Abstract
Purpose
To estimate the impact of increasing pre-pregnancy Body Mass Index (BMI) on the risk of adverse maternal and perinatal outcomes, in patients who delivered in an Italian tertiary care Obstetric department.
Methods
Data, related to women who delivered at Sant’Anna Hospital, Turin, between 2011 and 2015, were collected retrospectively from the hospital database. According to BMI, women were considered as normal weight, overweight, and class 1, 2 and 3 obese (WHO criteria). Logistic regression analysis studied the impact of BMI on maternal and neonatal outcomes, adjusting results for maternal age and parity. Adjusted absolute risks of each outcome were reported according to incremental values in pre-pregnancy BMI.
Results
A total of 27,807 women were included. 75.8% of pregnancies occurred among normal-weight women, whereas 16.7% were overweight, and 7.5% obese women (5.4% class 1, 1.7% class 2 and 0.4% class 3). A 10% decrease in pre-pregnancy BMI was associated with a reduction of at least 15% of Gestational diabetes mellitus (GDM), preeclampsia, maternal admission to intensive care unit (ICU), macrosomia, APGAR 5′ < 6 and neonatal admission to ICU. GDM and preeclampsia resulted in the highest reduction being almost 30%. Larger differences in BMI (20–25%) corresponded to at least a 10% in reduction of risk of preterm and very preterm delivery and emergency cesarean section. Differences in maternal pre-pregnancy BMI had no impact on the frequency of shoulder dystocia and stillbirth.
Conclusions
This study offers a quantitative estimation of negative impact of pre-pregnancy obesity on the most common pregnancy and perinatal complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past three decades, the prevalence of overweight and obesity has been increasing dramatically worldwide [1,2,3].
This trend has involved also women in reproductive-age and pregnant women [4,5,6]. In the United States, 12–38% of pregnant women are overweight, whereas 11–40% are obese, while in the UK, 33% of pregnant women are overweight or obese [7]. Whereas Italy is the European country with the lowest prevalence of adult obesity (9.8%), the proportion of obese women in reproductive age (25–44 years) has been reported as 18% [8, 9]. Several studies addressed the influence of maternal pre-pregnancy weight on pregnancy outcome: obesity can cause complications both for the woman and her offspring. Maternal risks include gestational diabetes (GDM), gestational hypertension and preeclampsia (PE), infectious morbidity, post-partum hemorrhage (PPH), macrosomia (LGA) [10,11,12], preterm delivery (PTD < 37 weeks gestational age), and very preterm delivery (VPTD < 32 weeks gestational age) [13, 14]. Fetuses of obese mothers are at increased risk of stillbirth [13,14,15] and congenital anomalies [12, 16]. Furthermore, intra-partum care, normal and operative deliveries, cesarean section, anesthetic and operative interventions in obese women demand extra care and extra costs [10, 11]. Besides, maternal obesity may impact on long-term offspring’s risk of metabolic disease [17].
The National Institute of Health (NIH) advises that a 10% reduction (achievable in 6 months of therapy) in body weight is associated with significant health benefits, in non-pregnant obese individuals [18]. Until then, the potential benefits of pre-pregnancy weight reduction on maternal and perinatal outcomes have not been studied properly. Indeed, the most extensive studies were focused on the management of weight gain during pregnancy, showing limited advantages of such an approach [19, 20].
The aim of the present study was to examine pregnancy outcomes in obese and overweight North-Italian women, and evaluate the adjusted risk of adverse maternal and neonatal outcomes as a function of increasing pre-pregnancy BMI. We also aimed to provide useful results to clinicians to calculate risk reductions associated with lowering of pre-pregnancy BMI.
Materials and methods
Study design
A retrospective population-based cohort study was conducted on 30,853 women giving birth to a singleton baby, over a period of 5 years.
Setting
All women admitted to Obstetrics departments of Sant’Anna Hospital in Turin, Italy, a tertiary referral hospital for antenatal care, from January 1st, 2011 to December 31st, 2015, were included in the study. Data were extracted from a database which was updated prospectively, using the software TrakCare (Informative Sanitary System used by the National Health System), and from Hospital Discharge Folders (SDO).
Eligibility criteria
All women with single pregnancies who had delivered between 23 + 1 and 42 + 0 weeks of gestational age were included.
Exclusion criteria
Patients with incorrect data registration and women whose BMI was not computable or whose pre-pregnancy BMI was < 18.5 kg/m2 were excluded. Pregnancies complicated by pre-existing diabetes and/or hypertension, were also excluded.
BMI was calculated from self-reported pre-pregnancy weight and height, obtained at the first antenatal visit. All women were categorized according to pre-pregnancy BMI following WHO guidelines: normal weight women (BMI 18.5–24.99 kg/m2), overweight women (BMI 25.00–29.99 kg/m2), class 1 obese women (BMI 30–35 kg/m2), class 2 obese women (BMI 35–40 kg/m2) and class 3 obese women (BMI 40 or higher kg/m2) [21].
Overweight and obese women were compared with normal weight women for all considered outcomes. Maternal and neonatal complications during pregnancy and delivery were classified according to the International Classification of Diseases 10th Revision (Inter class diseases) [22].
Outcomes
The following maternal outcomes were considered: (1) preeclampsia (PE), (2) gestational diabetes mellitus (GDM), (3) induction of labor, (4) cesarean section after failure of induction, (5) elective cesarean delivery, (6) emergency cesarean delivery, (7) post-partum hemorrhage (PPH), defined as more than 1000 mL of postpartum blood loss, (8) maternal admission to the intensive care unit (ICU), (9) pre-term and very preterm delivery (PTD < 37 weeks and VPTD < 32 weeks).
The following neonatal outcomes were considered: (10) shoulder dystocia, (11) small for gestational age (SGA) infants, defined as birthweight < 10th percentile for gestational age, (12) large for gestational (LGA) infants, defined as birthweight > 90th percentile for gestational age, (13) neonatal distress, defined as APGAR score < 7 at 5 min after birth, (14) congenital malformations, (15) admission to the neonatal intensive care unit (NICU), (16) stillbirth, defined as an APGAR score =0 five min after birth.
Statistics
The statistical approach aimed at providing a direct estimation of risks for each outcome of interest, stratified for each unit of BMI, as described by Shummers et al. [23].
Univariable analysis
The potential relationship between pre-pregnancy BMI and maternal and neonatal outcomes was explored in a crude analysis according to WHO-BMI category. For each outcome of interest, a χ2-test and a likelihood ratio test comparing a null logistic regression model with a model with a single quantitative variable (pre-pregnancy BMI) were performed.
Multivariable analysis
Logistic regression models were used to estimate the effect of increasing BMI on the most relevant outcomes, adjusting for maternal age and parity.
After performing each logistic regression analysis, the predicted risks and 95% confidence interval (95% CI) [risk=odds/(1 + odds)] at each BMI level were estimated. For those outcomes with a significant association with BMI adjusted risks (and 95% CI) were presented at the population average values of confounders (maternal age and parity) to represent the average risk of each outcome. Because of the small number of cases, the patients with a BMI > 40 were grouped together. For all analyses, a p value < 0.005 was considered significant.
Ethical approval
The study was carried out following the ethical rules of the hospital. Informed consent for using clinical data was given to each patient during hospital intake, before data were entered in a dedicated database. All data were treated as confidential.
Results
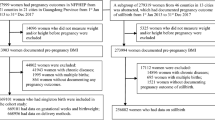
Out of 30,853 pregnancies, 2368 were excluded for incomplete follow-up. Pregnancies affected by pre-existing diabetes and/or hypertension (1.3% and 0.9% respectively) were also excluded. Therefore, the final study population included 27,807 pregnancies. Most pregnancies (75.8%) occurred among normal-weight women, whereas 16.7% occurred among overweight women, and 7.5% among obese women (5.4% in class 1, 1.7% in class 2 and 0.4% in class 3 obesity).
The demographic and clinical characteristics of the women included in the study, according to BMI category, are presented in Table 1.
Univariable analysis
The absolute risks of both maternal and neonatal complications are reported for each BMI subgroup in Table 2.
In the crude analysis, increasing BMI categories were associated with an increased proportion of pregnancies complicated by PE, GDM, cesarean section after failure of induction, elective cesarean section, emergency cesarean section, maternal ICU, PTD, VPTD and PPH. Shoulder dystocia and maternal mortality or severe maternal morbidity did not differ between BMI categories.
For what concerns neonatal outcomes, increasing BMI categories corresponded to increasing rates of LGA infants, APGAR score at 5′ < 7 and NICU admissions. In our population, shoulder dystocia and stillbirth incidences did not differ according to pre-pregnancy BMI.
Multivariable analysis
All the outcomes were directly associated with BMI and maternal age. PE, maternal ICU, APGAR 5′ < 7, NICU admission and emergency cesarean section were more frequently detected in nulliparae, while GDM, PPH, LGA, VPTD and PTD were more frequent in multiparous.
Table 3 quantifies the risks, adjusted for age and parity, of adverse maternal and perinatal outcomes according to pre-pregnancy BMI (with 95% CI). The following pregnancy outcomes were analyzed: PE, GDM, maternal ICU, PPH, LGA infants, APGAR 5′ < 7 and neonatal NICU admission. A sub-analysis of 20,206 pregnancies, not including elective cesarean sections, VPTD, PTD and emergency cesarean section, showed significant correlations with pre-pregnancy BMI.
Figure 1 shows the mean proportions of risk reduction (expressed as percentage) for each adverse outcome corresponding to a 10% decrease in pre-pregnancy BMI. A 10% difference in pre-pregnancy BMI was associated with reduction of at least 15% of GDM, PE, maternal ICU admission, LGA, APGAR 5′ < 6 and NICU admission risk. GDM and PE, in particular, resulted in the highest difference being almost 30%, instead PPH resulted in a 10% risk reduction.
Mean different percentage probability estimated for each outcome of interest according to a 10% BMI reduction. GDM gestational diabetes mellitus, PE preeclampsia, ICU maternal admission in intensive care unit, LGA large for gestational age, APGAR 5′ < 7 APGAR at 5′ min < 7, NICU neonatal intensive care unit admission, PPH post-partum hemorrhage (> 1000 mL), VPTD very preterm delivery (< 32 weeks), PTD preterm delivery (< 37 weeks), emergency CS emergency cesarean section
Larger differences in BMI (20–25%) corresponded approximately to 10% reduction in the risk of VPTD, PTD and emergency cesarean section.
Table 4 reports the number needed to “treat” assuming as “treatment” a 10% reduction in pre-pregnancy BMI. As an example, 35 and 51 women should be “treated” with a 10% reduction in BMI, to prevent one case of PE, considering basal BMI of 40 and 36, respectively.
Discussion
This study shows that overweight and obesity affects respectively 16.7% and 7.5% of women who gave birth at Sant’Anna Hospital, a tertiary care University hospital in Turin. These data in pregnant women are in line with the prevalence of female overweight and obesity in the Piedmont Region, as reported by the Italian Statistic Agency (ISTAT) [24].
As far as we know, this is the first report in a sample of Italian population regarding the association between pre-pregnancy BMI and the risk of several negative obstetric outcomes, including PE, GDM, LGA infants, emergency cesarean delivery, maternal ICU admission, NICU admission, PTD and VPTD. In general, our findings are consistent with those reported in previous studies, carried out on other populations [7, 11, 25, 26].
According to the crude analysis performed in BMI subcategories, VPTD and PPH seemed to have a less significant association with BMI in this large series of patients. As a matter of fact, the literature is not univocal on this aspect [13, 23]. Whereas some studies [27,28,29] found an association between obesity and PPH, mainly attributable to atonic uterus, others [23, 30, 31] failed to find any effect of BMI. Among the potential explanation for such conflicting results, different criteria for PPH definition (either blood loss > 500 mL or > 1000 mL, and/or PPH requiring intervention) could be advocated.
In contrast with many previous reports [31, 32], but in line with several studies [33, 34], we found a constant rate of shoulder dystocia across the BMI sub-groups. Several explanations could exist for these discordance, including the increased systematic use of ultrasonography to detect macrosomia and interventions, such as preterm induction of labor or cesarean delivery. The management of pregnancy in a tertiary referral hospital, as in this case, could indeed explain the low incidence of this severe complication, hence the lack of any correlation with BMI.
The present investigation was prompted by the study by Schummers et al. performed on a North American population. The Italian population is quite different from North American and Canadian in the prevalence of overweight and obesity [3, 35,36,37]. Furthermore, large differences exist in dietary habits and diet composition; in particular, compared with the American/North European diet, the Mediterranean dietary pattern is lower in red meats and saturated fatty acid and higher in olive oil and seafood, with high amounts of monounsaturated and polyunsaturated fatty acids [38].
We are aware that counseling about the clinical meaningful reduction is hard and challenging outcome and we believe, in accordance with Shummers, that incidence expressed as “risk” instead of “odds ratio” could be very useful for clinical counseling aimed to quantify benefits on health, deriving from weight loss. Additionally, we also reported a simple bar chart (Fig. 1) that describes the mean percentage reduction for each outcome considered for a 10% restriction of pre-pregnancy BMI. GMD and PE resulted in the highest reduction of their incidence followed by ICU admission and LGA. For these diseases, a 10% in BMI reduction would result in at least a 15–20% reduction of disease. For VPTD and emergency cesarean section, to reach a similar result, a BMI shifting of at least 15–20% is instead necessary.
Finally, as secondary results, we also provide information about the number of people to “treat” to prevent one case of the diseases here analyzed, at least in the Italian population.
Limit of the study
Since this is not a longitudinal study we cannot conclude that the inter-woman difference in BMI is clinically equivalent to the intra-woman BMI loss. No data about race have been available even if the racial composition of North Italy is 75.80% Caucasian with a much lower rate of black women in respect with UK and US population.
The pre-pregnancy BMI estimation is quite often self-managed and not performed by a healthcare professional, therefore, a bias of under or overestimation of unknown degree is expected. For women that culturally adopt pounds and feet and inches units, a higher possible degree of mistakes is also possible in the conversion to kilograms and centimeters.
Obese and overweight women investigated in this study were rather multiparous than nulliparous: previous pregnancies, in fact, may cause a weight-gain and specifically, women who were overweight and obese before pregnancy, are two to six times more likely to exceed the weight gain recommendations during pregnancy [39, 40]. Higher weight gain increases the risk of post-partum weight retention even in normal pre-pregnancy BMI women [41].
Strength of the study
This cohort study offers a tool for the physician to counsel more properly obese patients. The risks estimation is much more clinically useful than odds ratio and the use of quantitative BMI improves the clinical applicability. Moreover, information about the “number to treat” and the rate of risk reduction at a given BMI loss is a further message of encouragement for patients who want to deal with a pregnancy.
In conclusion, our study provides, for the first time, in a sample of Italian population, reference values of quantitative pre-pregnancy BMI and risks for the most common maternal and neonatal outcomes, focusing the attention on the importance of maternal weight loss in preventing pathological outcomes of the pregnancy and perinatal age. We acknowledge that similar recent studies with same or higher number of cases have been performed in other Countries including Puerto Rico [42] France [43] and China [44].
References
American Medical Association. American Medical Association House of Delegates Resolution: 420 (A-13)
World Health Organization (2015) World health statistics. WHO, Geneva, pp 101–111
Adilson M, Miguel P (2017) Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur J Public Health
Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD (2007) Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG 114:187–194
LaCoursiere DY, Bloebaum L, Duncan JD, Varner MW (2005) Population-based trends and correlates of maternal overweight and obesity, Utah 1991–2001. Am J Obstet Gynecol 192:832
Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491
McDonald SD, Han Z, Mulla S, Beyene J, Knowledge Synthesis Group (2010) Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 341:c3428.
https://www.epicentro.iss.it/problemi/obesita/EpidItalia.asp. Accessed 13 Aug 2018
https://www.istat.it/it/files//2016/07/Fattori-di-rischio_salute_def.pdf. Accessed 13 Aug 2018
Begum KS, Sachchithanantham K, De Somsubhra S (2011) Maternal obesity and pregnancy outcome. Clin Exp Obstet Gynecol 38:14–20
Robinson HE, O'Connell CM, Joseph KS, McLeod NL (2005) Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol 106:1357
Lim CC, Mahmood T (2015) Obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol 29(3):309–319
Cnattingius S, Bergström R, Lipworth L, Kramer MS (1998) Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 338(3):147–152
Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikström AK, Granath F (2013) Maternal obesity and risk of preterm delivery. JAMA 309(22):2362–2370
Persson M, Johansson S, Villamor E, Cnattingius S (2014) Maternal overweight and obesity and risks of severe birth-asphyxia-related complications in term infants: a population-based cohort study in Sweden. PLoS Med 11(5):e1001648
Stothard KJ, Tennant PW, Bell R, Rankin J (2009) Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 301(6):636–650
Yajnik CS (2014) Transmission of obesity–adiposity and related disorders from the mother to the baby. Ann Nutr Metab 64(Suppl 1):8–17
(1998) Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: the evidence report, NIH Publication No. 98-4083 September 1998
Dodd JM, Turnbull D, et al (2014) Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 348:g1285
Briley AL, et al (2014) A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 14:74
https://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed 13 Aug 2018
(2004) International Classification of Diseases 10th Revision. WHO, Geneva
Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP (2015) Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol 125(1):133–143
Istat, Report: Fattori di rischio per la salute: fumo, obesità, alcol e sedentarietà, Anno 2015, 26 luglio 2016 Sovrappeso e obesità in Piemonte, I dati del sistema di sorveglianza PASSI 2010–2013
Gross T, Sokol RJ, King KC (1980) Obesity in pregnancy: risks and outcome. Obstet Gynecol 56(4):446
Wolfe KB, Rossi RA, Warshak CR (2011) The effect of maternal obesity on the rate of failed induction of labor. Am J Obstet Gynecol 205(2):128.e1–7
Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B (2009) Effects of onset of labor and mode of delivery on severe postpartum hemorrhage. Am J Obstet Gynecol 201:273.e1–9.
Blomberg M (2011) Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol 118:561
Gollop ND, Childs CA, Coupe B, MacFarlane S, Burrell J, Kumar B (2014) Body weight, body image and primary postpartum haemorrhage: a review of the literature. J Obstet Gynaecol 34(5):373–382
Butwick AJ, Abreo A, Bateman BT, Lee HC, El-Sayed YY, Stephansson O, Flood P (2018) Effect of maternal body mass index on postpartum hemorrhage. Anesthesiology 128(4):774–783
Ovesen P, Rasmussen S, Kesmodel U (2011) Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 118(2 Pt 1):305–312
Jensen DM, Damm P, Sorensen B, Molsted-Pedersen L, Westergaard JG, Ovesen P et al (2003) Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. Am J Obstet Gynecol 189:239–244
Zhang C, Wu Y, Li S, Zhang D (2018) Maternal prepregnancy obesity and the risk of shoulder dystocia: a meta-analysis. BJOG 125:407–413
Harper LM, Renth A, Todd Cade W (2014) Impact of obesity on maternal and neonatal outcomes in insulin-resistant pregnancy. Am J Perinatol 31(05):383–388
Eurostat, Obesity rate by body mass index (BMI). https://ec.europa.eu/eurostat/web/products-datasets/-/sdg_02_10. Accessed 13 Aug 2018
www.worldobesity.org/data/. Accessed 13 Aug 2018
Gallus S, Odone A, Lugo A (2013) Overweight and obesity prevalence and determinants in Italy: an update to 2010. Eur J Nutr 52:677–685
Farmer B, Larson BT, Fulgoni VL 3rd, Rainville AJ, Liepa GU (2011) A vegetarian dietary pattern as a nutrient-dense approach to weight management: an analysis of the National Health and Nutrition Examination Survey 1999–2004. J Am Diet Assoc 111:819–827
Brawarsky P, Stotland NE (2005) Pre-pregnancy and pregnancy-related factors and the risk of excessive or inadequate gestational weight gain. Int J Gynaecol Obstet 91(2):125–131
Wells CS, Schwalberg R, Noonan G, Gabor V (2006) Factors influencing inadequate and excessive weight gain in pregnancy: Colorado, 2000–2002. Matern Child Health J 10(1):55–62
Artal R, Lockwood CJ, Brown HL (2010) Weight gain recommendations in pregnancy and the obesity epidemic. Obstet Gynecol 115(1):152–155
Eick SM, Welton M, Cordero JF (2019) Relationship between prepregnancy overweight, obesity, and preterm birth in Puerto Rico. Matern Child Health J (epub ahead of print)
Siddiqui A, Azria E, Howell EA, Deneux-Tharaux C, EPIMOMS Study Group (2018) Associations between maternal obesity and severe maternal morbidity: findings from the French EPIMOMS population-based study. Paediatr Perinat Epidemiol (epub ahead of print)
Shen J, Zhang Z, Chen K, Lu M, Qian Q, Liu P, Gao Q, Zhang C (2018) Prepregnancy obesity status and risks on pregnancy outcomes in Shanghai: a prospective cohort study. Medicine (Baltimore) 97:e12670
Funding
The authors received no specific funding for this study.
Author information
Authors and Affiliations
Contributions
BM: protocol/project development, data collection. VF: protocol/project development, data collection. CG: protocol/project development, data collection, manuscript writing. RA: protocol/project development, manuscript writing. GG: data collection, manuscript writing, data analysis. AL: data collection, data analysis, manuscript writing. AR: Protocol/project development, data collection, manuscript writing. CP: protocol/project development, data collection. EB: data collection, manuscript writing. AY: data collection, manuscript writing. TT: protocol/project development, manuscript writing. AF: protocol/project development, data collection, manuscript writing, data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The committee for this study is Dr. Bianca Masturzo.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masturzo, B., Franzè, V., Germano, C. et al. Risk of adverse pregnancy outcomes by pre-pregnancy Body Mass Index among Italian population: a retrospective population-based cohort study on 27,807 deliveries. Arch Gynecol Obstet 299, 983–991 (2019). https://doi.org/10.1007/s00404-019-05093-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05093-0