Abstract
Purpose
This study aimed to compare the effect of gonadotropin-releasing hormone agonist (GnRHa) trigger alone versus dual trigger comprising GnRHa and low-dose human chorionic gonadotropin (hCG) on reproductive outcomes in patients with polycystic ovary syndrome (PCOS) who received the freeze-all strategy.
Methods
A total of 615 cycles were included in this retrospective cohort study. Propensity score matching (PSM) was performed to control potential confounding factors between GnRHa-trigger group (0.2 mg GnRHa) and dual-trigger group (0.2 mg GnRHa plus 1000/2000 IU hCG) in a 1:1 ratio. Multivariate logistic regression was applied to estimate the association between trigger methods and reproductive outcomes.
Results
After PSM, patients with dual trigger (n = 176) had more oocytes retrieved, mature oocytes, and 2PN embryos compared to that with GnRHa trigger alone. However, the oocytes maturation rate, normal fertilization rate, and frozen embryos between the two groups were not statistically different. The incidence of ovarian hyperstimulation syndrome (OHSS) (14.8% vs. 2.8%, P < 0.001) and moderate/severe OHSS (11.4% vs. 1.7%, P < 0.001) were significantly higher in dual-trigger group than in GnRHa-alone group. Logistic regression analysis showed the adjusted odds ratio of dual trigger was 5.971 (95% confidence interval 2.201–16.198, P < 0.001) for OHSS. The pregnancy and single neonatal outcomes were comparable between the two groups (P > 0.05).
Conclusion
For PCOS women with freeze-all strategy, GnRHa trigger alone decreased the risk of OHSS without damaging oocyte maturation and achieved satisfactory pregnancy outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are still some concerns that the use of GnRH agonist single trigger may affect the reproductive outcomes of patients with high ovarian response. This study demonstrated that for PCOS patients with freeze-all strategy, the use of a GnRH agonist trigger without hCG will not damage the embryo quality and pregnancy outcome of infertile women, but decrease their risk of OHSS. |
Introduction
According to Rotterdam criteria, 11.2% of Chinese women aged 12–44 are affected by Polycystic ovary syndrome (PCOS) [1]. For PCOS patients suffering from infertility, in vitro fertilization (IVF) and/or intracytoplasmic sperm injection (ICSI) play an important role in achieving pregnancy. However, PCOS women usually exhibit higher sensibility and hyperstimulation responses [2], with more antral follicle count (AFC) and higher Anti-Müllerian hormone (AMH) levels [3, 4]. Meanwhile, the diagnosis of PCOS, elevated AMH values, and a large number of antral follicles are the risk factors of ovarian hyperstimulation syndrome (OHSS) [5]. Therefore, PCOS patients are at high risk of OHSS during the IVF/ICSI procedure.
OHSS is an iatrogenic complication, which will increase medical expense and even endanger the lives of patients in severe cases. Gonadotropin-releasing hormone (GnRH) antagonist protocol is recommended as the primary protocol of controlled ovarian stimulation for PCOS women [6, 7] to reduce the risk of OHSS [8]. Moreover, selecting an appropriate trigger scheme is crucial to prevent the incidence of OHSS when inducing final oocyte maturation. The releasing of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) triggered by GnRH agonist (GnRHa) is more physiologic than human chorionic gonadotropin (hCG). Unfortunately, GnRHa was related to a lower clinical pregnancy rate and higher miscarriage rate among patients with fresh embryo transfer [9], which is resulted from the short duration and low peak concentration of LH after GnRHa trigger [10]. Therefore, to support luteal phase function and improve endometrial receptivity, scholars have proposed the dual-trigger strategy consisting of GnRHa and low-dose hCG [11, 12].
Nevertheless, retrospective studies have reported that dual triggers would increase the risk of OHSS in high responders [13,14,15,16]. Currently, frozen embryo transfer (FET) becomes a routine treatment for high ovarian responders, and the freeze-all strategy is widely applied to prevent the OHSS among PCOS patients [7, 17]. When patients are administered by the freeze-all strategy, there seems to be no need for additional low-dose hCG to improve luteal phase defects, as fresh embryo transfer is not required and a possible higher risk of OHSS. And previous studies have revealed that GnRHa can significantly reduce the risk of OHSS for patients with the GnRH antagonist regimen [18, 19]. On the contrary, a prospective cohort study found that supplementing low-dose hCG in a single GnRHa trigger for high responders would have more high-quality embryos, lower abortion rate, and no increased risk of OHSS during FET cycles [20]. Moreover, there are still reservations about the use of GnRHa trigger due to concerns that the oocyte maturity of a small part of patients may decline [21, 22]. Hence, the effects of GnRHa trigger alone or dual trigger on reproductive outcomes among high responders are controversial.
Therefore, this study aimed to compare the effects of GnRHa trigger with or without low-dose hCG on reproductive outcomes, including oocyte maturation, OHSS rate, and pregnancy and single neonatal outcomes for PCOS patients when undergoing the freeze-all strategy.
Materials and methods
Study design and participants
This retrospective cohort study was conducted between March 2016 and December 2021 in the Reproductive Center of Xinan Gynecology Hospital in Sichuan, China. Medical records of PCOS patients were reviewed for possible inclusion. The diagnosis of PCOS was based on the Rotterdam criteria [23]. In this study, PCOS women were administrated with GnRH antagonist protocol and triggered by GnRHa alone or in combination with low dose hCG during IVF/ICSI cycles. Only the first FET cycle after the IVF cycle was included in this study. As a result, each patient received only one cycle of evaluation. The inclusion criteria were described as follows: (1) aged ≤ 40 years; (2) with freeze-all strategy. Women with the following conditions were excluded from the study: (1) donated oocytes; (2) preimplantation genetic testing; (3) recurrent spontaneous abortion; (4) untreated hydrosalpinx; (5) endocrine disease (diabetes mellitus, thyroid dysfunction, Cushing syndrome, hyperprolactinemia); (6) endometriosis, uterine adhesion, endometrial polyps, or unicorn uterus; and (7) missing data.
Ovarian stimulation, IVF/ICSI procedure, and vitrification
All PCOS patients were administered by GnRH antagonist protocol. The initial dose of gonadotropin (Gn) was based on the patient’s age, body mass index (BMI), and ovarian reserve (100–300 IU). The injection of Gn (recombinant FSH, Gonalfin, Merck Serono, Switzerland) was started on the 2nd–4th days of the menstrual cycle. When the diameter of dominant follicles reached 12–14 mm or on the 5th–6th days of ovarian stimulation, the GnRH antagonist (Cetrotide acetate, Merck Serono, Germany) at 0.25 mg/d was injected subcutaneously until the trigger day. The trigger was performed for final oocyte maturation when at least three follicles were ≥ 17 mm in diameter. The following trigger patterns were based on the patients’ BMI and the risk of OHSS (E2 level on hCG day): (1) GnRHa trigger: 0.2 mg triptorelin (Ferring Pharmaceuticals, Switzerland) alone; (2) dual trigger: 0.2 mg triptorelin plus 1000 or 2000 IU hCG (Lizhu Pharmaceutical, China). After 34–36 h, oocytes were retrieved by transvaginal ultrasound. Conventional IVF or ICSI insemination was performed according to the results of semen analysis.
The presence of two pronuclei (2PN) and two polar bodies (2 PB) in an oocyte was considered normal fertilization. Moreover, the development of zygotes was evaluated daily in the cleavage medium (Cook, Dublin, Ireland). A top-quality cleavage embryo was confirmed by the Istanbul consensus [24], which includes the following three criteria: (1) 7–8 cells at 3 days after fertilization, (2) < 10% fragmentation, and (3) symmetric blastomeres. Scoring for blastocysts was based on the Gardner classification [25]. Blastocysts graded as “grade 1” (AA) were of top-quality according to the morphology and appearance of the embryos. All available embryos were cryopreserved by vitrification for subsequent frozen-thawed cycles.
Endometrial preparation and frozen embryo transfer
According to the clinical assessment, all patients in this study underwent hormone replacement cycles to prepare the endometrium. Oral estradiol valerate (6 mg/d, Abbott, Holland) was administered on the 2nd–4th days of the menstrual cycle for 10–12 days. Endometrium thickness was detected by transvaginal ultrasound. The dose of estradiol was increased appropriately if the endometrial thickness was < 7 mm. When the endometrial thickness was ≥ 7 mm, the injection of progesterone (60 mg/d, Zhejiang, Xianju) and oral progesterone (20 mg/d, Duphaston, Abbott, Netherland) were administered daily to support luteal function until 14 days after FET. Once the pregnancy was confirmed, luteal support would last until 10 weeks of gestation.
The embryos were warmed by rapid thawing method on the fourth and sixth day after progesterone supplementation and then cultured in medium (Cook Medicine) until transplantation. On the day of thawing, a maximum of two embryos was transferred under the guidance of ultrasound.
Outcome measures
The diagnosis of OHSS was based on the criteria proposed by Golan et al. [26]. Oocyte maturation rate was the percentage of mature COCs and MII oocytes in the total oocytes retrieved. The normal fertilization rate was the percentage of 2PN and 2 PB oocytes in inseminated oocytes. The gestational sac with fetal heartbeat observed by ultrasound 28 days after embryo transfer was considered a clinical pregnancy. Miscarriage referred to the loss of clinical pregnancy within 24 weeks of gestation. The live birth rate was calculated by dividing the total deliveries with live infants after 24 weeks of gestation by the total number of transferred cycles. Preterm birth referred to the delivery of a live baby before 37 weeks of gestation. Low birth weight was defined as the birth weight of newborns being less than 2500 g. And small for gestational age referred to babies’ birth weight below the 10th percentile for the specific gestational age according to the latest birthweight curve in China [27].
Statistical analysis
All the statistical analyses were performed using the SPSS version 25.0 and R software version 4.0.5. In this study, we applied propensity score matching (PSM) [28]. PCOS women with different trigger method were randomly matched in a 1:1 ratio without replacement. Based on the following baseline variables, the propensity score was calculated by binary logistic regression: female age, BMI, primary infertility, duration of infertility, previous IVF/ICSI cycles, basic concentrations of FSH and LH, antral follicle count (AFC), anti-Mullerian hormone (AMH), LH and E2 levels on hCG day, number of follicles ≥ 14 mm on hCG day, the total dose of Gn, duration of stimulation, and fertilization method. We used the nearest neighbor matching with a 0.02 caliper.
We used the Shapiro–Wilk test to assess the normality of the data. Continuous variables with normal distribution were expressed as mean ± standard deviation (SD) and compared by Student’s t-test or one-way analysis of variance, whereas the non-normal distribution continuous variables were presented as median (interquartile range, IQR) and compared by Mann–Whitney U test. Categorical variables were presented as frequency (percentage) and compared by Chi-square test or Fisher’s exact test, as appropriate. Binary logistic regression analyses were carried out to further control possible confounding factors. P-values (two tailed) < 0.05 were considered indicative of statistical significance.
Results
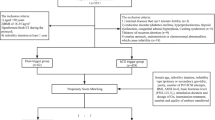
The flowchart of this study is shown in Fig. 1. The pretreatment characteristics and ovarian stimulation parameters of the two groups before and after PSM are listed in Table 1. Before matching, the BMI and dose of Gn used were significantly higher in dual-trigger group than in GnRHa-trigger group (24.4 ± 4.1 vs. 22.7 ± 3.3, P < 0.001; 1894 ± 757 vs. 1565 ± 491, P < 0.001). Women in dual-trigger group had less AFC (30.4 ± 8.4 vs. 31.9 ± 8.8, P = 0.023) and lower concentration of E2 (5795 ± 1818 vs. 7062 ± 1784, P < 0.001) compared to those in GnRHa-trigger group. And there were more patients in dual-trigger group who underwent previous IVF/ICSI cycles (5.9% vs. 1.7%, P = 0.004). After matching, the distribution of baseline and ovarian stimulation characteristics between the two groups was not statistically different.
Table 2 showed that compared with the GnRHa-trigger group, the dual-trigger group obtained more oocytes (22.1 ± 7.8 vs. 20.0 ± 6.9, P = 0.006), mature oocytes (19.2 ± 6.9 vs. 17.7 ± 6.3, P = 0.031), and 2PN embryos (15.1 ± 6.0 vs. 13.6 ± 5.4, P = 0.010). However, the oocytes maturation rate, and normal fertilization rate between the two groups were not significantly different. We also compared the embryology outcomes between the groups with different hCG dosages in Supplement Table 1. It was shown that the number of oocytes obtained and 2PN embryos were significantly different. But the oocyte maturation rate was comparable in the three groups.
Table 3 showed that dual-trigger method resulted in an increased risk of OHSS (14.8% vs. 2.8%, P < 0.001) and moderate/severe OHSS (11.4% vs. 1.7%, P = 0.001). When breaking down the dose of hCG (Supplement Table 2), the OHSS rate of patients with 1000(14.3%) or 2000(14.9%)IU hCG was still significantly higher than that patients without additional hCG.
Comparisons of the pregnancy outcomes after the first FET of the two groups are presented in Table 3. And there were no statistical differences between women who received additional hCG and those who did not (P > 0.05).
In this study, as shown in Table 4, the single newborn outcomes were not significantly different between the groups with or without hCG used.
Multivariate logistic regression analyses on pregnancy and singleton outcomes (Table 5), which adjusted the dosage of hCG on trigger day and the number of embryos transferred, demonstrated that the dual trigger increased the OHSS risk(OR = 5.971, 95% CI: 2.201–16.198, P < 0.001). But the trigger method did not significantly affect pregnancy and single newborn outcomes.
Discussion
Currently, there are still no conclusive results on whether GnRHa trigger can replace dual trigger to achieve sufficient oocyte maturation and prevent the occurrence of OHSS in patients with high ovarian response. The results of our study suggested that dual trigger with low-dose hCG was still related to a higher risk of OHSS. However, the oocyte maturation rate, normal fertilization rate, and pregnancy and singleton outcomes were comparable between the two groups, which may encourage the use of GnRHa trigger alone in PCOS women during IVF/ICSI cycles.
OHSS is characterized by multi-follicular development, enlarged ovaries, hemoconcentration, and increased vascular permeability [29]. Complications of severe cases are accompanied by effusion in serous cavity, renal insufficiency, and vascular embolism and are even life-threatening. Several strategies have been adopted to reduce OHSS risk. In addition to using GnRH antagonists protocol, the GnRHa trigger is also an effective method at present [30]. Compared to the extended half-life of hCG, the smaller peak amplitude and shorter duration of LH after GnRHa triggering may be conducive to avoiding the OHSS [5, 10]. Engmann et al. [18] suggested that combining GnRHa trigger and GnRH antagonists protocol can trigger oocyte maturation and effectively reduce the risk of OHSS in women with PCOS and other high responders. In addition, a randomized control trial and a prospective cohort study have shown that for PCOS patients with FET, the GnRHa was superior to hCG in eliminating OHSS risk [31, 32]. However, compared to GnRHa trigger, dual trigger was related to the risk of severe OHSS (6.0% vs. 0%) [13]. A recent study by He et al. [14] also found that adding low dose of hCG (1000/2000 IU) still increased the risk of OHSS among patients who adopted freeze-all strategy. The results of our study are consistent with the above studies’ conclusions that GnRH trigger alone can reduce the incidence of OHSS for hyper-responders. Therefore, for PCOS patients who do not need fresh embryo transfer, GnRHa single trigger seems to be desirable.
However, several groups supported the co-administration of low dose hCG in patients with high response, which conflicts with our results. One study used GnRHa with the administration of low dose hCG (1000 IU) among high responders with peak E2 < 4000 pg/ml and reported that supplementation with low-dose hCG improved the rate of pregnancy and live birth of fresh IVF cycles without increasing the risk of OHSS [33]. Yet the dual-trigger group occurred with one case of mild OHSS when no cases of OHSS in the GnRHa-alone group. And the peak estradiol levels in these patients were lower than in other highly responsive patients. At the same time, the E2 levels of those patients were also much lower than those of the patients in our study. Another group declared that 1000 IU hCG supplementation in high ovarian responders with FET would decrease the abortion rate and not increase the OHSS risk [20]. However, a significant difference is that the AFC of patients in their study is significantly lower than that ours. Shapiro et al. [34] also showed that dual trigger significantly increased the ongoing pregnancy rate while the prevalence of OHSS was similar to GnRHa alone in fresh autologous transfer cycles. Although the additional hCG could improve the outcomes of fresh embryo transfer cycles, it is still unclear whether the dual trigger benefits the pregnancy outcome for patients with FET. More importantly, it should be noted that since there is no unified guideline for high ovarian response, previous studies have different definitions for it, including the female age, number of oocytes retrieval, serum E2 concentrations on hCG day, and follicles development. The different definitions of high ovarian response may be one of the reasons for the inconsistent conclusions. Moreover, the differences in hCG dose, sample size, and the heterogeneity of the infertile population may also contribute to the inconsistency of their results.
Clinicians are concerned that some patients may have poor pregnancy outcomes because the low LH concentrations in these patients after GRHa trigger cannot induce sufficient oocyte maturation [21]. A modest increase of oocytes both in number and maturity after dual trigger has been reported by a retrospective cohort study [13], but the clinical pregnancy rate and miscarriage rate were not significantly different. In addition, the groups supplemented with hCG (1000, 2000, 3000 IU) obtained more oocytes and top-quality embryos than the group of single GnRHa trigger [20]. However, Jones et al. [35] revealed that the maturity of oocytes in normal and high response donors was not improved by dual trigger. Another study also suggested that oocyte maturation and embryo quality in groups supplemented with 1000 and 2000 IU hCG were not different compared with the GnRHa-trigger group [14]. For euploid embryos, Makhijani et al. [36] found that the final oocyte maturation triggered by GnRHa alone had no significant effect on the reproductive potential of euploid blastocysts. And whether triggered by hCG or GnRHa in the FET cycle, there were no differences in the probability of ongoing pregnancy and live birth. Furthermore, in patients with normal and low ovarian response who underwent planned oocyte cryopreservation, the oocyte maturation rate was also not increased by the addition of hCG [37]. In this study, although the results showed a moderate increase in number of oocyte retrieval in dual-trigger group, there was no significant difference in terms of oocytes maturation rate, normal fertilization rate, and number of frozen embryos between GnRHa-trigger and dual-trigger groups. Most importantly, the live birth rate and single newborn outcomes were comparable between the two groups. Based on the above results, adding low dose hCG may not significantly improve embryo quality. Therefore, our study suggested that GnRHa single trigger may be more beneficial to PCOS women who treated with the freeze-all strategy.
Compared with previous studies, the strength of our study is that we controlled the critical parameters on the day of hCG injection, which may influence clinical decisions. Thus, this study’s results are more reliable than other studies. Additionally, the patients with PCOS we selected were homogenous, while previous studies had different definitions of high responder. However, this study has several limitations. First, selection bias is inevitable because of its retrospective nature. Second, since we used the PSM, this led to a reduction in sample size. Third, only PCOS patients from a single center were included in this study, and this conclusion may not applicable to other high responders. Therefore, large multi-center prospective studies are required to verify the results of this study.
In conclusion, for PCOS women who were undergoing GnRH antagonist treatment with a freeze-all strategy, GnRH trigger alone is an effective method to induce follicular maturation, which can reduce the risk of OHSS without impairing oocyte maturity and achieve satisfactory pregnancy outcomes.
Data availability
The datasets used and analyzed during the current study are available from corresponding author on reasonable request.
References
Wang FF, Pan JX, Wu Y, Zhu YH, Hardiman PJ, Qu F (2018) American, European, and Chinese practice guidelines or consensuses of polycystic ovary syndrome: a comparative analysis. J Zhejiang Univ Sci B 19:354–363
Zeng R, Chen H, Zeng X, Qin L (2022) The essential role of body weight in adjusting gn dosage to prevent high ovarian response for women with PCOS during IVF: a retrospective study. Front Endocrinol (Lausanne) 13:922044
Homburg R, Ray A, Bhide P, Gudi A, Shah A, Timms P, Grayson K (2013) The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod 28:1077–1083
Sun B, Ma Y, Li L, Hu L, Wang F, Zhang Y, Dai S, Sun Y (2020) Factors associated with ovarian hyperstimulation syndrome (OHSS) severity in women with polycystic ovary syndrome undergoing IVF/ICSI. Front Endocrinol (Lausanne) 11:615957
Pfeifer S, Butts S, Dumesic D, Fossum G et al (2016) Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril 106:1634–1647
Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, Stener-Victorin E, Fauser BC, Norman RJ, Teede H (2016) The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 22:687–708
Ovarian Stimulation T, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, Kunicki M, La Marca A, Lainas G et al (2020) 2020 ESHRE guideline: ovarian stimulation for IVF/ICSI(†). Hum Reprod Open 2:hoaa009
Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, van der Veen F, van Wely M (2017) GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update 23:560–579
Youssef MA, Van der Veen F, Al-Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, Aboulfoutouh I, van Wely M 2014 Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev (10):Cd008046. https://doi.org/10.1002/14651858.CD008046.pub4
Casper RF (2015) Introduction: gonadotropin-releasing hormone agonist triggering of final follicular maturation for in vitro fertilization. Fertil Steril 103:865–866
Humaidan P, Engmann L, Benadiva C (2015) Luteal phase supplementation after gonadotropin-releasing hormone agonist trigger in fresh embryo transfer: the American versus European approaches. Fertil Steril 103:879–885
Lin MH, Wu FS, Lee RK, Li SH, Lin SY, Hwu YM (2013) Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril 100:1296–1302
O’Neill KE, Senapati S, Maina I, Gracia C, Dokras A (2016) GnRH agonist with low-dose hCG (dual trigger) is associated with higher risk of severe ovarian hyperstimulation syndrome compared to GnRH agonist alone. J Assist Reprod Genet 33:1175–1184
He Y, Tang Y, Chen S, Liu J, Liu H (2022) Effect of GnRH agonist alone or combined with different low-dose hCG on cumulative live birth rate for high responders in GnRH antagonist cycles: a retrospective study. BMC Pregnancy Childbirth 22:172
Karacan M, Erdem E, Usta A, Arvas A, Cebi Z, Camlibel T (2017) Gonadotropin-releasing hormone agonist triggering with concomitant administration of low doses of human chorionic gonadotropin or a freeze-all strategy in high responders. Saudi Med J 38:586–591
Santos-Ribeiro S, Mackens S, Popovic-Todorovic B, Racca A, Polyzos NP, Van Landuyt L, Drakopoulos P, de Vos M, Tournaye H, Blockeel C (2020) The freeze-all strategy versus agonist triggering with low-dose hCG for luteal phase support in IVF/ICSI for high responders: a randomized controlled trial. Hum Reprod 35:2808–2818
Qiu M, Tao Y, Kuang Y, Wang Y (2019) Effect of body mass index on pregnancy outcomes with the freeze-all strategy in women with polycystic ovarian syndrome. Fertil Steril 112:1172–1179
Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C (2008) The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril 89:84–91
DiLuigi AJ, Engmann L, Schmidt DW, Maier DB, Nulsen JC, Benadiva CA (2010) Gonadotropin-releasing hormone agonist to induce final oocyte maturation prevents the development of ovarian hyperstimulation syndrome in high-risk patients and leads to improved clinical outcomes compared with coasting. Fertil Steril 94:1111–1114
Shen X, Yang Q, Li L, Lu W (2021) Clinical pregnancy and incidence of ovarian hyperstimulation syndrome in high ovarian responders receiving different doses of hCG supplementation in a GnRH-agonist trigger protocol. Evid Based Complement Alternat Med 2021:2180933
Meyer L, Murphy LA, Gumer A, Reichman DE, Rosenwaks Z, Cholst IN (2015) Risk factors for a suboptimal response to gonadotropin-releasing hormone agonist trigger during in vitro fertilization cycles. Fertil Steril 104:637–642
Engmann L, Benadiva C, Humaidan P (2016) GnRH agonist trigger for the induction of oocyte maturation in GnRH antagonist IVF cycles: a SWOT analysis. Reprod Biomed Online 32:274–285
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology (2011) The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 26(6):1270–1283. https://doi.org/10.1093/humrep/der037
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73:1155–1158
Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E (1989) Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv 44:430–440
Capital Institute of Pediatrics, & Coordinating Study Group of Nine Cities on the Physical Growth and Development of Children (2020) Growth standard curves of birth weight, length and head circumference of Chinese newborns of different gestation. Zhonghua Er Ke Za Zhi 58:738–746
Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, Aldridge MD (2014) Methods for constructing and assessing propensity scores. Health Serv Res 49:1701–1720
Schirmer DA 3rd, Kulkarni AD, Zhang Y, Kawwass JF, Boulet SL, Kissin DM (2020) Ovarian hyperstimulation syndrome after assisted reproductive technologies: trends, predictors, and pregnancy outcomes. Fertil Steril 114:567–578
Dosouto C, Haahr T, Humaidan P (2017) Gonadotropin-releasing hormone agonist (GnRHa) trigger-State of the art. Reprod Biol 17:1–8
Deepika K, Suvarna R, Sumi M, Snehal D, Arveen V, Anuja K, Gautham P, Kamini R (2021) HCG trigger versus GnRH agonist trigger in PCOS patients undergoing IVF cycles: frozen embryo transfer outcomes. JBRA Assist Reprod 25:48–58
Krishna D, Dhoble S, Praneesh G, Rathore S, Upadhaya A, Rao K (2016) Gonadotropin-releasing hormone agonist trigger is a better alternative than human chorionic gonadotropin in PCOS undergoing IVF cycles for an OHSS Free Clinic: a Randomized control trial. J Hum Reprod Sci 9:164–172
Griffin D, Benadiva C, Kummer N, Budinetz T, Nulsen J, Engmann L (2012) Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril 97:1316–1320
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C (2011) Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril 95:2715–2717
Jones BP, Al-Chami A, Gonzalez X, Arshad F, Green J, Bracewell-Milnes T, Saso S, Smith R, Serhal P, Ben Nagi J (2021) Is oocyte maturity influenced by ovulation trigger type in oocyte donation cycles? Hum Fertil (Camb) 24:360–366
Makhijani R, Thorne J, Bartels C, Bartolucci A, Nulsen J, Grow D, Benadiva C, Engmann L (2020) Pregnancy outcomes after frozen-thawed single euploid blastocyst transfer following IVF cycles using GNRH agonist or HCG trigger for final oocyte maturation. J Assist Reprod Genet 37:611–617
Maslow BL, Guarnaccia M, Stefanacci C, Ramirez L, Klein JU (2020) The use of GnRH-agonist trigger for the final maturation of oocytes in normal and low responders undergoing planned oocyte cryopreservation. Hum Reprod 35:1054–1060
Funding
This work was supported by the National Health Commission Key Laboratory of Birth Defects and Reproductive Health (Chongqing Key Laboratory of Birth Defects and Reproductive Health, Chongqing Population and Family Planning Science and Technology Research Institute) (grant number:2021yjy-kfkt-0008) and the Platform of Chongqing Yidu Cloud (grant number: ZHYX202127).
Author information
Authors and Affiliations
Contributions
XT and YD: Conceptualization; XW and YH: Data curation. TL: Supervision; QW: Formal analysis; QW and QW: Writing—original draft; ZZ and KP: Writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics approval
The study was performed in accordance with the ethical standards of the Declaration of Helsinki (1964) and its subsequent amendments. The study protocol involved human participants were approved by the Ethics Committee of Chongqing Medical University (number: 2021060).
Consent to participate
Due to the retrospective nature, written informed consent was waived by the Ethics Committee of Chongqing Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Wan, Q., Li, T. et al. Effect of GnRH agonist trigger with or without low-dose hCG on reproductive outcomes for PCOS women with freeze-all strategy: a propensity score matching study. Arch Gynecol Obstet 309, 679–688 (2024). https://doi.org/10.1007/s00404-023-07285-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07285-1