Abstract
Background
In treatment of locally advanced squamous cell carcinoma of the head and neck (SCCHN), the use of docetaxel, cisplatin, and 5-fluorouracil (TPF) followed by high-dose cisplatin chemoradiotherapy (CRT) carries concerns over toxicity. We evaluated the feasibility of TPF as induction chemotherapy (IC) to Japanese patients and the tolerability of CRT with fractionated administration of cisplatin after IC.
Methods
Patients with unresectable stage III, IV SCCHN received IC followed by CRT. IC consisted of three 3-week cycles of docetaxel 70–75 mg/m2 on day 1, cisplatin 70–75 mg/m2 on day 1, and 5-fluorouracil 750 mg/m2 on days 1–5. Patients subsequently received IMRT concomitant with fractionated administration of cisplatin (20 mg/m2) on days 1–4, repeated every 3 weeks. The primary endpoint was completion of the three cycles of IC.
Results
Forty-eight patients were enrolled. The IC treatment completion rate was 85%. Grade 3–4 toxicities of TPF were neutropenia (79%) and febrile neutropenia (15%). Thirty-eight patients (79%) achieved a response after IC. Forty patients subsequently underwent CRT. Thirty-three patients (83%) completed the planned cycles of fractionated administration of cisplatin, but seven (18%) did not. Grade 3–4 toxicities during CRT were neutropenia (23%), mucositis (53%), and dysphagia (33%). With a median follow-up of 36.1 months, 3-year overall survival was 65%.
Conclusion
TPF IC is feasible and CRT with fractionated administration of cisplatin after IC is tolerable. IC followed by CRT appears to be a useful and safe sequential treatment. (Trial registration no. UMIN000024686).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Standard treatment for stage III or IV unresectable locally advanced squamous cell carcinoma of the head and neck (SCCHN) is chemoradiotherapy (CRT) [1]. Several trials have shown that the addition of chemotherapy to radiation therapy (RT) improves local control and overall survival compared with RT alone [2, 3]. The standard drug used concurrently with RT is high-dose cisplatin (CDDP), which is associated with both acute and late toxicities [1].
Induction chemotherapy (IC) may improve the prognosis of locally advanced SCCHN [4]. Several studies have shown that IC consistently results in a higher response and exerts a pronounced effect on distant metastases [5, 6]. The combination of docetaxel, cisplatin, and 5-fluorouracil (TPF) is considered a standard IC regimen based on the results of several phase III trials [7,8,9,10]. Although some retrospective data for IC with TPF in Japanese populations are available, no prospective trial has evaluated the safety of standard dose of TPF in Japanese patients [11, 12].
No consensus exists yet regarding the optimal post-IC regimen. Although previous studies demonstrated survival benefit with IC followed by RT alone versus RT alone in unresectable locally advanced SCCHN, the role of IC versus CRT alone in unresectable disease remain controversial, due to insufficient patient accrual or complicate study design [13, 14]. Furthermore, IC increased toxicity and compromises the subsequent CRT in some studies [15, 16]. Based on these results high-dose cisplatin CRT has not been recommended after induction TPF [17]. The recommended cumulative dose of cisplatin reported is approximately 200 mg/m2 independent of administration schedule [18,19,20,21]. Therefore, we decided to select the fractionated administration of cisplatin at a dose of 20 mg/m2 on days 1–4, repeated every 3 weeks.
Here, we evaluated two features of the treatment of unresectable locally advanced SCCHN, namely the feasibility of TPF as IC to Japanese patients and the tolerability of CRT with fractionated administration of CDDP after IC.
Patients and methods
Eligibility
Main inclusion criteria were defined as follows: histologically proven SCCHN; stage III or IV unresectable locally advanced disease; primary lesion located in the oropharynx, hypopharynx, or larynx; no distant metastases documented by computed tomography (CT); age 20 to 75 years; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; and adequate hematologic and organ function, namely a white-cell count of at least 2000/m3, platelet count of at least 100,000/m3, and creatinine clearance of > 60 mL/min.
Patients were deemed unsuitable for radical surgery after evaluation of a multidisciplinary team. Inoperability criteria were technical reasons (tumor fixation/invasion to either cervical vertebra, skull base, carotid artery or fixed lymph nodes), risk of significant postoperative dysfunction (T4 oropharyngeal cancer) and low surgical curability (T3–4, N2–3 excluding T1N2).
The trial was conducted under a multi-institutional design at three institutions in Japan: National Cancer Center Hospital East, The Jikei University School of Medicine, and Hyogo Cancer Center. The study protocol was approved by the National Cancer Center Hospital Protocol Review Committee and the institutional review board of each participating institution, and carried out in accordance with the Declaration of Helsinki. This trial was registered at the UMIN Clinical Trials Registry as UMIN000024686. All patients gave written informed consent before study entry in accordance with institutional guidelines.
Treatment plan: IC
The TPF regimen consisted of docetaxel at a dose of 70–75 mg/m2, administered as a 1-h infusion on day 1, followed by cisplatin at a dose of 70–75 mg/m2, administered as a 1-h infusion on day 1, and fluorouracil at a dose of 750 mg/m2/day, administered by continuous infusion on days 1–5. A range of 70–75 mg/m2 for the docetaxel and cisplatin doses was used, because their maximum allowable doses under Japanese health insurance regulations were changed in 2010 from 70 to 75 mg/m2. Treatment was administered every 3 weeks for up to three cycles. Dose modifications were determined based on hematological and non-hematological toxicities. During chemotherapy, patients were monitored clinically and by laboratory testing. Patients received prophylactic antibiotics (ciprofloxacin, at a dose of 300 mg given orally twice daily, or an alternative agent) from days 5 to 15 of each cycle. Prophylactic Granulocyte colony-stimulating factor(G-CSF) was not used but therapeutic G-CSF was used only if a patient had febrile neutropenia or infection, a delay in recovery of the absolute neutrophil count. After three cycles, radiological evaluation was performed. Patients achieving a complete response (CR), partial response (PR) or stable disease (SD) after three cycles were then directed to undergo CRT.
CRT
CRT with cisplatin was started between 4 and 6 weeks after the three cycles of TPF. A cisplatin dose of 20 mg/m2/day was administered for 4 days concomitantly with the start of radiotherapy. Treatment cycles were repeated every 3 weeks during the whole course of radiotherapy (three infusions during conventional RT). Cisplatin dose modifications were determined based on hematological and non-hematological toxicity.
RT was delivered in accordance with institutional preferences. All patients were treated with intensity-modulated radiation therapy (IMRT). Using an IMRT system with the Simultaneous Integrated Boost (SIB) method, high-risk areas received 70 Gy/33 fr, intermediate risk areas received 60 Gy/33 fr and prophylactic irradiation areas received 54 Gy/33 fr. Following the recommendations of the International Commission on Radiation Units reports 50 and 62, the gross tumor volume (GTV) including the primary tumor and involved lymph nodes and CTV was determined. The definition of involved lymph nodes (GTV) was as follows: Cervical lymph nodes with the shortest axial diameter of ≥ 10 mm and retropharyngeal lymph nodes with the shortest axial diameter of ≥ 5 mm on CT or MRI were defined as malignant. Margins of 3–5 mm for treatment set-up and internal organ motion error were added to the CTV to determine the planning target volume (PTV). For planning organ at risk volume, a margin of 3 mm was added to the spinal cord. For the parotid glands, no margin was added in treatment planning.
Clinical evaluations
Pretreatment evaluation consisted of complete history and physical examinations, complete blood counts, liver function tests, chest X-rays, chest CT, and ECGs. All patients were imaged with CT and magnetic resonance imaging (MRI) scans of the head and neck. Bone scans were performed when clinically indicated. PET-CT was not used because it was not allowed under Japanese health insurance regulations. Treatment responses were assessed radiographically according to the RECIST 1.1 criteria after the three cycles of IC and the completion of CRT.
IC and CRT toxicities were described using the National Cancer Institute Common Toxicity Criteria (version 4.0).
Statistical analysis
The primary endpoint was completion of the three cycles of IC. Based on the data available in the literature this was predicted to be approximately 70–80% of patients [7, 8]. A sample size of 35 patients was needed to accept the hypothesis that the true completion rate was > 85% with 80% power and to reject the hypothesis that the completion rate was < 70% with 10% significance. Assuming 10% non-evaluable patients, a total of 38 patients were required. After 38 patients were enrolled, one more ten patient cohort was enrolled. Because the dose of drug which was allowed to use in Japan was changed in 2010, we tried to use increased drugs of TPF (docetaxel at a dose of 75 mg/m2, cisplatin at a dose of 75 mg/m2). Secondary endpoints were progression-free survival (PFS), overall survival (OS), response rate (including CR and PR), acute toxicities, and the completion rate of CRT after IC.
The follow-up time for each patient was calculated as the time from the start of treatment to 31 March 2015. A survival curve was generated using the Kaplan–Meier method. Safety and efficacy analyses were both conducted on an intention-to-treat basis, defined as all patients enrolled in the study who received at least one dose of chemotherapy. Statistical data were obtained using commercial software (SPSS 11.0 Inc., Chicago IL, USA).
Results
Patient population
From 2010 to 2014, 48 patients were enrolled. Patient clinical and disease characteristics are summarized in Table 1. The patients consisted of 43 males and five females with a median age of 61 years (range, 35–74 years). Most patients had stage IVa (70%) disease; approximately 30% had stage IVb. The primary tumor sites were larynx (3/48), oropharynx (25/48), and hypopharynx (20/48).
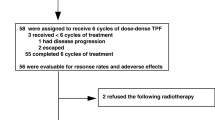
Treatment (Fig. 1)
Study diagram (n = 48). TPF, Docetaxel + Cisplatin + 5-FU; 70/70/750 = 70 mg/m2 doxetaxel/70 mg/m2 cisplatin/750 mg/m2 5-FU; 75/75/750 = 75 mg/m2 doxetaxel/75 mg/m2 cisplatin/750 mg/m2 5-FU; IMRT Intensity-modulated radiation therapy, RT radiation therapy, *All 40 patients received three cycles of TPF before CRT
A total of 41 patients (85%) completed the three cycles of planned IC (Table 2). This completion rate met the predefined criteria. Six patients received only one cycle of IC, of whom two developed grade 2 renal insufficiency, two could not abstain from alcohol, one had rapidly progressive disease, and one experienced drug allergy to 5-FU. One patient received two cycles of TPF because of grade 2 renal insufficiency. The median dose intensities relative to the target dose of docetaxel, cisplatin, and 5-FU were 93.3%, 87.1%, and 93.3%, respectively.
Among 48 patients receiving IC, 40 (83%) patients underwent CRT. Four patients received RT alone (one with lung abscess, two with renal insufficiency, one at drug allergy), and four patients received palliative care (two with progression of disease during IC, and two with alcoholism).
A total of 33 of the 40 patients (83%) completed the planned CRT with three cycles of fractionated cisplatin (Table 3). Seven patients received only two cycles of cisplatin due to fever (n = 1), poor clinical condition (n = 2), bone-marrow suppression (n = 1), physician’s judgment (n = 1), patient refusal (n = 1), and reason unknown (n = 1).
Adverse events
Acute toxicities experienced during TPF treatment and CRT are listed in Table 4. During TPF treatment, 38 patients (79%) experienced grade 3 or 4 neutropenia, 7 (15%) experienced grade 3 febrile neutropenia, and 7 (15%) experienced grade 3 anorexia. Toxicity was as expected and manageable.
During CRT, nine patients (23%) experienced grade 3 or 4 neutropenia, 21 (53%) experienced grade 3 mucositis, and 13 (33%) experienced grade 3 dysphagia.
Treatment outcomes
A total of 44 patients enrolled in the study were assessable for a response to TPF. Objective response rate (ORR) after IC was documented in 38 patients (79%), including 8 (17%) with complete response (CR) and 30 (63%) with partial response (PR) (Table 5).
After the completion of CRT, 40 patients were evaluable. ORR was documented in 37 patients (93%), including 27 with CR and 10 with PR (Table 5).
Also, among four patients who received RT alone, three patients achieved a CR and one patient achieved a PR.
The median follow-up time was 36.1 months (range, 1.5–62.5 months), and 3-year PFS and OS were 49% and 65%, respectively (Fig. 2). A total of 27 of the 48 patients were alive at the time of this report with no evidence of disease, while two patients were alive with disease.
Patterns of first treatment failure
Local recurrence developed in three patients. Median time to local recurrence was 14.4 months (range, 0.3–25.1 months). Of the three patients with local relapse, one is alive after salvage surgery, one is presently alive with disease and continues to receive chemotherapy, and one is alive with palliative therapy. Regional relapse developed in six patients, of whom one patient, who underwent elective neck dissection, is alive with no relapse. Local and regional failure developed in three patients. Finally, eight patients developed distant metastases. Median time to distant metastases was 12.3 months (range, 5.0–52.7 months). Six patients had metastases to lung, one to bone, one to a mediastinal lymph node, and one to brain.
Discussion
This is the first prospective study to evaluate the feasibility of TPF as IC to Japanese patients and that tolerability of CRT with fractionated CDDP after IC for the treatment of locally advanced SCCHN. Our study shows that IC with TPF is feasible to Japanese patients and fractionated administration of high-dose CDDP after three cycles TPF is tolerable.
Many trials of IC have been conducted, and the toxicity of TPF alone has been confirmed to be manageable. TPF is a standard IC regimen around the world and many institutions use it in accordance with past reports [7, 8]. On the other hand, reduced dose regimens have been used clinically due to safety or tolerability concerns [22, 23]. Because the effect of lower doses of TPF has not been studied, however, we consider that current regimens should be employed. Here, we studied the feasibility of three cycles of IC TPF, which is covered by the national health insurance program.
Although about half of the patients required dose reduction, many patients (85%) received the three planned cycles of TPF, and dose intensity was generally maintained at 90%. Our results are consistent with previous studies, which reported completion rates of 75.7–90.0% [8, 16, 24].
With regard to efficacy, overall response rate (ORR) to IC was 79% (CR: 17%, PR: 63%), again consistent with previous studies (67.8–80.1%) [8, 16, 24].
The main adverse events with this IC were hematological toxicity, febrile neutropenia, and anorexia. As expected, grade 3/4 leukopenia and neutropenia were seen with high frequency (leukopenia: 50%, neutropenia: 79%) but did not lead to frequent infectious complications, as the patients were on prophylactic antibiotics. In our study, febrile neutropenia occurred in 15%, which is again comparable to other reports (5.2–17%) [8, 16, 24]. Three patients (6%) stopped IC because of renal insufficiency, but most patients did not experience prolonged severe adverse events.
For CRT, the standard drug concurrent with RT is cisplatin. A meta-analysis revealed that platinum regimens might provide a survival advantage compared with non-platinum regimens [2, 3]. Currently, the standard regimen is 100 mg/m2 cisplatin every 3 weeks.
However, the reported rate of high-grade adverse events with this regimen has ranged from 77 to 85% for high-dose cisplatin. Common adverse events with CRT include mucositis, dysphagia, and anorexia. Additionally, renal toxicity from high-dose cisplatin is a relatively common but critical event, with an incidence of 5–8% in previous trials. The completion rate of this high-dose cisplatin regimen has been reported as 70–85% of patients in the CRT arm in several clinical trials [1, 25, 26].
Although high-dose cisplatin concurrent with RT after IC has not been recommended due to residual toxicity from IC, this study indicates that it is tolerable to use cisplatin concurrently with RT by changing the method of cisplatin administration.
Previous studies that used intensive induction strategies followed by radiotherapy alone reported significant numbers of loco-regional failure but few distant metastases [8, 24]. In contrast, studies of CRT showed that this treatment was associated with lower rates of loco-regional failure than radiotherapy alone but with a minimal effect on the rate of distant metastases [1, 25]. To improve these results, CRT with modified cisplatin after IC appears to be the treatment of choice.
Ang et al. [18]suggested that a cumulative cisplatin dose of approximately 200 mg/m2 would be the threshold for patients to yield a beneficial antitumor effect from CRT regardless of schedule [19]. A recent systematic review concluded that the recommended cumulative dose of cisplatin that should be administered during radiotherapy appears to be approximately > 200 mg/m2 [20, 21]. Based on these findings, the present study used a maximum cumulative dose of 240 mg/m2 as the target dose.
With regard to CRT after IC, the question here is whether patients tolerate an adequate amount of CDDP during CRT. Previous studies have reported that three cycles of concurrent cisplatin after TPF IC are infrequently administered, with rates reported as only 22–47% [16, 27]. On the other hand, in our study, the treatment completion rate was 83%, and 68% of patients received the minimum cumulative dose of cisplatin of approximately ≥ 200 mg/m2. We confirmed the good trend in response rate after CRT in the cohort of cumulative dose of cisplatin approximately ≥ 200 mg/m2 than lower (ORR: 96% vs 85%, respectively).
Although another concern is increased adverse events during CRT after IC, their incidence was not greater than that with CRT alone. The predominant adverse events were neutropenia (23%), mucositis (53%), and dysphagia (33%). Our results are consistent with a previous study, which reported neutropenia (35%) and mucositis (45%) [28].
Several limitations of this study should be noted. First, use of our study to assess the feasibility of a standard dose of TPF would be premature. Although the standard TPF regimen consists of docetaxel at a dose of 75 mg/m2, cisplatin at a dose of 75 mg/m2, and fluorouracil at a dose of 750 mg/m2/day, we used a 70-mg/m2 cisplatin regimen initially because the dose was restricted by insurance regulations at the time the study was started [7, 8, 24]. During the course of the study, approval of the higher dose of drug was received, allowing us to increase the dose to 75 mg/m2, hence the range of 70–75 mg/m2. Although the additional cohort of patients is small sample size (10 patients) and exploratory, similar trends were seen on feasibility of IC and tolerability of CRT after IC between initial TPF (70/70/750) and standard TPF (75/75/750). Both treatment completion rate of IC (84%, 90%, respectively) and CRT after IC (81%, 89%, respectively) were good. Second, the higher proportion of patients with oropharyngeal cancer may have affected the response [29]. Although about half of the patients had oropharyngeal cancer, we did not collect their HPV status because the primary objective of the current study was to assess the feasibility of induction TPF chemotherapy followed by CRT, not to assess its efficacy and the assessment of HPV infection was not allowed under Japanese health insurance regulations at that time.
The results showed that three cycles of TPF IC is feasible with acceptable toxicities to Japanese patients. CRT with fractionated administration of CDDP after IC is tolerable with acceptable toxicities. On the basis of these findings, TPF followed by CRT with fractionated administration of CDDP appears to be a useful and safe sequential treatment in selected patients. To make an accurate assessment of IC followed by CRT requires further investigation.
References
Adelstein DJ, Li Y, Adams GL et al (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21(1):92–98
Pignon JP, le Maitre A, Maillard E et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92(1):4–14
Pignon JP, Bourhis J, Domenge C et al (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet 355(9208):949–955
Ghi MG, Paccagnella A, Ferrari D et al (2017) Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol 28(9):2206–2212
Ma J, Liu Y, Huang XL et al (2012) Induction chemotherapy decreases the rate of distant metastasis in patients with head and neck squamous cell carcinoma but does not improve survival or locoregional control: a meta-analysis. Oral Oncol 48(11):1076–1084
Mak MP, Glisson BS (2014) Is there still a role for induction chemotherapy in locally advanced head and neck cancer? Curr Opin Oncol 26(3):247–251
Posner MR, Hershock DM, Blajman CR et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357(17):1705–1715
Vermorken JB, Remenar E, van Herpen C et al (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357(17):1695–1704
Janoray G, Pointreau Y, Garaud P et al (2016) Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, +/- docetaxel for larynx preservation. J Natl Cancer Inst 108 (4)
Lorch JH, Goloubeva O, Haddad RI et al (2011) Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol 12(2):153–159
Takahashi K, Nakajima K, Murata T et al (2016) Sequential chemoradiotherapy for advanced head and neck cancer: a clinical study with 33 cases. Nihon Jibiinkoka Gakkai Kaiho 119(5):734–740
Izawa N, Onozawa Y, Hikosaka T et al (2015) Efficacy and feasibility of docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy for locally advanced head and neck squamous cell carcinoma classified as clinical nodal stage N2c, N3, or N2b with supraclavicular lymph node metastases. Int J Clin Oncol 20(3):455–462
Cohen EE, Karrison TG, Kocherginsky M et al (2014) Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 32(25):2735–2743
Haddad R, O’Neill A, Rabinowits G et al (2013) Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 14(3):257–264
Lefebvre JL, Pointreau Y, Rolland F et al (2013) Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol 31(7):853–859
Hitt R, Grau JJ, Lopez-Pousa A et al (2014) A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 25(1):216–225
National Comprehensive Cancer Network Head and Neck Cancers (2018) https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 25 Oct 2018
Ang KK (2004) Concurrent radiation chemotherapy for locally advanced head and neck carcinoma: are we addressing burning subjects? J Clin Oncol 22(23):4657–4659
Nguyen-Tan PF, Zhang Q, Ang KK et al (2014) Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the radiation therapy oncology group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol 32(34):3858–3866
Alterio D, Cossu Rocca M, Russell-Edu W et al (2017) Feasibility of concurrent chemoradiotherapy with high-dose cisplatin after induction TPF chemotherapy in head and neck cancer: a critical review of the literature and the experience of the European Institute of Oncology. Med Oncol 34(5):86
Strojan P, Vermorken JB, Beitler JJ et al (2016) Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: a systematic review. Head Neck 38(Suppl 1):E2151–E2158
Komatsu M, Tsukuda M, Matsuda H et al (2012) Comparison of concurrent chemoradiotherapy versus induction chemotherapy followed by radiation in patients with nasopharyngeal carcinoma. Anticancer Res 32(2):681–686
Komatsu M, Shiono O, Taguchi T et al (2014) Concurrent chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) in patients with locally advanced squamous cell carcinoma of the head and neck. Jpn J Clin Oncol 44(5):416–421
Pointreau Y, Garaud P, Chapet S et al (2009) Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 101(7):498–506
Forastiere AA, Goepfert H, Maor M et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349(22):2091–2098
Bourhis J, Sire C, Graff P et al (2012) Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99—02): an open-label phase 3 randomised trial. Lancet Oncol 13(2):145–153
Driessen CM, de Boer JP, Gelderblom H et al (2016) Induction chemotherapy with docetaxel/cisplatin/5-fluorouracil followed by randomization to two cisplatin-based concomitant chemoradiotherapy schedules in patients with locally advanced head and neck cancer (CONDOR study) (Dutch Head and Neck Society 08—01): a randomized phase II study. Eur J Cancer 52:77–84
Zenda S, Onozawa Y, Tahara M et al (2007) Feasibility study of single agent Cisplatin and concurrent radiotherapy in Japanese patients with squamous cell carcinoma of the head and neck: preliminary results. Jpn J Clin Oncol 37(10):725–729
Hama T, Tokumaru Y, Fujii M et al (2014) Prevalence of human papillomavirus in oropharyngeal cancer: a multicenter study in Japan. Oncology 87(3):173–182
Acknowledgements
We thank all the patients, clinicians, and support staff who participated in this study.
Funding
No funding was received from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MT received fees from Merck Serono during the study; and grants and personal fees from MSD, Bayer, Eisai, Pfizer, Astra Zeneca, Ono Pharmaceutical, and Bristol-Myers Squibb, personal fees from Otsuka, grants from Boehringer Ingelheim, Novartis and NanoCarrier outside the submitted work. The other authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Okano, S., Enokida, T., Onoe, T. et al. Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer. Int J Clin Oncol 24, 789–797 (2019). https://doi.org/10.1007/s10147-019-01418-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01418-w