Abstract
Unresectable head and neck squamous cell carcinoma (HNSCC), non-metastatic, comprises a heterogeneous group of patients (pts), formed of stage III and IV pts. Since the available literature had not distinguished among these two groups, we prospectively addressed whether the recommended regimen involving cisplatin 100 mg/m2 concurrent to conventionally delivered radiotherapy (RT) is feasible in stage IV pts, based on the efficacy and safety of this regimen. A total of 30 pts were enrolled onto this study. Chemoradiation (CRT) consisted of RT 70 Gy, delivered in 35 daily fractions of 2 Gy, in 7 weeks, concurrent to cisplatin 100 mg/m2 on days 1, 22 and 43. Supportive treatment was provided as needed. Twenty-eight pts had tumors staged as T4 and 20 had N2 or N3 cervical involvement. The most common primary sites were the oral cavity and the oropharynx (23 pts). We observed six complete responses and 12 partial responses, with an overall response rate of 60%. A high rate of treatment-related toxicities was observed, with three deaths during CRT, and 26 pts suffering from one or more grade 3/4 toxicities, mainly dysphagia, mucositis, dermatitis, vomiting, infection or anemia. A prolonged treatment time was observed (63 days), as a result of unplanned treatment breaks. The lack of requirement of red blood cell transfusion was favorably related to the response to the treatment (93% vs. 50%, P = 0.033). For the whole population, with a median follow-up of 20.8 months, the median progression-free survival (PFS) was 8.0 months, and the median overall survival (OS) was 17.3 months. Longer median PFS and OS were seen in responding pts (12.8 vs. 4.1 months, P = 0.0001; and not reached (NR) vs. 10.4 months, P = 0.0037, respectively), as well as in those pts not requiring red blood cell transfusion (12.8 vs. 3.9 months, P = 0.0162; and NR vs. 10.4 months, P = 0.0176, respectively). In conclusion, this concurrent CRT regimen is hardly delivered in stage IV, unresectable, locally advanced HNSCC pts, due to treatment-related toxicities and longer RT duration. As a subset of pts may benefit from this regimen, adequate patient selection and aggressive supportive measures are essential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unresectable head and neck squamous cell carcinoma (HNSCC), non-metastatic, comprises a heterogenous group of patients, formed of stage III and IV patients. This is a very aggressive disease, with around 30–35% of patients being expected to achieve long-term progression-free survival (PFS) nowadays [1–4]. This scenario is probably worse in countries like Brazil, taking into account surveys indicating that more than 50% of newly diagnosed oral HNSCC patients present with stage IV disease, in the state of São Paulo [5].
For those patients with unresectable tumors, the Meta Analysis of Chemotherapy on Head and Neck Cancer (MACH-NC) Collaborative Group indicates that the standard regimen should include a platinum derivative, concurrent to conventionally delivered radiotherapy (RT). The recently updated results of the MACH-NC, including 50 studies, show that concurrent chemoradiation (CRT) offers a 8% gain in overall survival (OS) at 5 years, with a 19% reduction in death risk as compared to exclusive RT [hazard ratio (HR) 0.81, P < 0.0001]. The magnitude of the benefit was higher for platinum-based than for other chemotherapy regimens (HR 0.75 vs. 0.86, P < 0.01) [6]. The most used regimen for unresectable HNSCC is high-dose cisplatin (100 mg/m2 every 3 weeks) concurrent to standard RT, the only one validated in phase III trials [1, 7]. However, there are many conundrums concerning this recommendation.
To begin with, the definition of tumor resectability may vary among different authors. Indeed, there is a great variability related to the proportion of patients staged as III or IV and also related to the different chemotherapeutic agents, dosing and RT schemes [8–13]. Above all, the high toxicity associated to CRT is a major concern.
For these reasons, we aimed to prospectively address if cisplatin 100 mg/m2 every 3 weeks, concurrent to conventionally delivered RT is feasible in patients with stage IV, unresectable HNSCC, treated in our institution, based on the efficacy and safety of this regimen. Considering the very small magnitude of the survival gain with this regimen, 8% gain in OS at 5 years, it is crucial to define the tolerability and feasibility of this regimen in this subgroup of patients, separately.
Patients and methods
Thirty patients were consecutively included in this study, between March 2003 and August 2004 and followed-up until August 28, 2006. Eligibility requirements included the presence of histologically proven SCC of the oral cavity, oropharynx, larynx or hypopharynx; no prior treatment; stage IV disease according to the AJCC staging system [3]. Eligible patients should not be candidates for surgical resection as defined by the attendant head and neck surgeon, based on the impossibility of achieving margin-free resection without unacceptable cosmetic and/or functional results thereafter. In addition, patients were required to have measurable disease according to the RECIST criteria [14]; age between 18–70 years; ECOG performance status 0, 1 or 2; life expectancy superior to 3 months; and adequate hepatic, renal and bone marrow function. Main exclusion criteria were: distant metastasis, serious medical conditions, current or past history of malignant neoplasms, and pregnancy. All the management was provided by a multidisciplinary team. Pretreatment evaluation included a complete medical history, physical examination, blood tests, 24-h urinary creatinine clearance, chest X-ray, computerized tomography (CT) scans of the tumor site and the neck, and ear, nose and throat (ENT) and upper digestive endoscopies.
The original accrual goal was 67 patients, based on the Simon two-stage optimal design, with 17 responses expected after 27 patients included in the first stage. This was identified as necessary to detect a 15% difference in response rate as compared to the expected 60% response rate with RT alone, with alpha and beta probabilities of 5% and 20%, respectively. After the inclusion of first 30 patients, we conducted a scheduled interim analysis, and decided to close the accrual, taking into account the toxicity—there occurred three toxic deaths during treatment and another patient died in the first 30 days after treatment. All patients had given their written informed consent, prior to any study procedures. The study was conducted, after approval of the institutional ethics committee, in a single institution (Hospital das Clínicas da Faculdade de Medicina da USP, south-eastern Brazil), in accordance with the applicable guidelines of Good Clinical Practices and also with the Brazilian law.
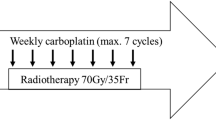
The treatment consisted of isocentrically delivered RT to the primary tumor and regional lymphatic nodes, with a megavoltage source, either cobalt-60 (17 patients) or 6-MV linear accelerator (13 patients), total 70 Gy in daily 2 Gy fractions. The targeted volume included the gross primary tumor and involved nodes with a margin of 1.0 cm at least, as well as the neck and supraclavicular fossae, using conventional technique. A reduction in the field was made at 50 Gy, and shielding of spinal cord at 44 Gy. Gross disease in the posterior cervical region was then boosted to the total dose of 70 Gy, with electron-beam of appropriate energy. Opposed lateral fields were used in the cervicofacial region, and a direct field was used for the supraclavicular fossae. All fields were to be treated every treatment day, 5 days a week, for 7 weeks, without any planned interruption. Cisplatin was administered concurrently on days 1, 22 and 43 of the RT as one-hour intravenous infusion of 100 mg/m2. All patients received vigorous hydration and anti-emetic therapy before cisplatin. Dose modifications for cisplatin were done in accordance with the levels of leucopenia, thrombocytopenia or nephrotoxicity. No chemotherapy was administered if any grade 3–4 toxicity had been detected.
The primary outcome measure was the response rate, defined as the proportion of patients with complete (CR) or partial (PR) response according to RECIST criteria [14], in the intent to treat (ITT) population. Tumor response was assessed 4 weeks after treatment completion, by means of CT scans, and these results were confirmed 4 weeks later. Other primary outcome measures were PFS and OS. PFS was defined as the time between the enrollment, (i.e., the date of the signed informed consent) and the date of disease progression or death, whichever came first. Patients who were alive and had not experienced disease progression, or were lost to follow-up, were censored for PFS at the date they were last known to be alive and progression-free. OS was calculated from the enrollment to the most recent follow-up or death. Secondary outcomes included toxicity assessment, changes in ECOG performance status, and symptomatic changes.
After the end of treatment, follow-up visits were scheduled every 3 months, and CT scans and ENT endoscopies were repeated every 6 months, or according to the clinician’s judgment. Chest X-rays were performed once a year. Pain control was evaluated at the enrollment and at the first visit after the end of the treatment, according to a numerical rating scale from 0 (no pain) to 10 (the worst possible pain) [15]. During the treatment, patients were evaluated on a weekly basis, and promptly diagnosed and treated for acute treatment-induced toxicities. Adverse events were recorded at each visit, weekly, and just before chemotherapy administration, in accordance with the Common Toxicity Criteria of the National Cancer Institute, version 2.0. Supportive measures included aggressive analgesia, hydration and antibiotics, whenever indicated. Patients presenting with grade 3 or 4 mucositis, pharyngeal dysphagia or impaired swallowing, were managed with the placement of a nasogastric tube, in order to ensure proper caloric supplementation and hydration. In case of grade 3 or 4 mucositis, RT was interrupted until recovery to grade 2.
Survival and safety analysis were conducted on the ITT population. Actuarial PFS and OS were calculated from the date of enrollment according to the Kaplan–Meier method [16] and curves were compared using the log-rank method. Patient characteristics and responses were compared using the two-tailed Fisher’s exact test, t test or Wilcoxon test, whenever considered appropriate. Exact binomial 95% confidence intervals were calculated. All reported P values were two-tailed and were considered to be statistically significant for P < 0.05. Analyses were done with the SPSS statistical software version 10.0.
Results
The clinical characteristics of the 30 patients with unresectable HNSCC enrolled onto the study, which comprises the ITT population, are shown in Table 1. The median age was 53 years and the majority was male. All patients had stage IV, unresectable tumors; 28 (93%) had tumors staged as T4 and 20 patients (67%) had N2 or N3 cervical involvement. Twenty-five patients received the full, prescribed dose of RT and were considered assessable for response.
The median treatment time was 63 days (range 54–86 days). Eight patients had unplanned interruptions in RT, varying from one to 22 days (median 9.5 days). The reasons for unplanned treatment breaks were: mucositis grade 3 (three patients), dermatitis grade 3 or 4 (three patients), infection grade 3 (two patients), anemia grade 3 (two patients), vomiting grade 3 (one patient), dysphagia grade 3 (one patient), acute renal failure (one patient). In the first cycle of chemotherapy, full dosage of cisplatin was administered to all patients. In the second and third cycles, it was given to 27 (90%) and 15 patients (50%), respectively. The median number of administered chemotherapy cycles was 2.5. The mean dose intensity of cisplatin was 27.8 ± 8.3 mg/m2/week, which corresponds to a relative dose intensity of 64.9%. Overall, 15 patients (50%) received all the scheduled treatment (i.e., 70 Gy RT and cisplatin 300 mg/m2).
All patients presented at least one adverse event and 26 patients (87%) presented some grade 3 or 4 treatment-related toxicity (Table 2). Acute grade 3 or 4 nausea and vomiting, interpreted as secondary to the cisplatin therapy, occurred in a minority of patients (four and three patients, respectively). Most patients had at least one of the most prominent toxicities, dysphagia, mucositis or dermatitis, which were generally attributed to RT. As a consequence, 17 patients (57%) required the placement of a nasogastric tube, in addition to four patients who had it already placed before enrollment. A weight loss of at least 10% during the treatment occurred in eight patients. The mean weight loss was 9% (range 1–18%). Of the hematological toxicities, grade 3 or 4 anemia and lymphopenia were the most prominent and both occurred in seven patients. Fourteen patients (47%) needed red blood cell transfusion, in order to maintain hemoglobin level above 10 g/dl during the treatment. No episodes of neutropenic fever were observed. Of the 25 patients who received full dose of RT, 11 (44%) did not receive the full dosage of cisplatin due to elevation of serum creatinine (n = 5), infection (n = 1), mucositis (n = 1), low performance status (n = 1) or chemotherapy refusal (n = 3). There were three deaths before RT completion, considered here as toxic deaths, caused by sepsis (n = 2) and one patient presented sudden death (no autopsy was performed). One more patient died of the disease in the first month after CRT. Two patients were discontinued from protocol before RT completion, one was found to present pulmonary metastasis, just after the first dose of cisplatin, possibly present before enrollment. A second presented pneumonia with respiratory failure, not treatment-related.
Four weeks after the end of the treatment, 23 patients were evaluated for response. Six patients presented complete response (20%; 95% CI 8–39%) and 12 presented partial response (40%; 95% CI 23–59%), with an overall response rate of 60% (95% CI 41–77%) in the ITT population. One patient presented stable disease and four patients presented progressive locoregional disease. One patient with PR due to persistence of nodal disease was rendered disease-free after therapeutic cervical lymph node dissection. Patients who did not receive red blood cell transfusion during CRT presented higher response rate (93 vs. 50%, P = 0.033, Fisher’s exact test). No other clinical-pathological parameters (primary site; tumor grade; nodal staging; ECOG performance status; number of administered cycles of chemotherapy; anemia or neutropenia; body mass index at diagnosis; or the use of nasogastric tube during the treatment) influenced response.
In terms of pain control, a median rate of 5.5 (range 0–10) was found before treatment as compared to the median rate of zero found after treatment in the ITT population (P = 0.0001, Wilcoxon test).
At the median follow-up of 20.8 months (range 8–36 months), 12 patients were alive. The median PFS was 8.4 months (95% CI 6.4–10.4 months), with a projected 1-year PFS rate of 36%. The median OS was 17.3 months (95% CI 2.4–32.2 months), with a projected 1-year OS rate of 57% (Fig. 1). Patients who achieved complete or partial response presented longer median PFS in comparison to non-responders (12.8 vs. 4.1 months, P = 0.0001, HR 0.173, 95% CI 0.00–0.12). The same occurred in relation to OS (NR vs. 10.4 months, P = 0.004, HR 0.21, 95% CI 0.02–0.44). We also observed longer median PFS (12.8 vs. 3.9 months, P = 0.016, HR 0.41, 95% CI 0.14–0.82) and also longer median OS (NR vs. 10.4 months, P = 0.018, HR 0.32, 95% CI 0.10–0.80) among patients that needed no red blood cell transfusion. Median PFS was also longer in patients treated with three cycles of chemotherapy (versus less than three) (11.3 vs. 4.6 months, P = 0.047, HR 0.47, 95% CI 0.19–0.99), but no difference in OS was seen. There was no difference in terms of PFS and OS among the diverse primary sites, pre-treatment hemoglobin levels, or need of RT interruption.
Discussion
We have examined the efficacy and safety of high-dose cisplatin concurrent to RT in non-metastatic, stage IV HNSCC patients, very representative of our daily practice in southeastern Brazil. Although considered as standard for these patients, based on the available meta-analysis [6] and prospective studies [1], there is much heterogeneity in the actual dosing of cisplatin to be combined to RT in the community setting. There are at least two reasons for that. First, the magnitude of reduction in the death risk offered by CRT: 19% reduction compared to exclusive RT, with only 8% gain in OS at 5 years and, second, most studies included in the meta-analysis as well as the prospective trials enrolled a mix of stage III and IV patients. Based on this, many argue that smaller chemotherapy doses on a more frequent basis, such as cisplatin (20 mg/m2 per day, 5 days) or weekly doses of 40 mg/m2 during RT, or even different combinations of carboplatin and taxanes, may be considered in a routine basis [17].
In this work, we have chosen cisplatin 100 mg/m2 every 3 weeks, concurrent to standard RT, because this was the only schedule validated in phase III trials [1]. We have found six patients with CR in 30 patients (20%) and a median survival of 17.3 months, below the 40% and 19.1 months, respectively, described by the Intergroup phase III study using the same CRT regimen [1]. Not taking into consideration differences in cisplatin scheduling, dosage, the addition of other drugs to the chemotherapy regimen and the proportion of different subsites included in different studies, the PFS and OS in our study were considerably worse than the previously reported in phase II and III trials. Vokes et al. [18] have found a median OS of 43 months, in a population with 93% stage IV HNSCC, using cisplatin, infusional 5-fluorouracil and hydroxyurea concurrent to hyperfractionated RT in a phase II study. In the category of similar regimens, Merlano et al. [12] had found a 43% CR rate and a median OS of 17 months in 80 unresectable patients (59 stage IV), using a regimen containing cisplatin and 5-fluorouracil plus RT. Using the carboplatin/5-fluorouracil doublet, Denis et al. [9] have found a median OS of 20 months in a group of 109 advanced oropharyngeal HNSCC, 73 of them stage IV.
The inferior results we have had, in terms of CR rate and OS duration, as compared to the literature, can be partially attributed to the single agent we had used, instead of the classic high-dose cisplatin/5-fluorouracil combination. However, it is difficult to consider that we should have used a more aggressive regimen, since 87% of our patients presented some grade 3 or 4 toxicity. This was also clear in terms of the median duration of the CRT, 63 days in our study, which was far superior to 47 days observed by others, using a similar CRT regimen, in a population comprised of 96% stage IV patients [1]. Similarly, our proportion of patients receiving the entire CRT, 50% in our study, was also inferior to the 78% (74 out of 95 patients) found in that study. The same occurred with the number of toxic deaths (13%), also superior to the observed in that study (4%) [1]. Additional confounding issues are the definition of resectability, which is different among studies [19] and differences in the proportion of patients in each primary site, among the different trials. Our proportion of oral cavity tumors (43%) grossly differs from other trials: 37% in Merlano’s study [12] and 13.3% in Aldelstein et al. [1]. On the other hand, the median OS here observed (17.3 months) approached the results described in the literature for other platinum-based CRT regimens [1, 12, 13]. If we consider the high response rate observed among patients diagnosed with HNSCC treated with chemoradiation, we could speculate that OS may be a better outcome than response rate for future studies.
Another aspect to be considered as having a possible detrimental effect on OS was the prolonged RT duration (63 days) consequent to unplanned treatment breaks. Mucositis and other treatment-related toxicities determined the radiotherapy breaks. Suboptimal supportive care allied to poor nutritional status, co-morbidities and adverse social aspects such as low income and low educational level, commonly observed in patients diagnosed with HNSCC, were the probable underlying causes. This situation would probably reflect the problems with this CRT schedule in the community setting. An alternative to the conventional once-daily RT we have used here is to use alternative RT dose fractionation schemes, in order to reduce overall treatment time. In the RTOG 90-03 trial [20], conventional once-daily RT was compared to hyperfractionated RT and to the concomitant boost technique and has shown to have no impact in OS despite the improved local-regional control rates. In parallel, acute toxicity was increased with altered fractionation. Late toxicity was comparable. Since prolonged radiation time is thought to accelerate tumor repopulation [21], we should speculate that longer RT duration might have impacted on tumor local control, exerting a deleterious effect on OS.
Clearly, to spare patients from toxicity, we need to better identify those who would benefit from this treatment. Of note, anemia is an important prognostic factor in these patients [9, 22]. We observed better OS and PFS in patients that did not need red blood cell transfusion to maintain hemoglobin levels above 10 g/dl along the treatment. Better patient stratification using clinical parameters such as age, TNM staging, performance status and pretreatment anemia into well-designed prospective trials will certainly help to define the optimal management of these patients [17, 22, 23]. Indeed, in a near future, the integration of translational research can refine this prognostication. Some of candidate markers under evaluation are epidermal growth factor receptor expression [24], plasma osteopontin levels [25], and TP53 mutational status [26].
A clear benefit in terms of pain control was observed in this study, suggesting that treatment was helpful to control the disease-associated pain, and may also contribute for a better quality of life. It was not our objective to distinguish the effect of the treatment from that provided by other analgesic measures. An experienced multidisciplinary team, familiar to the treatment-associated toxicities, and the best possible supportive care measures are essential during chemoradiation. Intensity-modulated RT [27, 28], palifermin [29], and low energy level laser [30] are under prospective evaluation as supportive treatments.
We concluded that high-dose-cisplatin CRT is a regimen difficult to administer in stage IV, unresectable, locally advanced HNSCC pts, with delays in total treatment time and high incidence of treatment related toxicities. Since a subgroup clearly benefited from these regimen, we reinforce the importance of optimal patient selection, aggressive supportive measures and the prompt management of toxicities in the daily clinical practice. Further efforts are required to identify new therapeutic alternatives. At this moment, other options of treatment can also be considered for this population such as hyperfractionated RT [20, 31, 32], induction chemotherapy [33, 34], association of epidermal growth factor receptor expression-targeted antibodies (as cetuximab) or hypoxic-cell-killing agents (such as nimorazole and tirapazamine) to RT [35–37], and even RT alone [38, 39].
References
Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, Schuller DE, Forastiere AA (2003) An Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Cooper JS, Farnan NC, Asbell SO, Rotman M, Marcial V, Fu KK, McKenna WG, Emami B (1996) Recursive partitioning analysis of 2105 patients treated in Radiation Therapy Oncology Group studies of head and neck cancer. Cancer 77:1905–1911
Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M (2002) AJCC cancer staging handbook. 6th edn. Springer, New York
Pignon JP, Bourhis J, Domenge C, Designé L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet 355:949–955
Available at http://www.fosp.saude.sp.gov.br, accessed in June 23rd, 2006
Bourhis J, Amand C, Pignon JP (2004) Update of MACH-NC (Meta-analysis of chemotherapy in head and neck cancer) database focused on concomitant chemoradiotherapy. J Clin Oncol 22(Suppl 14):5505
Rosenthal DI, Ang KK (2004) Altered radiation therapy fractionation, chemoradiation, and patient selection for the treatment of head and neck squamous carcinoma. Semin Radiat Oncol 14:153–166
Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, George SL, Huang AT, Prosnitz LR (1998) Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 338:1798–1804
Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Calais G (2004) Final results of the 94–01 French head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 22:69–76
Huguenin P, Beer KT, Allal A, Rufibach K, Friedli C, Davis JB, Pestalozzi B, Schmid S, Thoni A, Ozsahin M, Bernier J, Topfer M, Kann R, Meier UR, Thum P, Bieri S, Notter M, Lombriser N, Glanzmann C (2004) Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol 22:4665–4673
Jeremic B, Shibamoto Y, Milicic B, Nikolic N, Dagovic A, Aleksandrovic J, Vaskovic Z, Tadic L (2000) Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 18:1458–1464
Merlano M, Benasso M, Corvò R, Rosso R, Vitale V, Blengio F, Numico G, Margarino G, Bonelli L, Santi L (1996) Five-year update of a randomized trial of alternating radiotherapy and chemotherapy compared with radiotherapy alone in treatment of unresectable squamous cell carcinoma of the head and neck. J Natl Cancer Inst 88:583–589
Wendt TG, Grabenbauer GG, Rödel CM, Thiel HJ, Aydin H, Rohloff R, Wustrow TPU, Iro H, Papella C, Schalhorn A (1998) Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol 16:1318–1324
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Suntharalingam M, Haas ML, Van Echo DA, Haddad R, Jacobs MC, Levy S, Gray WC, Ord RA, Conley BA (2001) Predictors of response and survival after concurrent chemotherapy and radiation for locally advanced squamous cell carcinomas of the head and neck. Cancer 91:548–554
Vokes EE, Kies MS, Haraf DJ, Stenson K, List M, Humerickhouse R, Dolan ME, Pelzer H, Sulzen L, Witt ME, Hsieh YC, Mittal BB, Weichselbaum RR (2000) Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer. J Clin Oncol 18:1652–1661
Eisbruch A, Normolle DP (2005) Testing new chemoradiation regimens for head-and-neck cancer. Int J Radiat Oncol Biol Phys 61:5–6
Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, Ang KK (2000) A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 48:7–16
Withers HR, Taylor JM, Maciejewski B (1988) The harzard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27:131–146
Prosnitz RG, Yao B, Farrell CL, Clough R, Brizel DM (2005) Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 61:1087–1095
Bourhis J, Le Maître A, Pignon J, Ang K, Bernier J, Overgaard J, Tobias J, Saunders M, Adelstein D, O’Sullivan B (2006) Impact of age on treatment effect in locally advanced head and neck cancer: two individual patient data meta-analyses. J Clin Oncol 24(Suppl 18):5501
Bentzen SM, Atasoy BM, Daley FM, Dische S, Richman PI, Saunders MI, Trott KR, Wilson GD (2005) Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol 23:5560–5567
Overgaard J, Eriksen JG, Nordsmark M, Alsner J, Horsman MR (2005) Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol 6:757–764
Eriksen JG, Alsner J, Steiniche T, Overgaard J (2005) The possible role of TP53 mutation status in the treatment of squamous cell carcinomas of the head and neck (HNSCC) with radiotherapy with different overall treatment times. Radiother Oncol 76:135–142
de Castro G Junior, Federico MH (2006) Evaluation, prevention and management of radiotherapy-induced xerostomia in head and neck cancer patients. Curr Opin Oncol 18:266–270
Garden AS, Morrison WH, Rosenthal DI, Chao KS, Ang KK (2004) Target coverage for head and neck cancers treated with IMRT: review of clinical experiences. Semin Radiat Oncol 14:103–109
Brizel DM, Le QT, Rosenthal D, Meredith R, Brizel HE, Heard R, Yao B, Eng T, Sailer S, Chen Y, Murphy B, Mendenhall W (2002) Phase 2 study of recombinant human keratinocyte growth factor (rHuKGF) in head and neck cancer treated with standard or hyperfractionated irradiation and concurrent chemotherapy. Int J Radiat Oncol Biol Phys 54(Suppl):285–286
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100(Suppl 9):2026–2046
Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maitre A, Pajak TF, Poulsen MG, O’Sullivan B, Dobrowsky W, Hliniak A, Skladowski K, Hay JH, Pinto LH, Fallai C, Fu KK, Sylvester R, Pignon JP (2006) Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 368:843–854
Horiot JC, Bontemps P, van den Bogaert W, Le Fur R, van den Weijngaert D, Bolla M, Bernier J, Lusinchi A, Stuschke M, Lopez-Torrecilla J, Begg AC, Pierart M, Collette L (1997) Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol 44:111–121
Remenar E, Van Herpen C, Germa Lluch J, Stewart S, Gorlia T, Degardin M, Bernier J, Spirlet C, Vermorken JB (2006) A randomized phase III multicenter trial of neoadjuvant docetaxel plus cisplatin and 5-fluorouracil (TPF) versus neoadjuvant PF in patients with locally advanced unresectable squamous cell carcinoma of the head and neck. Final analysis of EORTC 24971. J Clin Oncol 24(Suppl 18):5516
Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, Rizo A, Isla D, Vega ME, Marti JL, Lobo F, Pastor P, Valenti V, Belon J, Sanchez MA, Chaib C, Pallares C, Anton A, Cervantes A, Paz-Ares L, Cortes-Funes H (2005) Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 23:8636–8645
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, Lindelov B, Jorgensen K (1998) A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother Oncol 46:135–146
Rischin D, Peters L, Fisher R Macann A, Denham J, Poulsen M, Jackson M, Kenny L, Penniment M, Corry J, Lamb D, McClure B (2005) Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol 23:79–87
Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, Bentzen J, Bastholt L, Hansen O, Johansen J, Andersen L, Evensen JF (2003) Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 362:933–940
Overgaard J, Mohanti BK, Bhasker S, Begum N, Ali R, Agerwal J, Kuddu M, Baeza M, Vikram B, Grau C (2006) Accelerated versus conventional fractionated radiotherapy in squamous cell carcinoma of the head and neck. A randomized international multicenter trial with 908 patients conducted by the IAEA-ACC Study Group. Int J Radiat Oncol Biol Phys 66(Suppl):13
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Castro , G., Snitcovsky, I.M.L., Gebrim, E.M.M.S. et al. High-dose cisplatin concurrent to conventionally delivered radiotherapy is associated with unacceptable toxicity in unresectable, non-metastatic stage IV head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 264, 1475–1482 (2007). https://doi.org/10.1007/s00405-007-0395-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-007-0395-9