Abstract
Purpose
To investigate whether gonadotropin-releasing hormone agonist (GnRH-a) combined with human chorionic gonadotropin (HCG) can improve pregnancy outcomes in patients with normal ovarian response (NOR).
Methods
In this retrospective cohort study, data of 404 NOR patients undergoing fresh embryo transfer (ET) from 2018 to 2022 were studied. Patients were divided into HCG group and HCG plus GnRH-a group according to trigger methods. After confounding factors were controlled by propensity score matching, 67 cases were included in HCG group and HCG plus GnRH-a group, respectively, and pregnancy outcomes were assessed. Basal data, ovarian stimulation, embryological data and pregnancy outcomes were compared. The effect of trigger methods on pregnancy outcomes was analyzed by binary logistic regression.
Results
There was no statistically significant differences in embryological data, embryo implantation rate, clinical pregnancy rate, live birth rate of ET, number of fresh embryos transferred and total number of embryos transferred after one cycle of oocyte retrieval. While, cumulative live birth rate (CLBR) was better in the dual-trigger group than in the HCG group. The binary logistic regression analysis indicated that the trigger methods had an independent influence on embryo implantation and cumulative live birth.
Conclusions
During IVF/ICSI, dual-trigger could potentially play a role in improving oocyte quality, ensuring embryo implantation rate, clinical pregnancy rate, live birth rate of ET and cumulative live birth rate at the end of one ovum pick-up (OPU) cycle, and reducing the physical, temporal and financial negative consequences due to repeated OPU cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
To investigate whether gonadotropin-releasing hormone agonist (GnRH-a) combined with human chorionic gonadotropin (HCG) improves pregnancy outcomes in patients with normal ovarian response (NOR) based on a propensity score matching (PSM) method. |
Introduction
Since the advent of test tube technology in the 1970s, in vitro fertilization/intracytoplasmic sperm injection and embryo transfer (IVF/ICSI-ET) has helped millions of infertile couples worldwide [1]. By the improvement of the ovulation induction strategy of the test-tube baby Technology, IVF/ICSI-ET have continued to improve in terms of efficiency and safety. The transition from IVF/ICSI to ovulation induction is a critical stage aimed at optimizing oocytes production and maturation. Ovulation induction protocol is a key step to help infertile women improve the quantity and quality of their oocytes during IVF/ICSI treatment. At this stage, the doctors will develop a personalized ovulation promotion protocol according to the specific situation of the patient to ensure optimal development and maturation process of the oocytes. The gonadotropin-releasing hormone antagonist (GnRH-ant) protocol, has greatly improved in recent years [2]. The GnRH-ant protocol is becoming favored by clinicians and patients because it is more congruent with the growth process of oocytes observed in a woman's natural cycle, requires a shorter ovulation induction time and lower gonadotropin (Gn) dosage, requires less time, and is more cost-effective [3]. In controlled ovarian stimulation (COS), the process of inducing the final maturation of oocytes to obtain mature oocytes with normal fertilization abilities is known as the trigger, which is critical for IVF/ICSI-ET pregnancy outcomes. As a frequently used trigger drug and natural alternative to luteinizing hormone (LH), Human chorionic gonadotropin (HCG) performs physiological functions similar to those of LH, rapidly activates the receptor, and has a long half-life, making it a common method to trigger the final maturation of oocytes in clinical practice [4]. However, some patients with high ovarian response (HOR) may lead to ovarian hyperstimulation syndrome (OHSS) after the HCG trigger, resulting in the inability to undergo fresh embryo transfer (ET) in the same month cycle, increasing the time cost and economic burden, and seriously threatening the patients’ lives [5].
The emergence of gonadotropin-releasing hormone agonist (GnRH-a) has allowed physicians to take a different approach. In contrast to HCG, GnRH-a induces follicle stimulating hormone (FSH) and LH peaks that mimic the normal physiological cycle of women [6], has a shorter half-life than HCG. The induction of FSH and LH peaks is milder and more effective as the expression of dual regulatory proteins and the regulatory epithelial protein mRNAs [7], which act as ligands for the epidermal growth factor receptor, is over-regulated and activated in ovarian granulosa cells in response to luteinizing hormone, induces transient and milder FSH and LH peaks, and induces the production of fewer vasoactive substances [8]. Therefore, it is a better trigger choice for patients with HOR [9, 10]. However, a proportion of patients who use GnRH-a trigger show a poor response, and their retrieved oocytes often remain in the follicular phase or first meiotic metaphase, making it harder to obtain mature oocytes and dramatically decreasing the rate of available embryos [11]. In addition, the rapid luteolytic effect of GnRH-a has a negative impact on luteal function and endometrial receptivity in patients receiving ET [12], making it difficult to achieve a desirable pregnancy outcome. Clinicians have modified the trigger approach to address this issue. A combination of GnRH-a and HCG has also been utilized [13]. This type of trigger is called a dual-trigger and plays an irreplaceable role in clinical practice. The dual-trigger combines the advantages of the two triggers, resulting in more healthy oocytes obtained without negatively affecting endometrial receptivity and subsequent luteal function, and reduces the potential risk of OHSS [14]. It has been found that adding a certain dose of HCG at the same time as a GnRH-a trigger can improve luteal function and pregnancy outcomes of ET cycles in patients with poor ovarian response (POR) [15]. Likewise, the antagonist protocol is the protocol that simulates the development and maturation of normal oocyte and maybe therefore more suitable for patients with good ovarian function and normal ovarian response (NOR). During COS, patients with NOR have a low probability of OHSS and relatively stable oocyte retrieval rate and number of cultured embryos. Previous studies have had inconsistent results on the use of dual triggers, with some studies reporting positive results while others have not demonstrated their effectiveness [16]. In addition, most of the studies about the effect of choice of trigger methods on IVF/ICSI outcomes have been retrospective, with confounding factors inevitably affecting the accuracy of the conclusions between test and control groups. To assess the effectiveness of HCG plus GnRH-a versus HCG in improving IVF/ICSI clinical pregnancy outcomes in patients with NOR is inconclusive. Therefore, further studies are needed to clarify its clinical value. Our study aimed to fill the gap by comparing single and dual trigger outcomes and determining which method is more effective on improving outcomes in patients with NOR. In this study, propensity score matching (PSM) was used to maximize the balance of basic characteristics between the two groups, and the effects of GnRH-a combined with HCG and HCG triggers on pregnancy outcomes were retrospectively studied.
Materials and methods
Study subjects
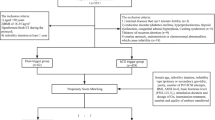
We searched the assisted reproductive technology system of the Department of Reproduction and Genetics of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine and selected patients admitted between 2018 and 2022 who could be followed up for the pregnancy outcome after ET and the first delivery outcome at the end of one ovum pick-up (OPU) cycle using the IVF/ICSI-ET antagonist protocol (Fig. 1). Inclusion criteria: (1) Indications for IVF or ICSI, such as fallopian tube disease caused by pelvic inflammatory disease or pelvic adhesions in women, or severe oligospermia or obstructive azoospermia in men; (2) Age between 20 and 45 years; (3) Regular menstrual cycles of 21–35 days; (4) Body mass index(BMI) less than 28 kg/m2; (5) Basal FSH level ≤ 12 IU/L; (6) The total number of basal antral follicles in both ovaries ranged from 7 to 29; (7) The serum estradiol(E2) level was between 500 and 3000 pg/ml, and the serum progesterone (Prog) level was less than 2 ng/ml on the trigger day; (8) The number of mature follicles (diameter ≥ 14 mm) on trigger day was between 4 and 15; (9)All patients received the trigger as HCG or HCG plus GnRH-a, and those who had no obvious OHSS symptoms after oocytes retrieval and were ready for ET. Exclusion criteria: (1) Patients with HOR: number of mature follicles > 15 on trigger day and/or high serum E2 level (3000–6000 pg/ml) on trigger day [17]. (2) Patients who meet the Bologna criteria with POR were following main clinical features: advanced age (age ≥ 40 years); number of oocytes obtained in the previous conventional IVF ovulation induction protocol was ≤ 3; or basal follicular count (AFC) < 7 (meeting more than two of the three requirements); and E2 level on the trigger day < 500 pg/ml and/or < 4 dominant follicles in mature oocytes [18]. (3) Infertility women with polycystic ovary syndrome [19]. (4) Patients with other endocrine disorders, such as diabetes, thyroid disorders, and pituitary lesions. (5) Previously received IVF/ICSI treatment at least three times. (6) Organic pathology of the uterus includes endometrial polyps, uterine malformations, uterine adhesions, uterine fluid, endometriosis, fibroids, and adenomyosis. (7) Recurrent miscarriage. (8) History of ovarian surgery. (9) Unilateral or bilateral hydrosalpinx as indicated by transvaginal ultrasonography or hysterosalpingography. (10) Chromosomal abnormalities.
Research methods
Protocol for controlled ovarian stimulation
All patients started the controlled ovarian stimulation protocol on the 2nd to 4th days of the menstrual period, and the reproductive physician administered recombinant human follicle-stimulating hormone for injection (Gonafine, Merck Serono, Switzerland) 150–300 IU according to the patients’ age, basal antral follicle number, basal reproductive hormone level, and BMI. During this period, the ovarian response was evaluated by ultrasound and measuring serum hormone levels; thus, the dosage was adjusted. Once the maximum follicle diameter reached 12 mm, 0.25 mg GnRH-ant (MerckSerono, Switzerland) was injected per day. Serum hormone levels and ultrasound findings were monitored every 1 to 3 days until the trigger day. When ultrasound showed three follicles larger than 17 mm in diameter in both ovaries, 6000–10,000 IU of urine-derived HCG(Li zhu, Zhuhai, China), or 0.1 mg GnRH-a (triptorelin acetate; France) combined with 2000–6000 IU of HCG were administered. For the choice of trigger methods, our reproductive center clinicians would decide according to the situation on the trigger day during ovulation induction, patients' past medical history, and doctors’ personal preferences, with the ultimate goal of improving the pregnancy rate and live birth rate. All patients underwent vaginal ultrasound-guided puncture oocyte retrieval within 35–36 h after triggering, then, standard IVF or ICSI fertilization was carried out. Finally all patients received ET on 3rd or 5th days after oocytes retrieval. From the day of oocytes retrieval, all patients received a combination of oral progesterone and vaginal progesterone sustained-release gel or intramuscular progesterone injections until 10 weeks of gestation. For patients with failed fresh embryo transfer, doctors would perform frozen-thawed embryo transfer (FET) based on the patient's remaining embryos.
Protocol for frozen-thawed embryo transfer
We homogenized FET protocols considering the influence of different intimal preparation protocols. There were two kinds of intima preparation protocols, which both required the following criteria for transplantation. (1) The endometrial thickness is greater than or equal to 8 mm. (2) E2 ≥ 150 pg/ml. (3) The professional titles of transplant doctors were all chief physicians. (4) Grade I or II embryos were given priority for transfer. The first was the natural cycle protocol: Ovulation was monitored around the 10th day of menstruation, inducing the follicles to grow. When the follicles grew to about 14 mm, the LH test strip should be measured every day, and the size of the follicle should be monitored after the LH peak appears in the urine or blood. When the follicles of more than 18–20 mm were discharged, progesterone was given for luteal support. 1–2 cleavage embryos were transferred on 3rd day or one blastocyst on 5th day after ovulation. Then luteal support continued after transplantation. The other is an alternative cycle protocol: On the 2–3rd day of menstruation, female patients would begin to use some drugs (such as Triptorelin, Leuprorelin, etc.) for artificial down-regulating, and after reaching the down-regulating standards, oral estrogen tablets (such as Estradiol Valerate, etc.) were started to further prepare the endometrium. When everything was ready, doctors would determine the embryo cryo-resuscitation and the specific transfer time based on the number of days of culture at the time of embryo freezing. After the transplant, they also gave luteal support drugs to preserve the fetus. Patients were tested for serum-HCG on the 14th days after FET to determine embryo implantation. Subsequent luteal support protocol was consistent with that of ET.
Embryo culture
The retrieved oocytes were subjected to IVF and ICSI according to the semen of the male partner, and the state of development was evaluated at approximately 16–18 h after fertilization. Three days after oocytes retrieval, embryos were graded according to the Racowsky Scoring Criteria [20]. If the number of cultured embryos was greater than 2, laboratory technicians selectively cultivated blastocysts according to the patient’s will and actual situation. In general, the lower grade-scoring embryos were cultivated and observed to the blastocyst stage, and then graded by the David Gardner Scoring Method [21]. Clinicians performed ET individually according to the patient's condition. In general, two embryos or one blastocyst were transferred, and the rest of embryos were cryopreserved for the next freeze–thaw embryo transfer until the end of the freeze–thaw embryo transfer cycle for all embryos in a single oocyte retrieval cycle.
Evaluation of results
The pregnancy outcomes of the two trigger methods were compared. Live birth rate of ET, is the primary outcome indicator of pregnancy in our study. And live birth is generally deemed as to be delivery after 28 weeks of gestation, with the baby breathing and a heartbeat at birth.
Secondary indicators: Clinical indicators included usage days of Gn, total dose of Gn, and number of mature follicles on trigger day. Laboratory indicators included the number of oocytes retrieved, 2PN fertilization rate, transferable embryo rate, total number of embryos transferred, cycle rate of high-quality embryos transferred, the number of fresh embryos transferred and the total number of embryos transferred at the end of one OPU cycle. Outcome indicators included embryo implantation rate, clinical pregnancy rate, and cumulative live birth rate (CLBR). 2PN fertilization is the appearance of two pronuclei and bipolar bodies 16–18 h after fertilization. The criteria for good-quality embryos were ≥ 6 cells, < 10% fragmentation, and symmetric blastomeres after 3 days of fertilization. Luteal phase support was started on the day of oocyte retrieval and continued until the day of embryo transfer on day 3 or 5. Two weeks after ET, serum β-HCG levels are determined among all patients. Embryo implantation is serum β-HCG levels above 10 IU/L. Five weeks after ET, the confirmation of an intrauterine gestational sac by gynaecological ultrasound is considered clinical pregnancy. The calculation formulas of pregnancy indicators in IVF/ICSI were shown in the supplementary file.
Statistical analysis
Data were matched and analyzed with the help of SPSS software (version 26.0). Whether the baseline data the of patients conformed to normal distribution was assessed using the Shapiro–Wilk test, histograms, or Q–Q plots. The mean ± standard deviation (SD) was used to describe the normally distributed or approximately normal distribution of the metric data, while the median plus quartile M (P25, P75) was used to describe the non-normal distribution of the metric data. A two-sample t-test or non-parametric test was adopted to analyze the two groups of data. Count data were presented as the rate or percentage, and the Chi-square test was adopted to analyze the data between two groups. Statistically and clinically significant variables between groups were included in the propensity score matching, and the matching tolerance was 0.02 with a 1:1 exact matching. Univariate analysis was adopted to baseline data, laboratory indicators, and outcome indicators of the two groups after successful matching. Finally, statistically significant indicators from the univariate analyses and clinically significant indicators were incorporated into binary logistic regression analyses for further analysis, and adjusted odds ratios (OR) were calculated using 95% confidence intervals (95% CI). A bilateral P value < 0.05 was considered statistically significant.
Results
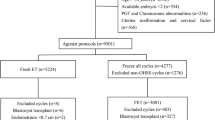
We selected 2178 women with infertility from the database, all of whom received an antagonist protocol for the IVF/ICSI cycle. References to the inclusion and exclusion criteria, 404 subjects were screened out (The data are presented in supplementary Table 1). According to the different trigger methods, they were divided into the HCG trigger group (337 cases) and the GnRH-a combined with HCG group (67 cases). The propensity score matching (PSM) method was applied to 1:1 exact matching, with a matching tolerance of 0.02. According to the statistically and clinically significant variables of the two groups, the matching factors were age, BMI, basal antral follicle number, and basal Prog level, and 67 pairs of study subjects were matched (The data are presented in supplementary Table 2). The matching success rate was 100%. This process is illustrated in Fig. 1.
The basic information of the patients in two groups before PSM is detailed in Table 1. There were no obvious differences in two groups as regards age, infertility duration, basal FSH, LH and E2 level, E2 and Prog level on trigger day, the number of mature oocytes on trigger day, and the number of oocytes acquired (P > 0.05). BMI, basal Prog level, usage days of Gn, total dose of Gn, and LH level on trigger day were statistically different in both groups. Both BMI and usage days of Gn were distinctly smaller in the single-trigger group than in the dual-trigger group, and the total dose of Gn was distinctly less than that of the dual-trigger group (P < 0.001). The number of basal antral follicles was more than that in the dual-trigger group (P = 0.005), and basal Prog and LH levels on trigger day were higher than those in the dual-trigger group (P < 0.001). All statistically significant indicators before PSM matching are shown in Fig. 2.
After PSM1:1 balancing of the variables of the two groups, the differences about else factors both in two groups were further compared (Table 2). We found statistically significant differences in the usage of Gn, total dose of Gn, and LH level on trigger day between the two groups. The dual-trigger group was lower in terms of LH level on the trigger day (P < 0.001), and the usage days of Gn and the total dose of Gn were higher (P < 0.05). The statistically meaningful indicators after PSM are shown in Fig. 3. After matching, we compared laboratory data and pregnancy outcome indicators between the two groups (Table 3). Three women who did not undergo follow-up for all frozen-thawed embryo outcomes after a single oocyte retrieval cycle until the end of 2022 were excluded from the calculation of the CLBR. And the dual-trigger group was significantly better than the HCG trigger group as the CLBR (64.6% vs. 43.9%, P = 0.018). In the matter of the 2PN fertilization rate (64.0% vs. 62.5%), transferrable embryo rate (60.7% vs. 57.3%), cycle rate of high-quality embryos transferred (46.3%v s. 43.3%), embryo implantation rate (55.2% vs. 40.3%), clinical pregnancy rate (50.7% vs. 38.8%), and live birth rate of ET (47.8% vs. 37.3%), probably because of small size of the included sample, there was no statistical significance in the above laboratory and outcome indicators between the two groups of patients with NOR (P > 0.05). Similarly, there was no statistically significant difference between the two groups in the number of fresh embryos transferred (χ2 = 0.153, P > 0.05) and the total number of embryos transferred after at the end of one OPU cycle (χ2 = 0.524, P > 0.05).
Multivariate logistic regression analysis after PSM
We included embryo implantation, clinical pregnancy, live birth, and cumulative live birth in binary logistic regression analysis, and potential confounding variables concerning age, BMI, basal antral follicle number, usage days of Gn, total dose of Gn, trigger methods, LH level on trigger day, and number of mature oocyte retrieval were controlled. The results are expressed as a regression coefficient, P value, OR value, and 95% CI of the OR value.
After adjustment for various confounding factors using binary logistic regression analysis, we explored whether dual-trigger had a positive effect on embryo implantation, clinical pregnancy, and live birth outcomes compared to single-trigger. Figure 4 shows the results of the different trigger groups. Table 4 shows that the dual-trigger was a positive independent factor affecting embryo implantation and cumulative live birth (OR: 2.547, 95% CI 1.040–6.239, p = 0.041; OR: 2.643, 95% CI 1.051–6.647, p = 0.039). The rate of implantation and cumulative live birth in the dual trigger group were 1.547 times and 1.643 times higher than those in the single trigger group, respectively. Unfortunately, there was no significant association between clinical pregnancy and live birth outcomes and trigger methods (p > 0.05).
Binary logistic regression results. It shows the results of the different trigger groups in terms of embryo implantation, clinical pregnancy, live births, and cumulative live births after adjusting for confounding factors such as age, BMI, basal antral follicle number, usage days of Gn, total dose of Gn, LH level on trigger day, E2 trigger day, basal FSH, basal LH, basal E2, basal P, and the number of oocytes acquired
Discussion
The aim of our study was to investigate whether GnRH-a combined with HCG improves pregnancy outcomes in patients with NOR compared to HCG triggering. The findings suggested that a dual-trigger strategy of HCG combined with GnRH-a in an antagonist protocol may improve embryo implantation and cumulative live birth rates to some extent in patients with NOR. However, there was no significant difference in terms of oocyte and embryo quality.
Decleer's study mentioned that dual trigger induced endogenous FSH and LH peaks that were more favourable to improve oocyte maturation and thus embryo quality [22]. Dual triggering increases the number of metaphase II oocytes and the number of high-quality embryos and reduce the probability of retrieving empty follicles [23,24,25]. Maged et al. similarly found that dual triggering increased the biochemical pregnancy rate (25% vs. 11.3%, P = 0.039) and the clinical pregnancy rate (22.5% vs. 8.8%, P = 0.028) in patients with POR [26]. In addition to this, several trials have confirmed the role of dual triggering in patients with HOR, showing an improvement in live birth and ongoing pregnancy rates, and a reduction in the risk of OHSS [27, 28]. Therefore, dual-trigger is a wise choice for patients who intend to transfer fresh embryos but are at risk of OHSS. And a previous meta-analysis showed that GnRH-a combined with HCG triggering was superior to HCG triggering alone in terms of clinical pregnancy and live birth rates in ET cycles, which again provides strong evidence for our findings [29]. Moreover, a retrospective study showed that in patients with NOR, the dual-trigger group (HCG 5000 IU + GnRH-a 0.1 mg) was significantly superior to the HCG alone group in terms of the number of mature oocytes (12.51 vs. 10.58, P = 0.019), the mean number of metaphase II oocytes (9.52 vs. 8.33, P < 0.01), and the number of normally fertilized oocytes (7.63 vs. 6.60, P < 0.01) [30]. These results suggest that the dual-trigger strategy can be an effective alternative to IVF/ICSI treatment and improve oocyte and embryo quality. In our study, we were unable to observe differences in oocyte maturation and embryo culture between the two groups, but this result is not surprising and may be related to the different patient inclusion criteria, small sample size, and non-uniformity in the dosage of HCG. The dual trigger may promote the proliferation and differentiation of granule cells, inhibit apoptosis of granule cells, and at the same time improve the follicular fluid microenvironment [31], thereby promoting follicle development and oocyte maturation, and reducing problems such as chromosome abnormalities and gene mutations during embryo culture [32]. A large amount of Gn and a long time of use will lead to changes of hormones within the patient's body in a short period of time, resulting in chromosome separation errors in the process of meiosis of oocytes, so that chromosome abnormalities in fertilized embryos and affecting the quality of embryos [33]. In this study, compared with the HCG trigger group, the pregnancy rate and live birth rate of the dual trigger group with larger total dose and longer use time of Gn were similar, and the embryo implantation rate and CLBR were higher, which also reflects that the dual trigger may compensate for the abnormal oocyte meiosis caused by excessive use of Gn to some extent. It reduces the probability of oocyte aneuploidy [34], reduces the probability of damaged oocyte nuclear maturation, improves the quality of oocytes, and thus reduces the IVF/ICSI of failures caused by poor embryo quality. Using the dual trigger strategy can effectively shorten the treatment cycle and improve the efficiency of treatment for patients, thus achieving pregnancy goals faster. Therefore, for patients with NOR, the dual-trigger strategy may be more suitable for them.
For patients using the dual trigger, the gene expression in granular cells and downstream luteinizing hormone signaling receptors may be different from those in the single trigger [35] This discovery provides a theoretical basis for successful pregnancy and delivery in NOR women with dual trigger therapy. The combination of Gn and GnRH-ant in IVF/ICSI cycles results in extremely low LH levels during COS [36]. In contrast, the dual-trigger allows for an immediate and significant increase in LH levels and triggers the induction of additional FSH peaks [37]. In the dual-trigger strategy, GnRH-a combined with HCG can simulate the secretion of LH and the production of LH peak, and the FSH and LH peak are more moderate [38]. This strategy may affect the gene expression profile of granular cells, including the expression of genes related to oocyte development, maturation, fertilization and luteal function [39, 40]. FSH affects granulosa gap junction and epidermal growth factor function during oocyte meiosis, increases hyaluronic acid synthesis, promotes oocyte nuclear maturation and cumulus enlargement [41]. In addition, FSH upregulates the expression of LH receptors in granulosa cells, which is related to the formation of LH peak and luteinization of granulosa cells before follicle maturation. The LH peak in mid-menstrual period before ovulation promotes the eventual maturation of oocytes and forces the cumulus oocyte cluster to be expelled from the ovaries to complete ovulation [42]. Multiple studies have shown that low LH level on trigger day is associated with low oocyte maturation [43, 44]. Low LH levels on trigger day may lead to an increased risk of miscarriage early in ET. Only when the LH level reaches a certain concentration can the oocytes complete the first meiosis, expel the first polar body, and become secondary oocytes. In our study, LH level on trigger day was 2.39 ± 1.07 IU/L, so clinicians would use GnRH-a combined with HCG trigger to improve oocyte quality and obtain satisfactory pregnancy and live birth rates. In this study, we found that the patients who received dual trigger had lower LH levels on the trigger day, but the pregnancy and live birth rates were similar to those in the HCG group, and the above mechanism may provide a plausible explanation for our results. In addition, the dual trigger strategy may also affect the expression and function of luteinizing hormone signaling receptors downstream of granular cells. In the dual-trigger strategy, the combined action of HCG and GnRH-a may affect the number, distribution, activation state and signal transduction of luteinizing hormone signal receptors, thus affecting oocyte development and luteal function [30, 45, 46]. In addition, surprisingly, the clinical pregnancy rate and live birth rate in the dual-trigger group were similar to those in the HCG group alone, but the CLBR were better than those in the single-trigger group. This may be inseparable from the synergistic effect of FSH and LH in granulosa cells and oocytes and their positive effects on oocytes. Furthermore, the embryo implantation rate of ET was higher in the dual- trigger group than in the HCG group, which may suggest that dual-trigger resulted in an increase in endometrial tolerance compared to HCG triggering alone. GnRH analogues have a direct effect on endometrial tolerance. In the GnRH-ant protocol, GnRH receptors in the endometrium preferentially bind GnRH-a, thus playing a key role in embryo implantation, trophoblast proliferation and invasion [29].
The strength of this study lies in the use of PSM to balance the distribution of confounders present in the two groups. This approach overcomes the bias error caused by individual heterogeneity of retrospective clinical study data, reduces the effects of bias and confounders, and helps to assess the effects of the two trigger methods on pregnancy outcomes in women with NOR in a more scientific and rigorous manner. In addition, we followed up the live birth rate of fresh embryo transfer and the cumulative live birth rate at the end of one cycle of egg retrieval in both groups of women, which provides an important reference for clinical assessment of the effect of dual trigger on embryo quality and pregnancy outcomes in women with NOR.
However, our study still has some shortcomings. Firstly, our study was a retrospective study, which suffered from small sample size and older age of included patients, so age was not reflected as a variable affecting clinical pregnancy and live birth, and there was a risk of bias and other potential confounders. Secondly, there may be some other potential variables that were not considered in the regression analysis, such as the FSH level on the trigger day that was not further followed up. In addition, some patients with good ovarian reserve were excluded from this study because the use of Gn may cause OHSS tendency and affect ET. Moreover, regarding the supplementary dose of HCG in the dual-trigger also due to the small sample size, we did not perform subgroup analyses. Finally, we did not follow up the neonatal outcomes in either group. Therefore, effects of the two trigger methods on oocyte and embryo quality in patients with NOR still need to be validated by large-scale mechanistic studies. We also recommend multicenter randomized controlled studies with larger sample sizes and longer follow-up periods to demonstrate the efficacy and safety of dual triggering.
Conclusions
In the present study, the CLBR was higher in the dual trigger group than in the HCG alone group. The 2PN fertilization rate, embryo implantation rate, clinical pregnancy rate and live birth rate of ET were also higher in the dual trigger group than in the HCG alone group, but the differences between the two groups were not statistically significant, probably due to the small sample size. We recommend multicenter randomized controlled studies with larger sample sizes and longer follow-up periods to demonstrate the efficacy and safety of dual-trigger.
Data availability
All data in this study were obtained from the assisted reproductive system database of the Department of Reproduction and Genetics, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, and all patients had signed written informed consent for assisted reproductive technology.
Abbreviations
- GnRH-a:
-
Gonadotropin-releasing hormone agonist
- HCG:
-
Human chorionic gonadotropin
- NOR:
-
Normal ovarian reaction
- CLBR:
-
Cumulative live birth rate
- Gn:
-
Gonadotropin
- IVF:
-
In vitro fertilization
- ICSI:
-
Intracytoplasmic sperm injection
- ET:
-
Fresh embryo transfer
- GnRH-ant:
-
Gonadotropin-releasing hormone antagonist
- COS:
-
Controlled ovarian stimulation
- OHSS:
-
Ovarian hyperstimulation syndrome
- HOR:
-
High ovarian response
- POR:
-
Poor ovarian response
- PSM:
-
Propensity score matching
- CLB:
-
Cumulative live birth
- OR:
-
Odds ratios
- BMI:
-
Body mass index
- 95% CI:
-
95% Confidence intervals
- FSH:
-
Follicle stimulating hormone
- LH:
-
Luteinizing hormone
- E2:
-
Estradiol
- Prog:
-
Progesterone
- FET:
-
Frozen-thawed embryo transfer
- OPU:
-
Ovum pick-up
References
Inhorn MC, Patrizio P (2015) Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 21(4):411–26. https://doi.org/10.1093/humupd/dmv016
Wei D, Liu JY, Sun Y et al (2019) Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 393(10178):1310–1318. https://doi.org/10.1016/S0140-6736(18)32843-5
Liu Y, Su R, Wu Y (2021) Cumulative live birth rate and cost-effectiveness analysis of gonadotropin releasing hormone-antagonist protocol and multiple minimal ovarian stimulation in poor responders. Front Endocrinol (Lausanne) 14(11):605939. https://doi.org/10.3389/fendo.2020.605939
Ludwig M, Doody KJ, Doody KM (2003) Use of recombinant human chorionic gonadotropin in ovulation induction. Fertil Steril 79(5):1051–1059. https://doi.org/10.1016/s0015-0282(03)00173-0
Hebisha SA, Aboelazm BA, Sallam HN (2017) GnRH antagonist cetrorelix administration before hCG for protection of ovarian hyperstimulation syndrome. J Obstet Gynaecol India 67(4):270–274. https://doi.org/10.1007/s13224-016-0952-5
Haas J, Bassil R, Samara N et al (2020) GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod 35(7):1648–1654. https://doi.org/10.1093/humrep/deaa107
Haas J, Ophir L, Barzilay E et al (2016) Standard human chorionic gonadotropin versus double trigger for final oocyte maturation results in different granulosa cells gene expressions: a pilot study. Fertil Steril 106(03):653–659. https://doi.org/10.1016/j.fertnstert.2016.06.002
Gonen N, Casper RF, Jurisicova A et al (2021) Does gonadotropin-releasing hormone agonist cause luteolysis by inducing apoptosis of the human granulosa-luteal cells? J Assist Reprod Genet 38(9):2301–2305. https://doi.org/10.1007/s10815-021-02226-w
Alama P, Bellver J, Vidal C, Giles J (2013) GnRH analogues in the prevention of ovarian hyperstimulation syndrome. Int J Endocrinol Metab 11(2):107–16. https://doi.org/10.5812/ijem.5034
Zeng C, Shang J, Jin AM et al (2019) The effect of luteal GnRH antagonist on moderate and severe early ovarian hyperstimulation syndrome during in vitro fertilization treatment: a prospective cohort study. Arch Gynecol Obstet 300(1):223–233. https://doi.org/10.1007/s00404-019-05163-3
He Y, Tang Y, Chen S et al (2022) Effect of GnRH agonist alone or combined with different low-dose hCG on cumulative live birth rate for high responders in GnRH antagonist cycles: a retrospective study. BMC Pregnancy Childbirth 22(1):172. https://doi.org/10.1186/s12884-022-04499-0
Eftekhar M, Mojtahedi MF, Miraj S et al (2017) Final follicular maturation by administration of GnRH agonist plus HCG versus HCG in normal responders in ART cycles: an RCT. Int J Reprod Biomed 15(7):429–434
Orvieto R (2015) Triggering final follicular maturation–hCG, GnRH-agonist or both, when and to whom? J Ovarian Res 21(8):60. https://doi.org/10.1186/s13048-015-0187-6
Zhou C, Yang X, Wang Y et al (2022) Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles. Hum Reprod 37(8):1795–1805. https://doi.org/10.1093/humrep/deac114
Haas J, Zilberberg E, Nahum R et al (2019) Does double trigger (GnRH-agonist + hCG) improve outcome in poor responders undergoing IVF-ET cycle? A pilot study Gynecol Endocrinol 35(7):628–630. https://doi.org/10.1080/09513590.2019.1576621
Chung RK, Mancuso AC, Summers KM et al (2021) Dual trigger protocol is an effective in vitro fertilization strategy in both normal and high responders without compromising pregnancy outcomes in fresh cycles. F S Rep 2(3):314–319. https://doi.org/10.1016/j.xfre.2021.05.008
Broer SL, Dólleman M, van Disseldorp J et al (2013) Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril 100(2):420-429.e7. https://doi.org/10.1016/j.fertnstert.2013.04.024
Ferraretti AP, La Marca A, Fauser BC et al (2011) ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26(7):1616–1624. https://doi.org/10.1093/humrep/der092
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group (2004) Revised 2003 consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome(PCOS) Hum Reprod 19(1): 41–47
Racowsky C, Machtinger R (2013) Morphological systems of human embryo assessment and clinical evidence. Reprod Biomed Online 26(3):210–221. https://doi.org/10.1016/j.rbmo.2012.10.021
Schoolcraft WB, Gardner DK, Lane M et al (1999) Blastocyst culture and transfer: analysis of results and parameters affecting outcome in two in vitro fertilization programs. Fertil Steril 72(4):604–609. https://doi.org/10.1016/s0015-0282(99)00311-8
Decleer W, Osmanagaoglu K, Seynhave B et al (2014) Comparison of hCG triggering versus hCG in combination with a GnRH agonist: a prospective randomized controlled trial. Facts Views Vis Obgyn 6(4):203–209
Zilberberg E, Haas J, Dar S et al (2015) Co-administration of GnRH-agonist and hCG, for final oocyte maturation (double trigger), in patients with low proportion of mature oocytes. Gynecol Endocrinol 31(02):145–147. https://doi.org/10.3109/09513590.2014.978850
Haas J, Zilberberg E, Dar S et al (2014) Co-administration of GnRH-agonist and hCG for final oocyte maturation (double trigger) in patients with low number of oocytes retrieved per number of preovulatory follicles–a preliminary report. J Ovarian Res 2(7):77
Alleyassin A, Ghasemi M, Aghahosseini M et al (2018) Final oocyte maturation with a dual trigger compared to human chorionic gonadotropin trigger in antagonist co-treated cycles: a randomized clinical trial. Middle East Fertil Soc J 23(3):199–204. https://doi.org/10.1016/j.mefs.2018.01.001
Maged AM, Ragab MA, Shohayeb A et al (2021) Comparative study between single versus dual trigger for poor responders in GnRH-antagonist ICSI cycles: a randomized controlled study. Int J Gynaecol Obstet 152(3):395–400. https://doi.org/10.1002/ijgo.13405. (Epub 2020 Oct 22)
Shapiro BS, Daneshmand ST, Garner FC et al (2008) Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril 90(1):231–233. https://doi.org/10.1016/j.fertnstert.2007.06.030. (Epub 2007 Nov 5)
Griffin D, Benadiva C, Kummer N et al (2012) Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril 97(6):1316–1320. https://doi.org/10.1016/j.fertnstert.2012.03.015. (Epub 2012 Apr 3)
Chen C, Tzeng C, Wang P et al (2018) Dual triggering with GnRH agonist plus hCG versus triggering with hCG alone for IVF/ICSI outcome in GnRH antagonist cycles: a systematic review and meta-analysis. Arch Gynecol Obstet 298(1):17–26. https://doi.org/10.1007/s00404-018-4751-3
Albeitawi S, Marar EA, Reshoud FA et al (2022) Dual trigger with gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves oocyte yield in normal responders on GnRH-antagonist cycles. JBRA Assist Reprod 26(1):28–32. https://doi.org/10.5935/1518-0557.20210039
Beck-Fruchter R, Weiss A, Lavee M et al (2012) Empty follicle syndrome: successful treatment in a recurrent case and review of the literature. Hum Reprod 27(5):1357–1367
Elias RT, Pereira N, Artusa L et al (2017) Combined GnRH-agonist and human chorionic gonadotropin trigger improves ICSI cycle outcomes in patients with history of poor fertilization. J Assist Reprod Genet 34(6):781–788. https://doi.org/10.1007/s10815-017-0917-3. (Epub 2017 Apr 13)
Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ (2015) Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril. 104(5):1145–52. https://doi.org/10.1016/j.fertnstert.2015.07.1151. (Epub 2015 Aug 18)
Demirel C, Celik HG, Tulek F et al (2022) Dual trigger with hCG plus GnRHa for final oocyte maturation in PGT-A cycles results in similar euploidy rates when compared to hCG-Only trigger. Reprod Sci 29(8):2265–2271. https://doi.org/10.1007/s43032-022-00954-7
Chern CU, Li JY, Tsui KH et al (2020) Dual-trigger improves the outcomes of in vitro fertilization cycles in older patients with diminished ovarian reserve: a retrospective cohort study. PLoS ONE 15(7):e0235707. https://doi.org/10.1371/journal.pone.0235707
Beckers NG, Macklon NS, Eijkemans MJ et al (2003) Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab 88(9):4186–4192. https://doi.org/10.1210/jc.2002-021953
Ding N, Liu X, Jian Q et al (2017) Dual trigger of final oocyte maturation with a combination of GnRH agonist and hCG versus a hCG alone trigger in GnRH antagonist cycle for in vitro fertilization: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 218:92–98. https://doi.org/10.1016/j.ejogrb.2017.09.004
Kasum M, Kurdija K, Orešković S et al (2016) Combined ovulation triggering with GnRH agonist and hCG in IVF patients. Gynecol Endocrinol 32(11):861–865. https://doi.org/10.1080/09513590.2016.1193141
Casarini L, Lispi M, Longobardi S et al (2012) LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS ONE 7(10):e46682. https://doi.org/10.1371/journal.pone.0046682. (Epub 2012 Oct 5)
Yung Y, Aizer A, Tieb S et al (2022) The in-vitro effect of gonadotropins’ type and combination on granulosa cells gene expressions. Reprod Biol Endocrinol 20(1):144. https://doi.org/10.1186/s12958-022-01017-x
Lamb JD, Shen S, McCulloch C et al (2011) Follicle-stimulating hormone administered at the time of human chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilization cycles: a randomized, double-blind, placebo-controlled trial. Fertil Steril 95:1655–1660. https://doi.org/10.1016/j.fertnstert.2011.01.019
Mizrachi Y, Horowitz E, Farhi J et al (2020) Ovarian stimulation for freeze-all IVF cycles: a systematic review. Hum Reprod Update 26(1):118–135. https://doi.org/10.1093/humupd/dmz037
Ganer Herman H, Horowitz E, Mizrachi Y et al (2022) Prediction, assessment, and management of suboptimal GnRH agonist trigger: a systematic review. J Assist Reprod Genet 39(2):291–303. https://doi.org/10.1007/s10815-021-02359-y
Benmachiche A, Benbouhedja S, Zoghmar A et al (2019) Low LH level on the day of GnRH agonist trigger is associated with reduced ongoing pregnancy and live birth rates and increased early miscarriage rates following IVF/ICSI treatment and fresh embryo transfer. Front Endocrinol (Lausanne) 18(10):639. https://doi.org/10.3389/fendo.2019.00639
Beck-Fruchter R, Baram S, Geslevich Y et al (2019) Gonadotropin releasing hormone agonist final oocyte maturation and human chorionic gonadotropin as exclusive luteal support in normal responders. Gynecol Obstet Invest 84(1):27–34. https://doi.org/10.1159/000490946
Wiweko B, Satria ML, Mutia K et al (2019) Correlation between luteinizing hormone receptor gene expression in human granulosa cells with oocyte quality in poor responder patients undergoing in vitro fertilization: a cross–sectional study. F1000Res 8:16. https://doi.org/10.12688/f1000research.17036.1
Funding
This work was supported by National Natural Science Fund of China (Grant numbers 81573778).
Author information
Authors and Affiliations
Contributions
Danyang Guo was responsible for study design, data collection, analysis and writing, and wrote the first draft of the manuscript. Conghui Pang was responsible for the review and revision of the manuscript, and for collated literature and the visual analysis. Kehua Wang was responsible for article design, manuscript revision and funding.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any conflict of interest.
Ethical approval
All patients had signed written informed consent for assisted reproductive technology. The study was retrospective and did not require ethical approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, D., Pang, C. & Wang, K. Comparison of pregnancy outcomes in women with normal ovarian response to the gonadotropin-releasing hormone agonist protocol using different trigger methods: a single-center retrospective cohort study based on propensity score matching. Arch Gynecol Obstet 309, 2153–2165 (2024). https://doi.org/10.1007/s00404-024-07404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-024-07404-6