveness of oxytocin and dexamethasone, which have known antioxidant activities, against cisplatin ototoxicity in intratympanic use.
Materials and Methods
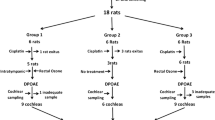
This study was carried out with the ethics committee approval of Experimental Animal Research Center of … (No: 14/20). A total of 30 female, adult, healthy, 3-month-old Albino-Wistar rats (60 ears) were used in our study. Rats were kept in an environment in experimental Animal Research Center where the temperature was 220C±20C, humidity 65–70%, with 12-hour light/12-hour dark and a free access to food and water, in addition to medication application times. External and middle ear examinations of the rats were performed under anesthesia. Ears with plugs were cleaned and rats with infection in the external auditory canal, opacification and perforation in the tympanic membrane and those with an infection in middle ear were excluded from the study.
Drug Application
All rats underwent general anesthesia with 60 mg/kg intraperitoneal (i.p) ketamine hydrochloride (Ketalar, Eczacibasi Parke-Davis, Istanbul, Turkey) and 10 mg/kg i.p xylazine HCl (Alfazyn, Alfas International B.V., Woerden, The Netherlands) before the procedures.
The groups were formed as follows: Group 1 (cisplatin) (n = 6), Group 2 (oxytocin) (n = 6), Group 3 (dexamethasone) (n = 6), Group 4 (atosiban) (n = 6) and Group 5 (0.9% NaCl (sodium chloride)) (n = 6). Group 5 was designated as the control group. In Group 1, 15 mg/kg i.p cisplatin (Cisplatin DBL, Hospira Australia Pty Ltd. Victoria, Australia) was administered via slow infusion. In Group 2, 5 I.U./ml oxytocin (Synpitan Forte, Deva Ltd, Istanbul, Turkey) was administered intratympanically in a dose of 0.05 ml to both tympanic membranes of each rat under the microscope. In Group 3, 4 mg/ml dexamethasone ampoule was administered intratympanically in a dose of 0.05 ml to both tympanic membranes of each rat under the microscope. In Group 4, 7.5 mg/ml oxytocin (Tractocile, Ferring Pharmaceuticals, Saint-Prez, Switzerland) was administered intratympanically in a dose of 0.05 ml to both tympanic membranes of each rat under the microscope. In Group 5, 0.9% NaCl was administered intratympanically in a dose of 0.05 ml to both tympanic membranes of each rat under the microscope. In Groups 2, 3, 4 and 5, i.p. 15 mg/kg of cisplatin was given 30 min after the administration of medication. Based on previous publications, ABR and DPOAE were performed when there was no residual drug in the middle ear (after 72 h) [9].
Distortion Product Otoacoustic Emission (DPOAE)
Distortion product otoacoustic emission recordings were taken with the Otodynamics OAE System device (Otodynamics Ltd, Hatfield, United Kingdom). Measurements were made before and 72 h after the medication administration. With the probe used for DPOAE, pure sound stimuli at 2 different frequencies (f1 and f2) were given simultaneously and the strongest emission in the cochlea was found with the formula 2f1-f2. These acoustic responses were obtained via the microphone inside the probe. The procedures were made in a quiet environment. The frequencies of 1416, 2002, 2832, 4004 and 5652 kHz were measured in DPOAE.
Auditory Brainstem Response (ABR)
Auditory brainstem response recordings were taken with the Interacoustics Eclipse EP15 (Interacoustics A/S, Middelfart, Denmark). Measurements were made before and 72 h after the medication administration. Newborn ear probes were inserted into the ear from the external ear canal of the measured side. Subdermal stainless-steel monops needle electrodes were placed on vertex (positive), mastoid region (negative) and dorsum (earth). Stimulations were produced in the first 10 milliseconds and all clicks were filtered (from 100 to 3000 Hz). Stimulation level started at 11 pps from 100 dB hearing level and reduced by 10 dB every step. Hearing threshold was defined as the visible, reproducible ABR produced at the lowest stimulation intensity. An average of 1500 click/stimulus was applied for all levels. The ABR I, ABR IV and ABR I-IV interval and threshold values were used in the measurements.
Statistical Analysis
SPSS Statistics 24.0 (IBM SPSS Inc, Chicago) program was used for statistical analysis. Descriptive statistics related to continuous data were stated as mean ± standard deviation. The statistical value of p < 0.05 was considered significant. Kolmogorov-Smirnov test and Shapiro–Wilk test were used as normality tests. Pre-treatment ABR I-IV interval values in Group 3, pre-treatment ABR threshold values in Group 3, pre-treatment 5652 frequencies values in Group 2 and post-treatment 2002 frequencies values in Group 1 were not normally distributed. Normally distributed data were compared with Paired Sample t-test. Comparison of normally distributed data between the groups was assessed with Independent samples t-test. Data without normal distribution were compared with Wilcoxon signed ranks test. Mann-Whitney U test was performed on the data that was not normally distributed among groups.
Results
Auditory Brainstem Response Outcomes
ABR was performed to all groups before and after the procedure. Pre-treatment and post-treatment ABR values of the groups are given in Table 1. The change in ABR pre- and post-treatment threshold median values of group is given in Fig. 1. The post-treatment ABR I, ABR IV and ABR threshold values of the groups were found to be significantly higher than the pre-treatment values (p < 0.001). Similarly, a significant prolongation of ABR I-IV interval values was observed in Group 1, Group 4 and Group 5 (p < 0.001). There was no significant prolongation of the post-treatment ABR I-IV interval in the oxytocin and dexamethasone groups (p = 0.441 and p = 0.871) (Table 1).
There was no significant difference between the groups’ pre-treatment ABR I, ABR IV, ABR 1–4 interval and ABR threshold values (p > 0.05). The difference in ABR I values in the oxytocin and dexamethasone groups was found to be significantly less than in Group 1, Group 4 and Group 5 (p < 0.001). There was no difference between the oxytocin and dexamethasone groups in terms of ABR I values (p > 0.05). The difference in ABR IV values was not significant between the groups (p > 0.05). The difference in ABR I-IV interval values in the oxytocin and dexamethasone groups was found to be significantly less than in Group 1, Group 4 and Group 5 (p < 0.001). Although there was a significant increase in post-treatment threshold values in all groups, the median value in Group 2 and Group 3 was found to be affected less than the other groups (p = 0.034 and p = 0.018). There was no difference between the oxytocin and dexamethasone groups in terms of threshold values (p > 0.05). When the differences in the atosiban group were compared with Group 1 and Group 5, no significant difference was found (p > 0.05).
Distortion Product Otoacoustic Emission Outcomes
DPOAE was performed to all groups before and after the procedure. Pre-treatment and post-treatment DPOAE values of the groups are given in Table 2. Pre-treatment values were higher than post-treatment values in all groups. Pre- and post-treatment changes in DPOAE 2832 and 4004 frequency are given in Figs. 2 and 3. There was a significant decrease in the 1416, 2002 and 5652 frequencies in all groups. There was no significant decrease in the frequencies of 2832 and 4004 after treatment in the oxytocin and dexamethasone group compared to pre-treatment (Table 2).
There was no significant difference between the groups’ pre-treatment at frequencies of 1416, 2002, 2832, 4004 and 5652 (p > 0.05). When the oxytocin and dexamethasone groups were compared with the other groups in terms of changes in all frequencies, the difference in Group 2 and Group 3 was found to be significantly less than the other groups (p < 0.001). When the oxytocin and dexamethasone groups were compared with each other, no significant difference was observed between the two groups in terms of changes in all frequencies (p > 0.05). When the differences in the atosiban group were compared with Group 1 and Group 5, no significant difference was found (p > 0.05).
Discussion
Cisplatin is one of about 130 ototoxic agents known to date [10]. Cisplatin enhances DNA damage and lipid peroxidation by increasing reactive oxygen radicals, furthermore blocking the ion transition channels causes hyperpolarization and auditory threshold elevation [11]. The deterioration in the antioxidant defense system causes an increase in lipid peroxidation and thereby leads to apoptosis in outer hairy cells [11]. Accordingly, cisplatin causes bilateral, irreversible and progressive sensorineural hearing loss. In our study, a prolongation in ABR values and a decrease in DPOAE frequencies were observed in each group given cisplatin.
Oxytocin receptors are found in many tissues [12, 13]. Kitano et al. reported that oxytocin receptor m-RNA is found in the inner ear [14]. The presence of oxytocin receptors in the inner ear makes oxytocin, which has anti-inflammatory and antioxidant properties, valuable for investigating ear diseases. In the study by Bekmez Bilmez et al., the protective effect of intratympanic and intraperitoneal oxytocin on cisplatin ototoxicity was demonstrated with DPOAE [15]. Especially in the group receiving intratympanic oxytocin, significantly less decrease in OAE values was observed compared to the intraperitoneal oxytocin group [15]. Akın Ocal et al. demonstrated the efficacy of intratympanic oxytocin in rats exposed to acoustic trauma with ABR and DPOAE [16]. In the study in which ABR thresholds were evaluated, no significant difference was found between the values on the 7th and 21st days after acoustic trauma and the values before acoustic trauma [16]. In our study, no significant prolongation was observed in the ABR I-IV interval value after cisplatin administration in the group receiving intratympanic oxytocin. Although the prolongation of the ABR I-IV interval was not significant in the oxytocin group, it was not as low as in the dexamethasone group. The difference in ABR I, ABR I-IV interval values in rats administered intratympanic oxytocin was found to be significantly less than the cisplatin, atosiban and control groups. When the dexamethasone and oxytocin groups were compared with the other groups, a significant increase was found in the ABR threshold values, but the increase in the dexamethasone group was less. In the group receiving oxytocin, there was no significant decrease in the post-treatment values at frequencies 2832 and 4004 compared to the pre-treatment values. In addition, the decrease in frequencies was significantly less than the cisplatin, atosiban and control groups. No difference was found when dexamethasone and oxytocin groups were compared with each other.
Atosiban is a reversible, competitive antagonist of the oxytocin receptor. Atosiban can reduce uterine contractions by decreasing intracytoplasmic calcium release and prostaglandin synthesis [17]. In many studies where oxytocin and atosiban are used together, it has been reported that atosiban reduces the anti-inflammatory and antioxidant effects of oxytocin [4, 18, 19]. Hussein and Mousa reported that in their study on acute myocardial injury in rats, atosiban decreased the antioxidant level increased by oxytocin [18]. Grzesiak et al. showed that atosiban given to pregnant women for tocolytic treatment increased oxidative stress [4]. In another study, oxytocin treatment was shown to alleviate stress-aggravated colitis, but atosiban reversed this effect [19]. In our study, no difference was observed between the atosiban, cisplatin and control groups for all values.
The efficacy of dexamethasone and methylprednisolone, which reduce reactive oxygen radicals, in cisplatin ototoxicity has been demonstrated in studies [20, 21]. Intratympanic steroid administration, which has no reported ototoxic effects, has the advantage of less side effects and higher perilymphatic concentration compared to systemic steroid administration [22]. In a meta-analysis, it was reported that combined steroid therapy (intratympanic steroid and systemic steroid) was significantly better than systemic steroid therapy [23]. Dexamethasone loaded nanoparticles have been shown to be effective in cisplatin ototoxicity [21]. Rauch et al. compared oral prednisolone with intratympanic methylprednisolone in a multicentered, prospective, randomized study of 250 patients with the unilateral sensorineural hearing loss [24]. Intratympanic methylprednisolone administration was shown not to be more ineffective than oral prednisolone therapy [24]. In our study, no significant prolongation of the ABR I-IV interval value was observed in rats receiving intratympanic dexamethasone after cisplatin administration. The difference in ABR I, ABR 1–4 interval and ABR threshold values in rats administered dexamethasone was found to be significantly less than in the cisplatin, atosiban and control groups. There was no significant decrease in post-treatment values compared to pre-treatment values in DPOAE frequencies of 2832 and 4004 in rats receiving dexamethasone. In addition, the decrease in frequencies was significantly less than in the atosiban, cisplatin and control groups. Studies can be detailed in larger series and with histopathological examinations.
Conclusion
In the literature, no study was found in which oxytocin, dexamethasone and atosiban were evaluated together in cisplatin ototoxicity and both ABR and DPOAE were used. When the dexamethasone and oxytocin groups were compared with the other groups, a significant increase was found in the ABR threshold values, but the increase in the dexamethasone group was less. However, no difference was found between the two groups. A similar situation was observed at 2832 and 4004 frequencies in DPOAE. As a result, it has been shown that intratympanic oxytocin may be an option that can be used in the treatment, although it is not as effective as dexamethasone in preventing cisplatin ototoxicity.
Data Availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Patient images are stored in the private archive of our faculty and can be shared by the corresponding author upon reasonable request.
References
McKeage MJ (1995) Comparative adverse effect profiles of platinum drugs. Drug Saf 13:228–244. https://doi.org/10.2165/00002018-199513040-00003
Kros CJ, Steyger PS (2019) Aminoglycoside- and Cisplatin-Induced Ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harb Perspect Med 9:a033548. https://doi.org/10.1101/cshperspect.a033548
Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH (2005) Naringenin attenuates cisplatin nephrotoxicity in rats. Life Sci 76:2125–2135. https://doi.org/10.1016/j.lfs.2004.11.005
Grzesiak M, Gaj Z, Kocyłowski R, Suliburska J, Oszukowski P, Horzelski W, von Kaisenberg C, Banach M (2018) Oxidative stress in women treated with Atosiban for Impending Preterm Birth. Oxid Med Cell Longev 2018:3919106. https://doi.org/10.1155/2018/3919106
Dusunceli F, Iseri SO, Ercan F, Gedik N, Yegen C, Yegen BC (2008) Oxytocin alleviates hepatic ischemia–reperfusion injury in rats. Peptides 29:1216–1222. https://doi.org/10.1016/j.peptides.2008.02.010
Tsuruya K, Tokumoto M, Ninomiya T, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Hirakata H, Iida M (2003) Antioxidant ameliorates cisplatin-induced renal tubular cell death through inhibition of death receptor-mediated pathways. Am J Physiol Ren Physiol 285:F208–218. https://doi.org/10.1152/ajprenal.00311.2002
Kim SH, Pohl O, Chollet A, Gotteland JP, Fairhurst AD, Bennett PR, Terzidou V (2017) Differential effects of Oxytocin receptor antagonists, Atosiban and Nolasiban, on Oxytocin receptor-mediated signaling in human amnion and myometrium. Mol Pharmacol 91:403–415. https://doi.org/10.1124/mol.116.106013
Martín-Saldaña S, Palao-Suay R, Trinidad A, Aguilar MR, Ramírez-Camacho R, San Román J (2016) Otoprotective properties of 6a-methylprednisolone-loaded nanoparticles against cisplatin: in vitro and in vivo correlation. Nanomedicine 12:965–976. https://doi.org/10.1016/j.nano.2015.12.367
Simşek G, Tokgoz SA, Vuralkan E, Caliskan M, Besalti O, Akin I (2013) Protective effects of resveratrol on cisplatin-dependent inner-ear damage in rats. Eur Arch Otorhinolaryngol 270:1789–1793. https://doi.org/10.1007/s00405-012-2183-4
Seligmann H, Podoshin L, Ben-David J, Fradis M, Goldsher M (1996) Drug-induced tinnitus and other hearing disorders. Drug Saf 14:198–212. https://doi.org/10.2165/00002018-199614030-00006
Liang F, Schulte BA, Qu C, Hu W, Shen Z (2005) Inhibition of the calcium- and voltage-dependent big conductance potassium channel ameliorates cisplatin-induced apoptosis in spiral ligament fibrocytes of the cochlea. Neuroscience 135:263–271. https://doi.org/10.1016/j.neuroscience.2005.05.055
Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG (1992) Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res 569:238–248. https://doi.org/10.1016/0006-8993(92)90635-m
Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683. https://doi.org/10.1152/physrev.2001.81.2.629
Kitano H, Takeda T, Suzuki M, Kitanishi T, Yazawa Y, Kitajima K, Kimura H, Tooyama I (1997) Vasopressin and oxytocin receptor mRNAs are expressed in the rat inner ear. NeuroReport 8:2289–2292. https://doi.org/10.1097/00001756-199707070-00038
Bekmez Bilmez ZE, Aydin S, Şanli A, Altintoprak N, Demir MG, Atalay Erdoğan B, Kösemihal E (2016) Oxytocin as a protective agent in cisplatin-induced ototoxicity. Cancer Chemother Pharmacol 77:875–879. https://doi.org/10.1007/s00280-016-2978-x
Akin Ocal FC, Kesici GG, Gurgen SG, Ocal R, Erbek S (2019) The effect of intratympanic oxytocin treatment on rats exposed to acoustic trauma. J Laryngol Otol 133:466–476. https://doi.org/10.1017/S0022215119001014
Akerlund M, Bossmar T, Brouard R, Kostrzewska A, Laudanski T, Lemancewicz A, Serradeil-Le Gal C, Steinwall M (1999) Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol 106:1047–1053. https://doi.org/10.1111/j.1471-0528.1999.tb08112.x
Hussien NI, Mousa AM (2016) Could nitric oxide be a mediator of action of oxytocin on myocardial injury in rats? (biochemical, histological and immunohistochemical study). Gen Physiol Biophys 35:353–362. https://doi.org/10.4149/gpb_2015049
Cetinel S, Hancioğlu S, Sener E, Uner C, Kiliç M, Sener G, Yeğen BC (2010) Oxytocin treatment alleviates stress-aggravated colitis by a receptor-dependent mechanism. Regul Pept 160:146–152. https://doi.org/10.1016/j.regpep.2009.11.011
Simsek G, Taş BM, Muluk NB, Azman M, Kılıç R (2019) Comparison of the protective efficacy between intratympanic dexamethasone and resveratrol treatments against cisplatin-induced ototoxicity: an experimental study. Eur Arch Otorhinolaryngol 276(12):3287–3293
Sun C, Wang X, Chen D, Lin X, Yu D, Wu H (2016) Dexamethasone loaded nanoparticles exert protective effects against cisplatin-induced hearing loss by systemic administration. Neurosci Lett 619:142–148. https://doi.org/10.1016/j.neulet.2016.03.012
Calli C, Pinar E, Oncel S, Alper Bagriyanik H, Umut Sakarya E (2012) Recovery of hearing in cisplatin-induced ototoxicity in the Guinea pig with intratympanic dexamethasone. Indian J Otolaryngol Head Neck Surg 64:46–50. https://doi.org/10.1007/s12070-011-0160-7
Han X, Yin X, Du X, Sun C (2017) Combined intratympanic and systemic use of steroids as a first-line treatment for Sudden Sensorineural hearing loss: a Meta-analysis of Randomized, controlled trials. Otol Neurotol 38:487–495. https://doi.org/10.1097/MAO.0000000000001361
Rauch SD, Halpin CF, Antonelli PJ et al (2011) Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA 305:2071–2079. https://doi.org/10.1001/jama.2011.679
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
No funds were received for this study.
Author information
Authors and Affiliations
Contributions
BMT: Planning, designing, data collection, literature survey, statistical analysis, interpretation of the results, writing. GŞ: planning, designing, data collection, literature survey, interpretation of the results. IÇK: planning, designing, literature survey, interpretation of the results. RK: planning, designing, literature survey, interpretation of the results. MA: planning, designing, literature survey, interpretation of the results, active intellectual support. All authors have contributed signifcantly, and that all authors are in agreement with the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study was carried out with the ethics committee approval of Experimental Animal Research Center of … (No: 14/20).
Conflict of Interest
The authors declared no potential conflicts of interest concerning the research, authorship and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taş, B.M., Özel, G., Azman, M. et al. Comparison of Intratympanic Oxytocin and Dexamethasone in Cisplatin Ototoxicity: An Experimental Study. Indian J Otolaryngol Head Neck Surg 76, 3405–3411 (2024). https://doi.org/10.1007/s12070-024-04701-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-024-04701-z