Abstract
Surgical approaches to the inner ear and internal auditory canal (IAC) are well known and well documented. The objective of this study is to analyze the morphology, and surgical and anatomic findings of an exclusive endoscopic transcanal approach (EETA) to the IAC. Cadaveric dissections were performed on 11 temporal bones, approaching the internal auditory meatus directly through the external ear canal and avoiding mastoidectomy. In all cases, it was possible to dissect the internal carotid artery and jugular bulb with a 0° endoscope, and with good control of these two structures. The medial wall of the bony labyrinth guaranteed good landmarks for IAC dissection, such as the spherical recess, and the labyrinthine tract of the facial nerve. The IAC can be thoroughly visualized in the cadaver using EETA, avoiding mastoidectomy, extensive temporal bone tissue removal and external incisions. Clinically based reports will be required in future to strengthen our preliminary results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The internal auditory canal (IAC) can be considered as one of the most inaccessible spaces to visualize and to operate in otoneurosurgery. Surgical approaches to the inner ear and IAC are well known and well documented. Up to the present time, the most popular methods can be classified as retrosigmoid, transmastoid–translabyrinthine and middle cranial fossa approaches. These approaches are mainly described in relation to acoustic neuroma (AN) surgery, and clinical indications, advantages, disadvantages and risks in terms of mortality and morbidity have been carefully described [1]. A particular aspect of all of the approaches described is an indirect approach to the inner ear, since both retrosigmoid and translabyrinthine methods approach the IAC posteriorly, while the middle cranial fossa method approaches the IAC superiorly.
Endoscopic instrumentation, techniques and knowledge have really improved during the last few years, and we believe that, in the future, endoscopic surgical techniques will gain increasing importance in otologic surgery. From our 7-year experience in endoscopic ear surgery, we believe that most of the spaces which are considered to be difficult to access with the microscopic technique could be easily visualized by endoscope-assisted surgery [2, 3], and we feel that new anatomic [2], physiologic [4, 5] and surgical concepts [6–9] should be introduced for this.
Moreover, dealing with middle ear pathology under endoscopic-guided surgery, most of the operations, which, using a microscopic view, would have required mastoidectomies, superficial soft tissue dissection, and retroauricular incisions, were performed by an exclusive endoscopic transcanal approach (EETA) [7–9] avoiding mastoidectomies and external incisions and passing only through the external ear canal (EAC). During several cadaveric dissections performed in recent years by our team to study middle ear anatomy and experiments with the endoscopic approach to the middle ear, with time, we have noted that the IAC and probably the whole temporal bone could also be accessed by an endoscopic view.
So the aim of our study was to analyze the morphology, and surgical and anatomic findings of an exclusive EETA to the IAC. The findings described and the results of our present experience, could form the basis for a direct lateral approach to the IAC, independent of endoscope use.
Methods
Between February 2011 and September 2011, a total of ten endoscopic cadaveric dissections were performed with an EETA to the IAC. During surgery, all the dissections were performed with 0° and 45° rigid endoscopes (Karl Storz, Tuttlingen, Germany), 15 cm in length and 3 mm in diameter. An AIDA three chip high-resolution monitor and camera (Karl Storz, Tuttlingen, Germany) were used for all of the procedures.
All dissections were recorded and stored in a digital database. During all of the dissections, by studying the transcanal endoscopic anatomy, we observed the anatomic variations, findings and accessibility of the IAC. In October 2010, video recordings from those dissections were reviewed, and the anatomic variations and accessibility of the inner canal were noted and analyzed. During the video revision, the anatomy of the facial nerve, internal carotid artery, jugular bulb, cochlea, vestibule, and internal auditory canal were noted.
The approach to the middle ear, carotid artery and jugular bulb
A circumferential tympanomeatal flap was created in the EAC with a round knife using a 0° endoscopic view, 2 cm from the tympanic membrane. The tympanomeatal flap was then elevated detaching the annulus from the bony ring, and the flap, pedicled on the umbus, was transposed laterally and then detached from the malleus with a micro-scissors. In this way, it was possible to remove all of the eardrum with the meatal skin flap of the EAC.
After this procedure, a good control of the tympanic cavity was achieved, and the ossicular chain, promontory region, protympanum, retrotympanum and hypotympanum, were detected endoscopically (Fig. 1).
Then, using a diamond bur under 0° view, the scutum was partially removed, uncovering the incudomalleolar joint. The incus was removed maintaining the integrity of the stapes, the tensor tendon was cut and the malleus was removed. These procedures allowed us to achieve a good control of the second tract of the facial nerve, cochleariform process and semicanal of the tensor tympani muscle. The cochleariform process and the bony wall of the semicanal of the tensor tendon were removed with a curette, uncovering the tensor tympani muscle (Fig. 2). A careful dissection of the muscle was performed using a dissector, and the tensor tympani muscle was transposed anteriorly: this procedure allowed a direct endoscopic view of the geniculate ganglion and great petrous nerve (Fig. 3). To gain optimal surgical access to the entire medial wall of the tympanic cavity, the bony ring and annulus were drilled until the hypotympanic space, the protympanum, and the eustachian tube were directly visible under 0° view. Then, dissection of the internal carotid artery and jugular bulb was performed. By drilling the protympanum just under the eustachian tube, it was possible to locate the vertical portion of the internal carotid artery, and this structure was followed cranially. The jugular bulb was located by drilling the hypotympanum just below the promontory.
The cochleariform process and the bony wall of the semicanal of the tensor tendon were removed with a curette, uncovering the tensor tympani muscle. Et eustachian tube, pr promontory, s stapes, fn facial nerve, rw round window, lsc lateral semicircular canal, ttc tensor tympani canal, ttm tensor tympani muscle, mcf middle cranial fossa
The tensor tympani muscle was transposed anteriorly: this procedure allowed a direct endoscopic view of the geniculate ganglion and great petrous nerve Et eustachian tube, pr promontory, s stapes, fn facial nerve, rw round window, lsc lateral semicircular canal, ttm tensor tympani muscle, mcf middle cranial fossa, pe pyramidal eminence, gg geniculate ganglion, gpn great petrosal nerve
The approach to the labyrinth and IAC
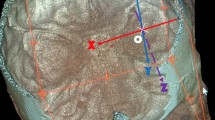
A transcanal transvestibular approach to the inner ear was then commenced. The stapes was removed opening the vestibule, and the medial wall of the vestibule was investigated endoscopically (Figs. 4, 5). The bony wall around the oval window was removed with a curette, uncovering the medial wall of the vestibule and paying special attention not to injure the facial nerve, running just above the oval window. In this way, the medial wall of the vestibule was visible endoscopically by direct view (Fig. 6). The exact positions of the saccular and utricular fossae were located, with respect to the other important anatomic structures. The vestibular crest dividing the saccular fossa from the utricular fossa was identified, and the spherical recess in the saccular fossa and the elliptical recess in the utricular fossa were noted. The presence of the labyrinthine opening in the vestibule was also observed. After those steps of exploring the medial wall of the vestibule, drilling just anteriorly to the vestibule on the promontory region was performed, looking for the basal turn of the cochlea (Fig. 7), and observing the position of this structure in relation to the vestibule. Both the cochlea turns and the medial wall of the vestibule were visible endoscopically at this point, and under the guidance of these landmarks, drilling into the vestibule over the spherical recess was commenced. This procedure allowed us to open the fundus of the IAC, removing the inferior vestibular nerve just attached to the spherical recess with its terminal nervous fibres; from this recess, a direct view of the intrameatal tract of the facial nerve was obtained (Fig. 8). The presence of all these landmarks further allowed the identification of an anatomical triangle between the geniculate ganglion superiorly, the basal turn of the cochlea anteriorly and the spherical recess IAC postero-inferiorly (Fig. 7), drilling into this anatomic triangle, the intralabyrinthine tract of the facial nerve from the IAC to the geniculate ganglion was identified, and the anatomic position and variations of the intralabyrinthine facial nerve were noted. Once the entire control of the first and second portions of the facial nerve had been obtained, the promontory was drilled until the cerebellopontine angle (CPA) was wide open with the possibility to explore further from there (Figs. 9, 10).
The stapes was removed opening the vestibule, and the medial wall of the vestibule was investigated endoscopically. pr promontory, s stapes, fn facial nerve, rw round window, lsc lateral semicircular canal, mcf middle cranial fossa, pe pyramidal eminence, gg geniculate ganglion, gpn great petrosal nerve, ow oval window, psc posterior semicircular canal, sph spherical recess
A scheme illustrating the medial aspect of the bony labyrinth. pr promontory, s stapes, fn facial nerve, rw round window, lsc lateral semicircular canal, mcf middle cranial fossa, pe pyramidal eminence, gg geniculate ganglion, ow oval window, psc posterior semicircular canal, jb jugular bulb, ca carotid artery, cho choclea, vc vestibular crest, sph spherical recess
The bony wall around the oval window was removed with a curette, uncovering the medial wall of the vestibule. pr promontory, s stapes, fn facial nerve, rw round window, lsc lateral semicircular canal, mcf middle cranial fossa, pe pyramidal eminence, gg geniculate ganglion, gpn great petrosal nerve, ow oval window, sph spherical recess, jb jugular bulb
Drilling just anteriorly to the vestibule on the promontory region was performed, looking for the basal turn of the cochlea (a, b). The identification of an anatomic triangle (yellow triangle) between the geniculate ganglion superiorly, the basal turn of the cochlea anteriorly and the spherical recess (IAC) postero-inferiorly (c, d). pr promontory, s stapes, fn facial nerve, rw round window, lsc lateral semicircular canal, mcf middle cranial fossa, pe pyramidal eminence, gg geniculate ganglion, gpn great petrosal nerve, ow oval window, sph spherical recess, ch choclea
Opening the fundus of the IAC, a direct view of the intrameatal tract of the facial nerve was obtained. pr promontory, s stapes, fn facial nerve rw round window, mcf middle cranial fossa, pe pyramidal eminence, gg geniculate ganglion, gpn great petrosal nerve, ow oval window, sph spherical recess, ch choclea
Once the entire control of the first and second portions of the facial nerve had been obtained, the promontory was drilled until the cerebellopontine angle was wide open. pr promontory, s stapes, fn facial nerve, rw round window, mcf middle cranial fossa, lsc lateral semicircular canal, pe pyramidal eminence, gg geniculate ganglion, gpn great petrosal nerve, ow oval window, sph spherical recess, cho choclea, iac internal auditory canal
Visualization of internal auditory canal by EETA, showing the relationship between facial and cochlear nerve. pr promontory, s stapes, fn facial nerve, rw round window, lsc lateral semicircular canal, mcf middle cranial fossa, pe pyramidal eminence, gg geniculate ganglion, ow oval window, psc posterior semicircular canal, jb jugular bulb, ca carotid artery, chon choclear nerve
Results
The following findings were noted during video review of the dissections.
EAC and ossicular chain findings
In all temporal bones, the dimensions of the external auditory canal allowed us to perform a dissection of the tympanomeatal flap removing en bloc the eardrum with the skin flap of the external canal and maintaining the integrity of the ossicular chain. In comparison with operations in living patients, these steps were made much easier by the absence of bleeding. The evaluation of the structure of the EAC facilitated the further steps to the middle and internal ear.
Carotid artery and jugular bulb findings
In all cases, it was possible to dissect the internal carotid artery and jugular bulb with a 0° endoscope, obtaining good control of these two structures.
Any anatomic variations or morphology with regard to the position of these vessels were not noticed, but we noted some anatomic variations with regard to the dimensions of the bony wall covering the vascular structures:
-
In 6/10 subjects, the vertical segment of the carotid artery was immediately visible endoscopically, with a dehiscence of the artery under the eustachian tube, and in these subjects, it was possible to dissect all of the vertical segment of the carotid artery just using a curette to remove the surrounding bone.
-
In 4/10 subjects, the internal carotid artery was not visible endoscopically lying under a thick bony wall. In these cases, it was necessary to drill the protympanic bone just anteriorly to the promontory region and inferiorly with respect to the eustachian tube to look for the artery.
-
In 2/10 subjects, the bulging of the jugular bulb was visible endoscopically without any drilling of the hypotympanic bone.
-
In 8/10 subjects, the jugular bulb was covered with a bony wall under the promontory region. In these cases, it was necessary to drill to remove the hypotympanic bone covering the jugular bulb.
Cochleariform process and tympanic facial nerve findings
In all ten subjects, it was possible to dissect the entire second tract of the facial nerve, geniculate ganglion and greater petrous nerve, but this procedure could only be performed by removing the cochleariform process and tensor tendon muscle.
In all temporal bones, the cochleariform process was removed by curetting the bony segment of this anatomic structure, to uncover the tensor tympani muscle. In all temporal bones, it was possible to elevate the tensor tympani muscle from anterior and medial aspect of the middle ear by a curved dissector, uncovering the geniculate ganglion and greater petrosal nerve area; in 7/10 subjects, we found a close relationship between the anterior and superior portions of the muscle and the geniculate ganglion. In these cases, we had to dissect the muscle from the nerve paying attention not to injure the nerve.
In 4/10 subjects, a dehiscence of the middle cranial fossa dura was found in the anterior epitympanic space: in all cases, the dura of the middle cranial fossa was in a close relationship with both the geniculate ganglion and the greater petrosal nerve while in 6/10 subjects, we had to drill to find the dura of the middle cranial fossa.
Vestibule and medial wall of the vestibule
After stapes removal and checking the oval window endoscopically, in all cases, the conformation of the medial wall of the vestibule could be visualized using the 0° endoscope. In all of the subjects, the saccule remnants lay just anteriorly and medially with respect to the footplate of the stapes. In 5/10 subjects, it was possible to view the spherical recess in the saccular fossa without any drilling by observing endoscopically through the oval window. In the other five subjects, we had to drill the oval window niche to enlarge it inferiorly and anteriorly: after this operation, it was possible to observe the spherical recess in the saccular fossa.
After enlarging the oval window by drilling its niche, the relationship between the saccular and the utricular fossa could be noted in all temporal bones: a vestibular crest was present dividing the saccular from the utricular fossa. In all subjects, the position of the utricular fossa lay medially with respect to the second tract of the facial nerve and just superiorly and posteriorly with respect to the saccular fossa.
The spherical recess composed of a thin bony wall where the inferior vestibular nerve fibres were attached. Just using a curette, it was possible to remove this bone, so as to enter the IAC. In all of the specimens, the intrameatal facial nerve lay just medially with respect to this opening.
Cochlea and intralabyrinthine facial nerve
In all subjects, we were able to find the basal turn of the cochlea by drilling just anteriorly with respect to the opening of the vestibule and inferiorly to the cochleariform process area. The medial turn of the cochlea represents an important landmark for the intralabyrinthine facial nerve, since this segment of the facial nerve runs just superiorly to the cochlea from the geniculate ganglion superiorly and anteriorly, and the intrameatal facial nerve inferiorly and posteriorly in all subjects.
In all subjects, it was possible to dissect the intralabyrinthine facial nerve by drilling the bony wall between the geniculate ganglion and the cochlea anteriorly, and the medial wall of the vestibule, and the IAC posteriorly.
In all subjects, the intralabyrinthine facial nerve was covered by a compact bony wall and the dissection of this component of the facial nerve was quite difficult. The bone over the facial nerve allowed us to reach the nerve, but in all subjects, the nerve was extremely fragile: in three cases, an injury to the intralabyrinthine facial nerve was provoked while performing this step.
In 7/10 subjects, we were able to dissect the entire first and second segments of the facial nerve, having control of the major vessels (carotid artery, jugular bulb). We were able to remove all of the promontory bone and the protympanic bone to perform a transcochlear approach to the inner ear and apex.
Discussion
Retrosigmoid, transmastoid–translabyrinthine and middle cranial fossa approaches are the most widely used for removal of IAC pathology, in particular, in AN surgery. The choice of the right approach depends on factors related to the surgeon’s preferences and habits, dimensions and extent of the pathology, goal of hearing preservation, risk to the facial nerve and post-operative complications [1]. The middle cranial fossa approach is classically recommended in the case of small acoustic neuromas [1]: it allows access to the IAC superiorly, drilling on the superior aspect of the petrous bone. It guarantees a limited access to the CPA, so it cannot be used in large acoustic neuromas, extending widely outside the IAC. A retrosigmoid approach guarantees optimal control of the CPA, although its control of the canal requires drilling of the posterior aspect of the petrous bone, and in most cases, IAC fundus control is difficult to obtain [1]. When hearing preservation is attempted, the retrosigmoid and middle cranial fossa approaches are chosen [10]; this frequently occurs for small ANs. The translabyrinthine approach results in loss of hearing and extensive drilling of the petrous bone, while it guarantees a good exposure of both IAC and CPA [11].
The first introduction of the endoscopic technique in AN surgery has been in combination with the retrosigmoid approach [12]: after the removal of the CPA extension of the neoplasm, under endoscopic control, the intracanalicular extension is removed, trying to avoid extensive drilling of the posterior aspect of the petrous bone. In our institution, such a technique has been used since 2003.
A gradual introduction of endoscopic techniques to treat middle ear pathologies began in 2004. Endoscopies were primarily used for the visualization of hidden areas such as the posterior epitympanum during classic microscopic tympanoplasties [13]. Gradually, it was also used during operations, to replace the microscope as the main tool in middle ear surgery [3–7, 9]. Development of the endoscopic technique required several cadaveric dissections, to better understand the anatomy and to determine appropriate instruments for this purpose. During these dissections, some steps were taken to explore the internal ear, from the labyrinth, jugular and carotid, to the IAC, until a procedure was roughly documented. The first step in the procedures resembles middle ear approaches described in the past for cholesteatoma treatment, for exploration and study of the middle ear [2, 7–9]. In EETA for IAC, the skin of the EAC must be removed en bloc as a glove finger, so as to obtain the widest space for surgical maneuvering of instruments and optics during the procedures. All middle ear structures can be visualized this way, even hidden areas, using angled optics. In addition, when required, annulus removal can be performed to gain optimal access to hidden areas: this is a very important procedure, not to visualize the structures, but to manoeuvre using appropriate instruments in the most difficult areas. In some cases, the jugular bulb, lying in the hypotympanum, and the carotid artery, lying in the protympanic spaces are easily visualized by EETA, due to the absence of a bony layer covering them, as mentioned above. If some bone hides those structures, it is possible to uncover them using appropriate instruments (burrs). The discovery of the great vessels and their relationship to the surrounding structures are very important for the following steps, which are performed more safely if the position of the great vessels is obtained. Moreover, the great vessels can represent landmarks for other structures.
As also described in an earlier article [7], the cochleariform process and the tensor tympani muscle represent fundamental landmarks for the tympanic tract of the facial nerve, but to obtain an adequate exposure of that nerve they must be removed. Even the geniculate ganglion and greater petrosal nerve could be identified by an EETA, and their relationships with the labyrinthine tract of the facial nerve and the middle cranial fossa dura could be evaluated. This could be relevant and clinically significant if an EETA to the facial nerve is attempted.
As mentioned above, by removing the stapes, it is possible to visualize a portion of the medial aspect of the vestibule by moving the optic tip close to the oval window. In particular, in some cases, the spherical recess in the saccular fossa can be seen. The spherical recess represents an important landmark for further steps since it is the exact place where the inferior vestibular nerve fibres are attached, representing a kind of door for the IAC entrance procedures. In all of the specimens, the intrameatal facial nerve lay just medially and anteriorly with respect to this opening.
After the removal of the lateral aspect of the otic capsule by drilling centrifugally from the oval window, it is possible to obtain complete access to the whole medial aspect of the vestibule, and the basal turn of the cochlea can be visualized this way: the medial wall of the vestibule represents the starting point for access to the IAC, just as in the classic translabyrinthine approach. As stated above, the medial turn of the cochlea represents an important landmark for the intralabyrinthine facial nerve, since this segment of the facial nerve runs just superiorly to the cochlea between the geniculate ganglion superiorly and anteriorly, and the intrameatal facial nerve inferiorly and posteriorly in all subjects. Although the intralabyrinthine facial nerve was covered by a compact bony wall, it can be exposed and visualized by drilling between the geniculate ganglion and the cochlea anteriorly, and the medial wall of the vestibule, and the IAC posteriorly. The dissection of this component of the facial nerve is probably the most difficult point in the procedure, since it is well known that the facial nerve has its smallest diameter at this point, and actually in few cases it was damaged during drilling. However, in the majority of cases, we were able to dissect the entire tympanic and labyrinthine segment of the facial nerve.
Finally, after enlarging the IAC opening, starting from the spherical recess, the position and relationship of the acoustic-facial bundle could be studied.
To our knowledge, this is the first description of the surgical anatomy of the temporal bone from a viewpoint that is not just ‘endoscopic’, but is also entirely ‘from lateral to medial’, and completely direct from EAC to IAC. The main principle of middle ear endoscopic approaches is to avoid external incisions, soft tissue dissection, and to operate only transcanally, and we maintained these principles when we approached the middle ear, i.e. medially toward the internal ear structures. Although improvements and refinements must be made, the importance of the knowledge of such anatomy is that it may represent a basis for the clinical application of this kind of approach. Actually, although the present paper is focused on cadaveric dissections, the present authors have already performed some of the steps described herein in living patients, and some of the concepts described can be used for approaches to temporal bone pathologies, such as cholesteatomas and cholesterol granulomas, without reaching the IAC. So we believe that these preliminary experiences may possibly be applied more extensively in future to surgery of the lateral skull base, with some consequential benefits on temporal bone tissue sparing and improved visualization of the temporal bone structures.
It is worth to underline that in authors’ opinion, microscopic based approaches are still at present the gold standard for lateral skull base procedures, and clinical indication for exclusive endoscopic approaches are very rare. Anyway more frequently, combined techniques could be already applied, which will be based on microscopic dissection, with the aid of endoscope for hidden area visualization.
The debate about natural orifice transluminal surgery (NOTES) is nowadays real in every sector of the surgery [14], and we should also consider the EETA as NOTES. Considering the possible future indications of the EETA to the IAC, we may consider AN surgery when there will not be attempts at hearing preservation, or in general the removal of all kinds of pathology involving the IAC. In future, development of instruments and techniques (e.g. robotic surgery) may allow an extended EETA, with the purpose to explore and operate on the CPA by this approach, although facial nerve preservation and post-operative function will be crucial issues for the validation of the technique in an extended clinical application. CSF leakage post-operatively could be managed in the same way as in a translabyrinthine approach, although a sectorial and limited tissue removal should at least theoretically reduce this kind of complication.
Conclusions
The carotid artery, jugular bulb, bony labyrinth, labyrinthine tract of the facial nerve and the IAC can be thoroughly visualized in the cadaver using an EETA, avoiding mastoidectomy, extensive temporal bone tissue removal and external incisions. Although microscopic procedures must be considered at present, still the gold standard for lateral skull base surgery This may possibly have clinical significance in future, for example, in AN surgery or in the treatment of every pathology involving the IAC, although clinically based reports will be needed to strengthen our preliminary results.
References
Bennett M, Haynes DS (2007) Surgical approaches and complications in the removal of vestibular schwannomas. Otolaryngol Clin North Am 40(3):589–609
Marchioni D, Alicandri-Ciufelli M, Piccinini A, Genovese E, Presutti L (2010) Inferior retrotympanum revisited: an endoscopic anatomic study. Laryngoscope 120(9):1880–1886
Tarabichi M (2004) Endoscopic management of limited attic cholesteatoma. Laryngoscope 114(7):1157–1162
Marchioni D, Grammatica A, Alicandri-Ciufelli M, Aggazzotti-Cavazza E, Genovese E, Presutti L (2011) The contribution of selective dysventilation to attical middle ear pathology. Med Hypotheses 77(1):116–120
Marchioni D, Alicandri-Ciufelli M, Molteni G, Artioli FL, Genovese E, Presutti L (2010) Selective epitympanic dysventilation syndrome. Laryngoscope 120(5):1028–1033
Alicandri-Ciufelli M, Marchioni D, Grammatica A, et al. Tympanoplasty: an up-to-date pictorial review. J Neuroradiol. 2011. (Epub ahead of print)
Marchioni D, Alicandri-Ciufelli M, Piccinini A et al (2011) Surgical anatomy of transcanal endoscopic approach to the tympanic facial nerve. Laryngoscope 121(7):1565–1573
Marchioni D, Villari D, Alicandri-Ciufelli M, Piccinini A, Presutti L (2011) Endoscopic open technique in patients with middle ear cholesteatoma. Eur Arch Otorhinolaryngol 268(11):1557–1563
Marchioni D, Alicandri-Ciufelli M, Molteni G, Genovese E, Presutti L (2010) Endoscopic tympanoplasty in patients with attic retraction pockets. Laryngoscope 120(9):1847–1855
Staecker H, Nadol JB Jr, Ojeman R, Ronner S, McKenna MJ (2000) Hearing preservation in acoustic neuroma surgery: middle fossa versus retrosigmoid approach. Am J Otol 21(3):399–404
Day JD, Chen DA, Arriaga M (2004) Translabyrinthine approach for acoustic neuroma. Neurosurgery 54(2):391–395
Magnan J, Chays A, Lepetre C, Pencroffi E, Locatelli P (1994) Surgical perspectives of endoscopy of the cerebellopontine angle. Am J Otol 15(3):366–370
Presutti L, Marchioni D, Mattioli F, Villari D, Alicandri-Ciufelli M (2008) Endoscopic management of acquired cholesteatoma: our experience. J Otolaryngol Head Neck Surg 37(4):481–487
Berney CR (2011) Natural orifice transluminal endoscopic surgery: Where should we draw the line? Ann Surg (Epub ahead of print)
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marchioni, D., Alicandri-Ciufelli, M., Mattioli, F. et al. From external to internal auditory canal: surgical anatomy by an exclusive endoscopic approach. Eur Arch Otorhinolaryngol 270, 1267–1275 (2013). https://doi.org/10.1007/s00405-012-2137-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-012-2137-x