Abstract
Background/aims
Preeclampsia is a pregnancy-specific disease with the increased risk of maternal morbidity and mortality. It is characterised by placental vascular dysfunction. Despite the numerous studies on preeclampsia, studies evaluating proliferation of villous trophoblasts in preeclamptic placentas are limited. Ki67 is a proliferation marker that expresses in the nuclei of proliferating cells. In this study, we examined the proliferation of villous trophoblasts in placentas of preeclamptic patients by using Ki67 and compared it with placentas of normal pregnant patients.
Material and methods
The current study is a prospective one, including 15 placentas from preeclamptic patients and 14 placentas from normal pregnancies as controls. For detection of proliferation in villous trophoblasts, Ki67 was used.
Results
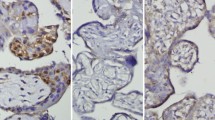
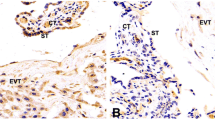
The Ki67 index was 11.48±1.67% in normal patients and 15.53±2.28% in preeclamptic patients. There was a difference in Ki67 index between the two groups (p < 0.001).
Conclusion
Our results support the opinion that trophoblasts undergo regeneration hyperplasia as a result of injuries arising on the villous surface in preeclampsia. Proliferation of trophoblasts may contribute the development of preeclampsia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia is a pregnancy-specific disease that complicates 2–7 % of all pregnancies [1]. It remains a major cause of maternal and perinatal morbidity and mortality [2]. Preeclampsia is a complex syndrome potentially involving all organs [3]. It is a pregnancy pathology caused by the deficient placental development, which has a complex mechanism [4]. Defects in the formation and development of placenta may cause negative results for mother and foetus, such as preeclampsia.
In normal development of the placenta, extravillous trophoblasts invade the muscular layer of spiral arteries [5]. This remodelling of spiral arteries leads to their transformation into uteroplacental arteries, characterised by low resistance and high capacity. For the first time, Browsens and colleagues showed in placental bed biopsies that physiological changes in spiral arteries did not occur in preeclampsia [6]. In preeclampsia, incomplete invasion of trophoblasts into the maternal spiral arteries, proliferation of non-invading trophoblasts with multiple nuclei in the interstitial space, thick-walled spiral arteries, thrombosis, and atheromatous plaques in the lumen are seen [7].
Because of defects in the remodelling of spiral arteries, hypoxia may occur in the extravillous space. Moreover, ischaemia–reperfusion damage may lead to oxidative stress in placenta, the release of free radicals, defective secretion of cytokines and growth factors, and the activation of leukocytes and macrophages, which result in preeclampsia [8–10].
Although the aetiology of preeclampsia remains incompletely understood, there are some theories. In recent years, the number of trophoblasts in the placenta has been proposed as a trigger mechanism for preeclampsia. However, there are limited studies on the proliferation of villous trophoblasts in this syndrome [11].
Ki67, which is seen in the nucleus of proliferating cells, is a marker for proliferation. Ki67 shows a good correlation with the number of mitotic cells. It is also known as the antigen identified by monoclonal antibody and is usually used for mitotic index and tumour grading [12].
In this study, we examined the proliferation in villous trophoblasts in term placentas of preeclamptic patients by using the proliferation marker Ki-67 and compared it with the term placentas of normal pregnant controls.
Materials and methods
15 preeclamptic patients in third trimester and 14 normotensive, uncomplicated pregnant controls who admitted to Tepecik Training and Research Hospital between 2007 and 2008 were included and trophoblastic tissues obtained from the placentas of these patients were examined in this study. Gestational age was established on the basis of menstrual dates and confirmed by first trimester ultrasonography.
All participants were informed about the survey and freely signed and dated the consent form. The protocol was approved by the Ethics Committee of the hospital and conducted in accordance with the Declaration of Helsinki.
Diagnosis of preeclampsia was made according to the American College of Obstetricians and Gynecologists (ACOG) guidelines [13]. These guidelines define preeclampsia as sustained pregnancy-induced hypertension with proteinuria. Hypertension was defined as sustained blood pressure readings of ≥140/90 mmHg (with reading taking place >6 h apart). ACOG defines proteinuria as urine protein concentrations of ≥300 mg/day (or 1+ on a urine dipstick) on two or more random specimens collected >4 h apart. As it has recently been suggested that early-onset (<34 completed weeks gestation) and late-onset PE (>34 completed weeks gestation) may have different etiologies [14], only late-onset preeclamptic women were enrolled in this study. Preeclamptic women did not take any anti-hypertensive therapy. Ethnical origins were similar among the study group.
Exclusion criteria for all subjects were tobacco use, multiple pregnancies, preexisting maternal chronic medical problems (such as maternal heart disease, chronic hypertension, diabetes mellitus and renal disease), chromosomal or suspected ultrasound foetal abnormalities, use of anti-hypertensive medication. Patients were followed until delivery to verify the foetal, neonatal and maternal outcomes. All patients were delivered by elective caesarean section(C/S). The indications were prior C/S, malpresentation, dystocia and prior myomectomy for caesarean delivery.
Of all the preeclamptic group, HELLP syndrome was seen in one patient and intrauterine growth retardation (IUGR) was seen in four patients. HELLP syndrome was diagnosed by the presence of hemolysis, elevated liver enzymes and low platelet counts with severe preeclampsia. IUGR was defined as an estimated intrauterine weight below the fifth centile after gestational age had been confirmed by an early pregnancy ultrasound and was confirmed after delivery.
Term placentas from preeclamptic women (n = 15) and from healthy controls (n = 14) were obtained immediately following caesarean deliveries. Samples of trophoblastic tissue were obtained from the peripheral and central parts of the placentas. Sections were deparaffinised on slides with lysin. Then they were kept in xylene for 10 min, in 100 % alcohol for 5 min, and washed with distilled water. Slides tamponated in 10 % citrate solution were kept in a microwave oven for 30 min. Then they were kept in H2O2 for 20 min. After the slides were washed with distilled water, they were kept in phosphate buffered saline (PBS) solution for 5 min. Then the slides were incubated with Ki-67 antibody for 20 min. They were washed with PBS solution for 5 min, then incubated with biotinylated secondary antibody for 20 min and washed in PBS solution for 5 min. Peroxidase- conjugated antibody was applied for 20 min. The slides were again washed in PBS solution for 5 min and kept in 3,3′-diaminobenzidine (DAB) solution for 15 min. Then they were washed with distilled water. The slides were stained with haematoxylin for 10 s and then dried and covered.
For each section, by using a projection microscope, at 400× magnification, Ki-67 positive and negative trophoblasts were countered in systematically randomised areas. Ki-67 index was expressed as a percentage of the number of Ki-67 positive trophoblasts against the number of total trophoblasts [15–17].
A power calculation was performed according to the preliminary results (results of first 5 samples from each group), Ki-67 index was found approximately 11.72 in control group and it was approximately 14.16 in preeclamptic group. Accepting power 0.80, it was calculated that 10 patients in each group would be required to detect a significant difference. The statistical package for social sciences for Windows 10.0 package (SPSS Inc., Chicago, IL) was used for statistical analysis of all data. Student’s t test, Mann–Whitney U test and Fischer’s exact test were used for comparison of qualitative data. Data were presented as mean ± standard deviation (SD) and statistical significance was set at p < 0.05.
Results
The clinical characteristics of patients are presented in Table 1. The mean maternal age in the preeclamptic group was 27.8 ± 5.04 and 26.42 ± 5.95 years in the control group. There was no significant difference in maternal age between the groups (p > 0.05). We did not find any difference between two groups for parity and body mass index (BMI) (p > 0.05). The mean gestational age was 37.33 ± 2.46 weeks for preeclamptic patients and 39.64 ± 1.08 weeks for control patients. There was no significant difference in gestational age between the groups (p > 0.05). The mean birthweight in preeclamptic group (2,990.00 ± 887.65 g) showed statistically significant difference compared to the mean birthweight in control group (3,525.00 ± 380.45 g) (p < 0.03).
IUGR (4/15; 23.7 %), ablatio placenta (2/15; 13.3 %), oligohydramnios (5/15; 33.4 %) and HELLP syndrome (1/15; 6.68 %) were seen in preeclamptic group. On the other hand, any negative obstetrical outcome was observed in the normal controls.
Levels of Ki-67, seen in villous trophoblasts of the placentae of normal and preeclamptic patients, are shown in Fig. 1. The Ki-67 index was 11.48 ± 1.67 % in normal patients, while it was 15.53 ± 2.28 % in preeclamptic patients. There was a difference in Ki67 index between the two groups (p < 0.001) (Fig. 2).
Discussion
In our study, we demonstrated an increase in villous trophoblast proliferation in preeclamptic term placentas compared to normal term placentas. Normal placental development depends on the cell proliferation, differentiation and invasion in a proper and simultaneous manner [18]. Information on factors that control these events is limited. It is known that preeclamptic placentas do not undergo normal development. However, the aetiology of preeclampsia is still unknown. Therefore, to understand the role of changes in cell cycle in pathologic human placentas will be extremely useful in this regard.
Currently, in both normal and complicated pregnancies, the mechanism that regulates the proliferation of villous cytotrophoblasts is not known. Villous trophoblasts are maintained until term [19]. However, it is controversial if the amount of these cells changes or not during pregnancy. By using stereological methods, some studies reported that the number of these cells increased constantly until term [20]. However, results on the number of cytotrophoblasts are controversial. It was observed in some studies (in which the proliferation markers such as Ki-67 and proliferating cell nuclear antigen (PCNA) were used) that the proliferative index of these cells increased in the first trimester and was reduced during pregnancy, till the last trimester [21, 22]. This decrease is thought to be relative, as a result of the rapid growth of villi and separation of cells during placental maturation [19].
In preeclampsia, an injury to the syncytiotrophoblast seems to lead to a repair hyperplasia of the cytotrophoblast [23]. This is not specific for preeclampsia. It can also be seen in cases where maternal blood flow and oxygen pressure are reduced, such as diabetes mellitus, maternal anaemia, and hypertensive diseases [19]. While tension at the maternal site of the placenta increases to supply nutrition for foetus, increased proliferation of trophoblasts seems to be achieved at the foetal site of the placenta to use this nutrition and oxygen effectively. PCNA, Kİ67 staining intensities were found increased in diabetic placentas compared to normal placentas [24]. But, decreased Ki67 index was reported in all regions of IUGR placentas [25]. On the other hand, Jesckhe et al. [26] researched Ki67 in 27 placentae (8 of preeclampsia, eight of normal, six of IUGR complicated pregnancy, and five of HELLP syndrome) and reported that there was no significant difference in Ki67 expression in preeclampsia and normal pregnancy, but they observed an increased Ki67 index in placentas of HELLP. Similar results were given by Thomas et al. [27] and Prusac et al. [28].
In literature, there are limited studies in which the detection of proliferation markers by immunohistochemical methods for preeclampsia has been performed. Unek and colleagues have recently showed that Ki67 staining intensities significantly increased in villous parts of placentas in preeclamptic patients [29]. Arnholdt and colleagues compared Ki67 and bromodeoxyuridine indexes between preeclamptic patients and normal pregnant controls [20]. They only observed an increased Ki67 index in villous trophoblasts of preeclamptic patients. Similarly, we demonstrated an increased number of villous cytotrophoblasts in preeclampsia than in normal pregnancy. Our data are consistent with the findings of other researchers [23, 30–32]. However, Kaltenbach and colleagues did not observe an increase in cell proliferation [33].
In recent years, it has been suggested that there is a threat for preeclampsia in all pregnancies, depending on the amount of trophoblasts, nature of the maternal immune response, and underlying renovascular sensitivity, and especially an adequate amount of trophoblast antigens may trigger an immune response, resulting in endothelial injury [11]. Therefore, an increased number of trophoblasts may be important for the development of the syndrome. That the number and proliferative activity of trophoblasts are higher in molar pregnancies, resulting in preeclampsia, compared with normal pregnancies supports this hypothesis. The increased proliferation index of villous cytotrophoblastic cells in preeclampsia is thought to be a result of regeneration hyperplasia which occurs because of the injury in syncytiotrophoblasts. Our findings confirmed that the proliferation of trophoblasts in preeclamptic placenta is indeed increased.
The present study has a number of limitations. First, we only examined small numbers of term placentas from late-onset preeclamptic patients. Placentas obtained from early-onset preeclamptic pregnants should be included in the study. Second, we did not divide preeclamptic patients into groups due to severity of the disease to determine the effect of such factors on Ki67 index.
However, the present study also has a few strengths. Although, our sample size seems to be small, it exceeds the size of such published researches on this topic in number. Besides, we prospectively examined proliferation of villous trophoblast in preeclamptic placentas compared to normal placentas.
In conclusion, our results support the opinion that trophoblasts undergo regeneration hyperplasia as a result of injuries arising on the villous surface in preeclampsia. However, it is not possible to relate the increased Ki67 index of villous trophoblasts in the last trimester in preeclampsia with one cause. To elucidate the etiopathogenesis of preeclampsia, we need further researches with large number of samples concerning other factors that are necessary in proliferation of villous trophoblasts.
References
Sibai BM (2005) Magnesium sulfate prophylaxis in preeclampsia: evidence from randomized trials. Clin Obstet Gynecol 48(2):478–488
Mohaupt M (2007) Molecular aspects of preeclampsia. Mol Asp Med 28(2):169–191
Higgins JR, de Swiet M (2001) Blood pressure measurement and classification in pregnancy. Lancet 357(9250):131–135
Redman CWG, Sargent IL (2001) The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil 29(7–8):518–522
Granger JP, Joey P, Alexander BT, Llinas MT, Benett WA, Khalil RA (2002) Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 9(3):147–160
Brosenes IA, Robertson WB, Dixon HG (1972) The role of spiral arteries in the pathogenesis of preeclampsia. In: Wynn RM (ed) Obstetrics and gynecology annual. Appleton-Century-Crofts, New York, pp 177–191
Madazlı R, Budak E, Calay Z, Aksu MF (2000) Correlation between placental bed biopsy findings, vascular cell adhesion molecule (VCAM-1) and fibronectin levels in preeclampsia. BJOG 107(4):514–518
Madazlı R, Benian A, Aydın S, Uzun S, Tolun N (2002) The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in preeclampsia. J Obstet Gynecol 22(5):477–480
Madazlı R, Somunkiran A, Calay Z, Ilvan S, Aksu MF (2003) Histomorphology of the placenta and the placental bed of growth restricted foetuses and correlation with the Doppler velocimetries of the uterine and umbilical arteries. Placenta 24(5):510–516
Hung TH, Burton GJ (2006) Hypoxia-reoxygenation; a possible mechanism for placental oxidative stress in preeclampsia. Tawain J Obstet Gynecol 45(3):189–200
Raymond W, Redline MD, Patterson BS (1995) Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol 26(6):594–600
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182(3):311–322
ACOG Committe on Obstetric Practice, ACOG practice bulletin (2002) Diagnosis and management of pre-eclampsia and eclampsia. In J Gynaecol Obstet 77(1):67–75
Raymond D, Peterson E (2011) A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv 66(8):497–506
Benirschke K, Kaufmann P (1995) Basic structure of the villous tree. In: Kaufmann P (ed) Pathology of the placenta, 3rd edn. Springer Verlag, New York, pp 71–79
Simpson RA, Mayhew TM, Barnes PR (1992) From 13 weeks to term, the trophoblast of human placenta grows by continuous recruitment of new proliferative unit: a study of nuclear number using dissector. Placenta 13(5):501–502
Mochizuki M, Maruo T, Samoto T, Ishihara N (1998) Biology of human trophoblast. Int J Gynaecol Obstet 60(Suppl 1):S21–S28
Genbacev O, McMaster MT, Fischer SJ (2000) A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am J Pathol 157:1337–1351
Suresh UR, Hale RJ, Fox H, Buckley CH (1993) Use of proliferation cell nuclear antigen immunoreactivity for distinguishing hydropic abortions from partial hydatidiform moles. J Clin Pathol 46(1):48–50
Arnholdt H, Meisel F, Fandrey K, Löhrs U (1991) Proliferation of villous trophoblast of the human placenta in normal and abnormal pregnancies. Virchow Archiv B Cell Pathology 60(6):365–372
Soma H, Yoshida K, Mukaida T, Tabuchi Y (1982) Morphologic changes in the hypertensive placenta. Contr Gynecol Obstet 9:58–75
Teadstale F (1985) Histomorphometry of the human placenta in maternal preeclampsia. Am J Obstet Gynecol 152(1):25–31
Hustin J, Foidart JM, Lambotte R (1984) Cellular proliferation in villi of normal and pathological pregnancies. Gynecol Obstet Invest 17(1):1–9
Unek G, Ozmen A, Mendilcioglu I, Simsek M, Korgun ET (2014) Immunohistochemical distribution of cell cycle proteins p27, p57, cyclin D3, PCNA and Ki67 in normal and diabetic human placentas. J Mol Hist 45:21–34
Unek G, Ozmen A, Ozekinci M, Sakinci M, Korgun ET (2014) Immunolocalization of cell cycle proteins (p57, p27, cyclin D3, PCNA and Ki67) in intrauterine growth retardation (IUGR) and normal human term placentas. Acta Histochem 116:493–502
Jeschke U, Schiessl B, Mylonas I, Kunze S, Kuhn C, Schulze S et al (2006) Expression of the proliferation marker Ki-67 and p53 tumor protein in trophoblastic tissue of preeclamptic, HELLP, and intrauterine growth-restricted pregnancies. Int J Gynecolo Pathol 25(4):354–360
Thomas SZ, Prusac IK, Roje D, Tadin I (2011) Trophoblast apoptosis in placentae from pregnancies complicated by preeclampsia. Gynecol Obstet Invest 71(4):250–255
Prusac IK (2011) Zekic Tomas S, Roje D. Apoptosis, proliferation and Fas ligand expression in placental trophoblast from pregnancies complicated by HELLP syndrome or preeclampsia. Acta Obstet Gynecol Scand 90(10):1157–1163
Unek G, Oxmen A, Mendilcioglu I, Simsek M, Korgun ET (2014) The expression of cell cycle related proteins PCNA, Ki67, p27 and p57 in normal and preeclamptic human placentas. Tissue Cell 46:198–205
Elpek GÜ, Karaveli Ş, Keleş N (2000) Preeklampsili olguların term plasentalarında villöz trofoblast proliferasyonunun incelenmesi (Evaluation of proliferation of villous trophoblasts in term placentae of preeclamptic patients). Turkish J Pathol 16(1–2):10–12
Lyall F, Myatt L (2002) The role of placenta in pre-eclampsia-a workshop report. Placenta 23:142–145
Redline RW, Patterson P (1995) Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol 26:594–600
Kaltenbach FJ, Fettig O, Krieger ML (1974) Autoradiographische untersuchungen über das proliferationsverhalten der menschlichen placenta unter normalen und pathologischen bedingungen [Radioautographic observations on DNA-synthesis in the human placenta under normal and pathologic conditions (author’s transl). Arch Gynakol 216(4):369–386
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaya, B., Nayki, U., Nayki, C. et al. Proliferation of trophoblasts and Ki67 expression in preeclampsia. Arch Gynecol Obstet 291, 1041–1046 (2015). https://doi.org/10.1007/s00404-014-3538-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3538-4