Abstract
Purpose
To determine the accuracy of multi-detector CT (MDCT) compared with the surgical findings, such as peritoneal seeding and metastatic lymph nodes, in ovarian cancer patients.
Methods
Fifty-seven FIGO stage IA–IV ovarian cancer patients, who underwent MDCT before primary surgery, were included in this study. Two radiologists evaluated the following imaging findings in consensus: the presence of nodular, plaque-like or infiltrative soft-tissue lesions in peritoneal fat or on the serosal surface; presence of ascites; parietal peritoneal thickening or enhancement; and small bowel wall thickening or distortion. We also evaluated the presence of lymph node metastases. To allow region-specific comparisons, the peritoneal cavity was divided into 13 regions and retroperitoneal lymph nodes were divided into 3 regions. Descriptive statistical data were thus obtained.
Results
The MDCT sensitivity, specificity, positive predictive values, and negative predictive values were 45, 72, 46, and 72%, respectively, for detecting peritoneal seeding and 21, 90, 52, and 69%, respectively, for detecting lymph node metastasis.
Conclusions
MDCT is moderately accurate for detecting peritoneal metastasis and lymph node metastasis in ovarian cancer patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is a fifth most common cause of cancer death and a leading cause of death from gynecologic malignancy in women worldwide. Although the prognosis of FIGO stage I disease can indicate a better survival rate of 80–90%, currently 60–65% of patients present with stages III or IV disease at the time of diagnosis. This is the main reason that ovarian cancer is one of the most lethal cancers in female. For these advanced ovarian cancer patients, the optimal cytoreduction of tumor bulk to as less as possible and platinum- and taxane-based chemotherapy is the standard treatment. In this patient group, the amount of residual disease is the most important prognostic factor [1]. The presence of tumor in a specific location, such as suprarenal lymph node, porta hepatis, liver parenchymal metastasis, and small bowel mesenteric involvement can prohibit optimal cytoreduction in ovarian cancer patients [2]. For this reason, delineation of the precise location of the peritoneal implant is crucial in the management of advanced ovarian cancer patients.
CT has been extensively used in the preoperative diagnosis and postoperative surveillance of ovarian cancer patients. Several investigations have been performed to evaluate the diagnostic performance of CT for detecting peritoneal seeding in ovarian cancer patients [3–7]. In these studies, the reported sensitivity and specificity were 63–93 and 73–100%, respectively. However, these studies reported patient-based CT diagnostic performance for the detection of peritoneal seeding, and as far as we know there have been no studies reporting the region-based diagnostic performance of CT for detecting peritoneal seeding in ovarian cancer patients.
The aim of our study is to prospectively determine the diagnostic performance, i.e. sensitivity, specificity, positive predictive value, and negative predictive value of CT compared with the surgical findings for detecting peritoneal seeding and metastatic lymph nodes in primary ovarian cancer patients.
Materials and methods
Study population
This study used a prospectively collected database. It included 57 consecutive patients with histologically confirmed FIGO stages IA–IV ovarian cancer as determined by surgery; 6, 5, 38, and 8 patients were identified and comprised the current FIGO stages I, II, III, and IV groups, respectively. These patients were recruited between July 2006 and January 2008, were between 30 and 72 years of age (mean age 53.1 years), had no contraindications to the surgical procedure, and had an Eastern Cooperative Oncology Group performance state of 0–2 before surgery. Their serum CA-125 levels ranged from 12.3 to 8510 U/mL (mean 794.8 U/mL). The histology of our study patients’ ovarian carcinoma is as follows: serous carcinoma (n = 38); mucinous carcinoma (n = 12); endometrioid carcinoma (n = 2); clear cell carcinoma (n = 2); transitional cell carcinoma (n = 1); and other (n = 2). Architecture grades 1, 2, and 3 tumors existed in 3 (10%), 14 (47%), and 13 patients (43%), respectively. All patients were classified into two groups according to the whether or not they had undergone neoadjuvant chemotherapy. Twenty-seven (47.4%) had undergone neoadjuvant chemotherapy before surgery and 30 patients (52.6%) had not. No statistical difference of the clinical variables was observed between the neoadjuvant group and the non-neoadjuvant patients group.
CT technique
All CT data were obtained using a four-channel MDCT scanner (Mx 8000, Marconi Medical System, Israel). Preoperative CT scans were obtained at a single institution an average of 17.6 days before surgery (range 2–44 days). IV contrast material (Iopromide, Ultravist 300, Schering, Berlin) was administered via an antecubital vein using a mechanical injector (140 mL at 2.3 mL/s). Scanning began 70 or 80 s after the start of IV contrast injection (from the lower thorax to the lower pelvis). Scanning parameters included the following: detector array 1.25 mm × 4; beam pitch 1.35; section thickness 3.2 mm; and a reconstruction increment of 3 mm.
Classification of regions
The peritoneal cavity was classified into 13 regions, i.e. right subdiaphragmatic area, left subdiaphragmatic area, porta hepatis, lesser sac, small bowel mesentery, splenic hilar area, omentum, right paracolic gutter, left paracolic gutter, right pelvic cavity, left pelvic cavity, sigmoid mesentery, and bladder dome area (Fig. 1).
Classification of the abdominopelvic cavity. 1 Right subdiaphragmatic area, 2 left subdiaphragmatic area, 3 porta hepatis, 4 lesser sac, 5 small bowel mesentery, 6 splenic hilar area, 7 omentum, 8 right paracolic gutter, 9 left paracolic gutter, 10 right pelvic cavity, 11 left pelvic cavity, 12 sigmoid mesentery, 13 bladder dome area
Lymph nodes were classified into three groups: para-aortic (including right and left para-aortic lymph nodes), right pelvic, and left pelvic areas.
Surgical technique
All patients underwent preoperative antibiotic bowel preparation for 2 days prior to surgery. Perioperative antibiotics, thromboembolic prophylaxis with lower molecular heparin, and pressure stockings were routinely used. Patients were placed in the Trendelenburg position with straight, extended legs, and gel pads were placed under their shoulders and heels. A midline vertical incision was made from the pubic symphysis to the xiphoid process.
All surgeries used the same instruments and techniques and were performed by experienced gynecologic oncologists assisted by a gynecology-board-certified fellow and a resident in training. First, the pelvic and abdominal organs and the peritoneal surfaces were thoroughly explored. Prior to dissection, peritoneal cytology samples were obtained from the abdominopelvic cavity. Cytoreductive surgery was performed from the upper abdomen to pelvic cavity. Omentectomy with resection of both gastro-epiploic vessels was usually the first cytoreductive procedure. Cytoreductive surgery was then performed in the upper abdomen. Subdiaphragmatic peritoneal stripping and/or diaphragmatic resection, hepatic resection, cholecystectomy, splenectomy, distal pancreatectomy, large and small bowel resection, appendectomy, and urinary tract resection and anastomosis were selectively performed as clinically indicated to minimize residual tumor. Then, tumor resection in the pelvis was performed, such as hysterectomy, bilateral salpingooophorectomy, low anterior resection, or pelvic peritonectomy. Lymph node dissection in the pelvis and para-aortic area was performed, and lastly, bowel anastomosis was performed. After the removal of all visible tumors, random peritoneal biopsies were obtained from the diaphragm, paracolic gutters, pelvic side walls, posterior cul-de-sac, bladder peritoneum, and from suspicious lesions or adhesions.
Analysis and statistics
CT images were interpreted by consensus of two radiologists (C.H.J. and J.D.C.) with 7 and 5 years of clinical experience, respectively, in gynecologic imaging including CT. The two readers were unaware of any of the surgical results. Evaluated imaging findings for peritoneal seeding were as follows: 1, presence of a nodular, plaque-like or infiltrative soft-tissue lesion in the peritoneal fat or surface bowel serosa; 2, presence of ascites; 3, parietal peritoneal thickening or enhancement; and 4, small bowel wall thickening or distortion [3–5, 8]. The criterion for suspicion of malignancy on CT was a pelvic or para-aortic lymph node with a short-axis diameter ≥1 cm. All images were examined using the picture archiving and communication system. Information regarding the CT imaging was entered into a prospective database, and the surgical staging information was stored in a separate file.
Descriptive statistical data, including sensitivity, specificity, positive predictive value, and negative predictive value, were calculated. The χ2 test was used to compare the sensitivity, specificity, positive predictive value, and negative predictive value. QuickCalc (GraphPad Software Inc) software was used for analysis. A P value of 0.05 or less was considered to represent a significant difference.
Results
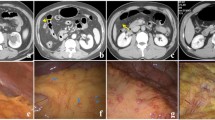
Of the 57 patients with primary ovarian cancer, who underwent laparotomy and CT, peritoneal seeding was present in 50 of the 57 patients in the all-patient group (87%), in 25 of the 30 patients in the non-neoadjuvant group (83%), and in 25 of the 27 patients in the neoadjuvant group (93%) (Fig. 2a–g). The splenic hilar area was the most common site of peritoneal seeding, and both pelvic cavities were the least common sites of peritoneal seeding in the all-patient group. The distribution of peritoneal seeding is summarized in Table 1.
A 54-year-old woman with primary serous cystadenocarcinoma of the ovary. a Axial contrast-enhanced CT scan shows a heterogeneously enhancing solid mass (arrow) in the spleen, suggestive of a left subdiaphragmatic peritoneal implant with invasion of the spleen. Note the large amount of ascites (asterisks) in both subdiaphragmatic area. b Intraoperative photograph shows a yellowish, bulging mass in the spleen (arrows), which turned out to be splenic metastasis form ovarian cancer. Note the disseminated, sub-centimeter-sized peritoneal implants (arrowheads) in the left subdiaphragmatic area. c Axial, contrast-enhanced CT scan shows a large amount ascites in both of the subdiaphragmatic areas without peritoneal implants (asterisk). Note the omental cake in the left anterior abdominal cavity (arrow). d Intraoperative photograph shows a disseminated, subcentimeter-sized peritoneal implant in the left subdiaphragmatic area. e Intraoperative photograph shows multiple, conglomerated, peritoneal implants forming an omental cake. f Axial, contrast-enhanced CT scan shows misty mesentery (asterisk), although no definite mass or implants are shown in the CT scan. Note the peritoneal thickening (arrowheads), omental cake (arrow), and large amount ascites. g Intraoperative photograph shows multiple peritoneal implants in the mesenteric root (arrowheads)
The diagnostic performance of CT for detecting peritoneal seeding and lymph node metastasis is summarized in Table 2. There were no statistical differences in the diagnostic performance of CT in the non-neoadjuvant and neoadjuvant groups. Sensitivity and specificity (35.1 and 68%) were lower for the detection of peritoneal seeding in patients with peritoneal implants smaller than 1 cm in maximum diameter compared with the detection of peritoneal implants 1 cm or larger (52.4 and 75%), and there was a statistically significant difference in sensitivities (p = 0.037) (data not shown). The region-specific diagnostic performance of CT in the non-neoadjuvant group, neoadjuvant group, and all-patient group are summarized in Table 3.
Discussion
The presence of peritoneal seeding is an important prognostic factor in ovarian cancer patients as it is the most common pathway of dissemination in ovarian cancer and is found in approximately 70% of patients on their initial diagnosis. Although ovarian cancer patients are surgically staged with laparotomy according to the recommendations of the International Federation of Gynecology and Obstetrics (FIGO) [9], it is important for the proper triage of ovarian cancer patients who will be suboptimally resected to be placed in a more appropriate neoadjuvant chemotherapy group to detect peritoneal seeding preoperatively [10]. In addition, recognizing the location of peritoneal seeding is also important for predicting the possibility of optimal resection [11, 12]. For this reason, it is important to know the region-based diagnostic performance of CT for detecting peritoneal seeding in ovarian cancer patients. However, the moderate accuracy of MDCT in the current study still leaves much to be desired. To completely know whether the patients underwent optimal cytoreduction or not, the current standard, exploratory laparotomy, should be performed. Image-based selection for neoadjuvant chemotherapy still remains experimental.
The primary purpose of our study was to prospectively evaluate the region-based diagnostic performance of post-contrast CT for the detection of peritoneal seeding in ovarian cancer patients. To date, some studies have been conducted based on the performance of CT for detecting peritoneal seeding in ovarian cancer patients [3–7]. However, all of these studies reported the patient-based diagnostic performance of CT. Tempany et al. [7] performed a multicenter prospective study and did a region-based comparison, but the obtained sensitivity (92%) and specificity (82%) of CT were based on patient-based diagnostic performance. On the other hand, in our study, the region-based diagnostic performance of CT was assessed as we thought it would form a baseline study for the evaluation of the usefulness of CT for predicting optimal tumor resection and peritoneal seeding in ovarian cancer patients.
In our study, the sensitivity and specificity of CT for detecting peritoneal seeding were 45 and 72%, respectively. In time of evaluating the accuracy of CT for the peritoneal seeding especially in upper abdomen, we must consider the surgical aggressiveness and surgical extent. Because even if surgeons keep the principle of surgical approach for ovarian cancer, such as thorough exploration of the whole abdomen on inspection and palpation and careful biopsy of all suspicious tissues via lower midline incision from the sternum to the symphysis pubis, surgical extensiveness and approach for each area could be different [13]. Evaluation and/or biopsy for the area of porta hepatis, less sac, or diaphragm at hepatophrenic junction which has been firstly evaluated in the current study as far as we know may be performed or not according to surgeons’ policy. This results in different outcomes in terms of evaluating the accuracy of CT. We did all our best to minimize the residual tumor adopting extensive cytoredutive surgical procedures such as intrathoracic debulking, diaphragmatic stripping, partial gastrectomy, hepatectomy, cholecystectomy, adrenalectomy, splenectomy, distal pancreatectomy, colectomy, and small bowel resection [14–18]. Therefore, the sensitivity in the current study might low than previous studies because of more microscopic tumor in more resected area. In the future, the study on the accuracy of CT for the peritoneal seeding of ovarian cancer is recommended to include such information, such as surgical efforts, surgical extent, and degree of optimal cytoreduction for comparison with previous studies.
Although statistically insignificant, the higher sensitivity and lower specificity seen in the non-neoadjuvant group when compared with those in the neoadjuvant group was observed. As ascites is a common finding in patients with peritoneal seeding and it makes the other CT findings of peritoneal seeding, such as a nodular or plaque-like, enhancing, soft-tissue mass, more prominent, the absent or decreased ascites may obscure an accurate diagnosis of peritoneal seeding in the neoadjuvant patient group.
The region-based sensitivity and specificity of CT for detecting lymph node metastasis were 22, 90% in all included patients and 30, 60 and 38, 100% in the non-neoadjuvant and neoadjuvant groups, and there was no statistical difference in diagnostic accuracy between the neoadjuvant and non-neoadjuvant group. These were relatively lower than previously reported sensitivities and specificities (41–44 and 64–89%) in our study [7, 19, 20], however, as there was only a relatively small number of metastatic nodes, we believe that, in general, it is difficult to arrive at a definite conclusion regarding the diagnostic performance of CT for detecting metastatic lymph nodes in ovarian cancer patients.
In our study, the common sites of peritoneal seeding were the splenic hilar surface (15%) and both subdiaphragmatic areas (right subdiaphragmatic area, 14%; left subdiaphragmatic area 14%) as these are the sites where peritoneum reflections and peritoneal fluid tend to remain longer [21]. In our region-based comparison, CT shows the highest sensitivity in the splenic hilar surface (71%, 27/38) and the lowest sensitivity in the bladder dome (0%, 0/16). As in our study, the coronary and sagittal reconstructions were not performed, and the lower sensitivity of CT for detecting peritoneal seeding in the bladder dome may be attributable to this factor. The sensitivity of CT for detecting peritoneal implants in the right and left paracolic gutter and right and left subdiaphragmatic area, was 77, 69, 72, and 61%, respectively, in the non-neoadjuvant group and 56, 29, 17, and 16%, respectively, in the neoadjuvant group. These decreased sensitivities may be due to the decreased or no longer existing ascites which may facilitate the detection of peritoneal implants.
Our study has some limitations. First, the number of patients involved was relatively small to be able to carry out a subgroup analysis, such as determining the difference in the diagnostic performance for detecting peritoneal seeding in the neoadjuvant and non-adjuvant groups. We believe that next study including a larger patient number may have resulted in a more reliable result on this issue. A second limitation of our study was that multi-planar reformatted images, which may facilitate the detection of peritoneal seeding [22], were not reconstructed.
In conclusion, although multi-detector CT is useful for detecting peritoneal implants and lymph node metastasis in ovarian cancer patients, it has only moderate, region-based diagnostic performance.
References
Bristow RE, Puri I, Chi DS (2009) Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol 112:265–274
Chi DS, Zivanovic O, Palayekar MJ, Eisenhauer EL, Abu-Rustum NR, Sonoda Y et al (2009) A contemporary analysis of the ability of preoperative serum CA-125 to predict primary cytoreductive outcome in patients with advanced ovarian, tubal and peritoneal carcinoma. Gynecol Oncol 112:6–10
Buy JN, Moss AA, Ghossain MA, Sciot C, Malbec L, Vadrot D et al (1988) Peritoneal implants from ovarian tumors: CT findings. Radiology 169:691–694
Coakley FV, Choi PH, Gougoutas CA, Pothuri B, Venkatraman E, Chi D et al (2002) Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology 223:495–499
Halvorsen RA Jr, Panushka C, Oakley GJ, Letourneau JG, Adcock LL (1991) Intraperitoneal contrast material improves the CT detection of peritoneal metastases. AJR Am J Roentgenol 157:37–40
Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH (1993) Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer 72:1631–1636
Tempany CM, Zou KH, Silverman SG, Brown DL, Kurtz AB, McNeil BJ (2000) Staging of advanced ovarian cancer: comparison of imaging modalities—report from the Radiological Diagnostic Oncology Group. Radiology 215:761–767
Walkey MM, Friedman AC, Sohotra P, Radecki PD (1988) CT manifestations of peritoneal carcinomatosis. Am J Roentgenol 150:1035–1041
(1987) Changes in definitions of clinical staging for carcinoma of the cervix and ovary: International Federation of Gynecology and Obstetrics. Am J Obstet Gynecol 156:263–264
Qayyum A, Coakley FV, Westphalen AC, Hricak H, Okuno WT, Powell B (2005) Role of CT and MR imaging in predicting optimal cytoreduction of newly diagnosed primary epithelial ovarian cancer. Gynecol Oncol 96:301–306
Dowdy Samkrbbjhwac Sean C (2004) The utility of computed tomography scans in predicting suboptimal cytoreductive surgery in women with advanced ovarian carcinoma. Cancer 101:346–352
Bristow RE, Duska LR, Lambrou NC, Fishman EK, O’Neill MJ, Trimble EL et al (2000) A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer 89:1532–1540
Lim MC, Bae J, Park SY (2009) In reply: different role of secondary cytoreductive surgery by surgeon’s experience and hospital facility. J Gynecol Oncol 20:199
Lim MC, Lee HS, Jung DC, Choi JY, Seo SS, Park SY (2009) Pathological diagnosis and cytoreduction of cardiophrenic lymph node and pleural metastasis in ovarian cancer patients using video-assisted thoracic surgery. Ann Surg Oncol 16:1990–1996
Song YJ, Lim MC, Kang S, Seo SS, Park JW, Choi HS et al (2009) Total colectomy as part of primary cytoreductive surgery in advanced Mullerian cancer. Gynecol Oncol 114:183–187
Lim MC, Kang S, Lee KS, Han SS, Park SJ, Seo SS et al (2009) The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol 112:28–34
Lim SW, Lim SB, Park JY, Park SY, Choi HS, Jeong SY (2008) Outcomes of colorectal anastomoses during pelvic exenteration for gynaecological malignancy. Br J Surg 95:770–773
Lim MC, Lee HS, Kang S, Seo SS, Lee BY, Park SY (2010) Minimizing tumor burden by extensive cytoreductive surgery decreases postoperative venous thromboembolism in ovarian clear cell carcinoma. Arch Gynecol Obstet 281:329–334
Carnino F, Fuda G, Ciccone G, Iskra L, Guercio E, Dadone D et al (1997) Significance of lymph node sampling in epithelial carcinoma of the ovary. Gynecol Oncol 65:467–472
Sakai K, Kamura T, Hirakawa T, Saito T, Kaku T, Nakano H (1997) Relationship between pelvic lymph node involvement and other disease sites in patients with ovarian cancer. Gynecol Oncol 65:164–168
Mironov S, Akin O, Pandit-Taskar N, Hann LE (2007) Ovarian cancer. Radiol Clin North Am 45:149–166
Franiel T, Diederichs G, Engelken F, Elgeti T, Rost J, Rogalla P (2009) Multi-detector CT in peritoneal carcinomatosis: diagnostic role of thin slices and multiplanar reconstructions. Abdom Imaging 34:49–54
Acknowledgments
The authors would like to thank all of the anesthesiologists, surgeons, and surgery nurses at the Center for Uterine Cancer for their invaluable help, patience, and active participation in this study. The authors also would like to thank Wha-Eun Lee and Soo Yeon Jung, research nurses at the National Cancer Center, Korea, for their efforts in gathering and organizing the data and Myungeun Lim (Jessica Lim) for drawing the illustration (Fig. 1). This work was supported by Grants (1010111-1) offered by the National Cancer Center.
Conflict of interest statement
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. J. Choi and M. C. Lim contributed equally to this work.
Rights and permissions
About this article
Cite this article
Choi, H.J., Lim, M.C., Bae, J. et al. Region-based diagnostic performance of multidetector CT for detecting peritoneal seeding in ovarian cancer patients. Arch Gynecol Obstet 283, 353–360 (2011). https://doi.org/10.1007/s00404-010-1442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1442-0