Abstract
Psoriasis (PsO) has been associated with lipoprotein abnormalities, visceral adiposity, atherosclerosis, and coronary artery disease (CAD) in several studies; however, data concerning the risk of psoriasis relevant to these parameters is not well established. We aimed to evaluate the relation between PsO and small dense low-density lipoprotein cholesterol (sd-LDL-C), serum lipid profile (SLP), blood pressures, anthropometric measurements, intima media thickness of the common carotid artery (CIMT), distribution of visceral adipose tissue (VAT; evaluated at 3 different measurement sites including VATa, VATb, VATc) along with subcutaneous (Sc-d1) and preperitoneal (Pre-d2) adipose tissue, and disease characteristics, so as to define relevant risk factors for PsO. In this cross-sectional and observational study, 62 patients with plaque-type PsO and 31 age- and sex-matched controls were enrolled. Data about metabolic profile, CIMT and VAT were obtained. There was a significant association between PsO and hypertension, smoking, diastolic blood pressure, sd-LDL-C/LDL-C ratio, CIMT, VATc, and Pre-d2. Following adjustments for hypertension and smoking, sd-LDL-C/LDL-C ratio, CIMT, and Pre-d2 still remained different between patients and controls (P = 0.03, P = 0.043, and P = 0.05, respectively). Each 0.1 unit increase in the CIMT increased the risk of PsO 1.51-fold (95%CI: 1.08 − 2.12, P = 0.016). PsO associates with a predisposition to develop thick preperitoneal fat tissue and thick intima of carotid arteries, all of which contribute to the increased risk of atherosclerosis and subsequent CAD. CIMT was considered as an independent risk factor for PsO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Psoriasis (PsO) is a chronic inflammatory skin disorder affecting skin, nails, and joints. Psoriatic inflammation can have systemic effects beyond skin inflammation that can lead to and/or exacerbate comorbidities including obesity, dyslipidemia, insulin resistance (IR), diabetes mellitus (DM), hypertension (HT), metabolic syndrome, and subsequently atherosclerosis [1]. On the other hand, obesity, DM, HT, and metabolic syndrome are found to be risk factors for development of psoriasis [2,3,4,5].
An increase in visceral adipose tissue (VAT) leads to abdominal obesity, which contributes to an increase in the risk of carotid and coronary atherosclerosis, IR, and an increase in small-sized low-density lipoprotein cholesterol (sd-LDL-C) [6]. Accumulation of excess and disproportionate VAT contributes to the progression of metabolic and atherosclerotic diseases, regardless of obesity, as VAT releases pro-inflammatory cytokines [7]. Balci et al. (8) recently reported that there is an increase in VAT in PsO patients, and Han et al. [2] observed that the risk of PsO is higher in individuals with abdominal obesity. Pro-inflammatory cytokines and activation of inflammatory cells might act a significant part in the development of psoriatic plaques and breakdown of atherosclerotic plaques, indicating similar pathogenetic mechanisms for PsO and atherosclerosis [9].
Carotid intima–media thickness (CIMT) is well known to be associated with early atherosclerosis predictive of future coronary artery disease (CAD), myocardial infarction, and stroke [10]. Several studies have reported higher rates of increased CIMT in patients with PsO than in healthy controls, suggesting the existence of subclinical atherosclerosis in PsO patients [11]. In contrast, Norata et al. [12] noted that sd-LDL-C can predict the CIMT variance, as sd-LDL-C particles are implicated in endothelial dysfunction related to accelerated atherogenesis.
The present study aimed to determine the relationship between PsO, and sd-LDL-C, the lipid profile, fasting blood glucose (FBG), CIMT of the common carotid arteries, blood pressure, BMI, visceral fat accumulation, visceral fat volume, and disease characteristics, to identify the related risk factors for PsO.
Material and methods
Participants
The study protocol was approved by the Hacettepe School of Medicine Ethics Committee for Non-Interventional Studies (code E-17–1189, 11 October 2017). All study strategies were performed according to the ethical principles of the 1964 Declaration of Helsinki, and all subjects provided written informed consent before being included.
The study included 62 PsO patients (35 male and 27 female) aged 18–65 years and 31 age- and gender-matched controls (15 male and 16 female) who presented to the Hacettepe University Dermatology Outpatient Clinic, Ankara, Turkey, between December 2016 and January 2018. PsO was diagnosed based on clinical and/or histopathological findings. All patients had chronic plaque psoriasis. A positive history of cancer, age < 18 years and > 65 years, pregnancy, lactation, familial hypercholesterolemia, concomitant active or chronic infection, and present use of lipid-lowering drugs were exclusion criteria. Controls were recruited from among patients who presented for common dermatological complaints, including seborrheic keratoses, actinic keratoses, warts, and nevi. In the patient group, six patients had DM, nine had HT, four had hypothyroidism, two had rheumatologic disease, two had CAD, and two had psychiatric disease. In the control group, two controls had DM, four had hypothyroidism, two had CAD, and two had psychiatric disease.
Age, gender, personal and family medical history, smoking habit, alcohol consumption, and participation in regular physical activity (exercise for ≥ 45 min per d 3 times per week) were recorded for all participants. Body weight (kg), height (m), and waist circumference (WC) in centimeters were measured, and BMI (kg m–2) was calculated using the Quetelet Index [13]. Cutoff points for BMI and WC are based on World Health Organization report [14]. Obesity is defined as having a body mass index of 30 or higher. Clinical characteristics, including duration and type of PsO, the Psoriasis Area Severity Index (PASI) score, PsO localization, the presence of nail and joint involvement, and current treatment for PsO were noted. A sphygmomanometer was used to measure blood pressure (ERKA, The Original, VARIO, Germany) after 10 min of seated rest.
Laboratory measurements
Blood samples were collected from all participants in the morning following 8 h of fasting. The serum lipid profile (SLP), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), and triglycerides (TG), FBG, and sd-LDL-C were measured. Measurement of FBG, TC, TG, and HDL-C was performed via the enzymatic method and the Friedewald formula (LDL-C = TC – HDL-C − TG/5.0) was used to calculate LDL-C.
Blood samples were centrifuged at 1200g (g = relative centrifugal force) for 10 min, to measure sd-LDL-C. The blood samples were stored at − 80 °C until analysis. An Abbott c8000 analyzer was used to calculate the sd-LDL-C level via a two-step automated colorimetric method. The first step was to precipitate the lipoprotein with a density < 1.044 g mL–1 using heparin–magnesium and the second step was direct LDL-C determination based on the supernatant fraction.
Imaging methods
Ultrasonic measurements were obtained by two radiologists using an ultrasound device (Aplio500, Toshiba, Japan). A 3.5 MHz convex-array probe was used to evaluate adipose tissue parameters, as ‘a’, ‘b’, ‘c’, and a 7.5 MHz linear-array probe on the same device was used to evaluate parameter ‘d’, as follows: a: the distance between the splenic vein and the internal surface of the abdominal muscle; b: the distance between the posterior wall of the aorta and the internal surface of the abdominal muscle at the level of the umbilicus; c: right posterior perirenal adipose tissue thickness; d: subcutaneous (Sc-d1) and pre-peritoneal (Pre-d2) adipose tissue thickness at the level of the xiphoid process [15]. Parameters ‘a’, ‘b’, and ‘c’ refer to VAT.
Visceral fat volume was calculated using the equation: [visceral fat volume] = [− 9.008] + [1.191 × the distance between the splenic vein and the internal surface of the abdominal muscle (mm)] + [0.987 × the distance between the posterior wall of the aorta and the internal surface of the abdominal muscle at the level of the umbilicus (mm)] + [3.644 × right posterior perirenal adipose tissue thickness (mm)].
In addition, CIMT was measured using an 11 MHz linear-array probe on the same device. CIMT values were surveyed at the end of diastole and evaluated in the longitudinal view, considering non-plaque segments of each of the common carotid arteries. All measurements were obtained following overnight fasting, which is crucial for abdominal parameter measurement, and all data were obtained in the supine position and at the end of expiration. A convex transducer was located perpendicularly on the skin and the pressure was minimized to prevent compression of adipose tissue. The final mean CIMT was calculated as the sum of the scores of left and right CIMT, and subsequent division of the total value by 2.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows v.21.0. (IBM Corp., Armonk, NY). Data are shown as mean ± SD or median (range), as appropriate. Descriptive statistics were used to describe demographic characteristics of participants. Categorical variables were given in percentage and frequency. The Chi-square test was used to compare the differences in categorical variables between the patient and control groups. To determine the normality of the distribution of numeric variables, the Shapiro–Wilk test was used. When parametric assumptions were not seen, the Mann–Whitney U test was used to compare numeric variables between the patient and control groups, whereas when parametric assumptions were met the independent samples t test was used. HT and smoking were selected as confounding factors can by themselves improve the thickness of the CIMT, and also HT and smoking are not part of the causal chain linking exposure to outcome. The reason for this can be explained as exposure is lipid improvement and the outcome is PsO in this study. Therefore, the ANCOVA test was used to determine the effect of variables on PsO after adjusting for smoking and HT. Multivariate logistic regression analysis was used to analyze variables with P value cutoff point of 0.15 to determine whether the relevant variables were a risk factor for PsO after elimination of confounding factors. The strength of the associations between the relevant parameters and PsO risk was estimated via the odds ratio (OR) and corresponding 95% confidence interval (CI). Spearman's correlation is a nonparametric statistic and there is no requirement of normality. Since significant associations were found between psoriasis and sd-LDL-C, mean CIMT, Pre-d2 and VATc, these variables were tested by Spearman’s correlation. The level of statistical significance was set at P < 0.05.
Results
The study included 62 plaque-type PsO patients and 31 controls. In the present study, PsO mostly involved the upper and lower extremities, followed by the trunk and scalp. The median age of patients and controls were 41.5 years (range, 18–65 years) and 37 years (range, 18–65 years), respectively. Median disease duration was 78 months (range, 3–744 months) and median PASI score was 5.5 (range, 0.2–36.8). In all, 22 patients were not currently receiving treatment for PsO, whereas 21 were using a topical treatment and the remaining 19 were using systemic treatment, primarily methotrexate and biologics. In addition, 46.8% and 12.7% of the patients had nail involvement and psoriatic arthritis (PsA), respectively. In the psoriasis group, 18 of 62 (29%) patients were obese and 44 of 62 patients (71%) were not obese. In the control group, 8 of 31 (26%) participants were obese and 23 of 31 (74%) controls were not obese. In terms of obesity, univariate analysis revealed that there were no differences between patients and controls (P = 0.74) (Table 1).
Univariate analysis showed that there was a significant association between PsO, and hypertension (P = 0.02). Following adjustments for HT and smoking, the sd-LDL-C/LDL-C ratio, and mean CIMT and Pre-d2 remained significantly different between the patients and controls (P = 0.03, P = 0.043, and P = 0.05, respectively). Although univariate analysis showed an association between PsO and VATc, after adjustments for hypertension and smoking, VATc did not remain significant between patients and controls (P = 0.09) (Tables 2 and 3). There was no difference in the sd-LDL-C/LDL-C ratio, or mean CIMT and Pre-d2 between the patients who were currently receiving a systemic treatment for PsO and those that were not (P = 0.57, P = 0.60, and P = 0.10, respectively), as well as between patients with and without PsA (P = 0.93, P = 0.76, and P = 0.17, respectively) (Tables 1, 2).
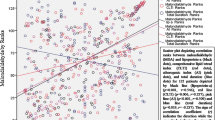
In the patient group, there was a moderate to strong positive correlation between CIMT and age (rs = 0.74, P < 0.001 and rs = 0.66, P < 0.001, respectively). There was a weak to moderate positive correlation between CIMT and FBG, BMI, WC, SBP, DBP, VATb, Sc-d1, and VFV. Additionally, a moderate to strong positive correlation was found between CIMT and VATc (rs = 0.51, P < 0.001). There was no significant correlation between the sd-LDL/LDL-C ratio and CIMT, blood pressures, and adipose fat tissue parameters. VATc was positively correlated in a moderate to strong relationship with BMI, WC, VATb, and VFV (rs = 0.73, P < 0.001, rs = 0.72, P < 0.001, rs = 0.66, P < 0.001, and rs = 0.78, P < 0.001, respectively, Table 4). Logistic regression analysis showed that there was a significant correlation between PsO, and mean CIMT; each 0.1-unit increase in CIMT (P = 0.02; 95% CI, 1.08–2.12) increased the risk of PsO 1.51-fold. As such, mean CIMT was considered an independent risk factor for PsO.
Discussion
There is strong evidence that chronic inflammation is the hallmark of atherosclerosis. Patients with chronic inflammatory diseases including PsO are predisposed to accelerated atherosclerosis and subsequent cardiometabolic diseases including obesity, type 2 DM, HT, and dyslipidemia [16]. On the other hand, obesity, type 2 DM, HT, and metabolic syndrome are found to be risk factors for development of PsO (2–5). Evaluation of different metabolic parameters, along with radiological alterations by the present study, may help to identify risk factors in terms of PsO development even if these data exist in other populations.
Adipose tissue functions as an endocrine and secretory organ, producing several pro-inflammatory cytokines and adipokines [17]. Obesity leads to excessive production of pro-inflammatory adipokines and a concurrent decrease in the production of adipokines with anti-inflammatory features. As such, cumulative inflammatory burden in obese psoriatic patients supports a critical role in all phases of atherosclerosis and cardiovascular events, with the onset of endothelial dysfunction in PsO [18]. On the other hand, irrespective of obesity, in PsO, the disproportionate accumulation of VAT may facilitate the development of atherosclerosis due to the excretion of pro-inflammatory cytokines from VAT. [7]. Although the present study findings revealed that there was no difference between the patients and controls in terms of obesity, the difference of some VAT parameters between these groups may reflect a probable link between altered adipose tissue distribution and PsO.
Due to the distinctive metabolic profile of visceral adiposity from subcutaneous adiposity, visceral adiposity has been shown to be associated with subclinical cardiovascular disease. In PsO, beyond an increase in visceral adiposity, Rivers JP et al. have shown that PsO is associated with vascular inflammation based on 18-fluorodeoxyglucose positron emission tomography (18-FDG PET) [19]. The authors added that vascular inflammation by 18-FDG PET/CT has been shown to precede subclinical atherosclerosis and predict the risk for development of future cardiovascular events [18]. Therefore, in the light of these studies, it may be speculated that increased visceral adiposity accompanied by altered vascular inflammation may play a role in the development of atherosclerosis and subsequent cardiovascular disease in PsO.
Although higher LDL-C levels are among the most significant risk factors for CAD, many patients with CAD have a normal LDL-C level. Therefore, the smaller and more atherogenic subfraction of LDL-C known as sd-LDL-C was thought to cause an increased risk of CAD and progression of atherosclerosis [12]. One study found a positive association between PsA and subclinical atherosclerosis [20]. However, there is no study investigating the association between the factors of lipid metabolism, such as sd-LDL-C and PsO. Significant difference in the sd-LDL-C levels was observed between patients with PsO and controls in the present study. To confirm this finding, it is necessary to repeat the assessment in different ethnic groups and with a greater number of participants.
In the recent years, there has been a growing interest in adipose tissue distribution rather than quantity of adipose tissue as a risk factor for metabolic disorders and cardiovascular diseases. Although obesity is a classical risk factor for atherosclerosis, it is not clear whether intra-abdominal fat accumulation has an association with cardiovascular disease in non-obese subjects. Accordingly, Tadokoro et al. [21] reported that preperitoneal fat tissue is significantly correlated with the coronary artery stenosis score and serum lipid levels in non-obese subjects. Liu et al. [22] noted that there is a positive association between preperitoneal fat thickness and CIMT. In the present study univariate analysis showed that VATc and Pre-d2 are significantly higher in the PsO patients than in the controls. Following adjustments for HT and smoking, Pre-d2 remained significantly correlated with PsO. The present findings show that although preperitoneal fat tissue was thick in psoriatic patients, it is not clear if cellular composition, impaired VAT functions, or the VAT volume has a significant effect on the development of PsO and subsequent atherosclerosis.
Carotid intima media thickness is considered an indicator of atherosclerosis, cardiovascular morbidity, and mortality. Since PsO is an auto-inflammatory disease associated with increased burden of atherosclerosis as well as an increase in the risk of cardiovascular morbidity, several studies have examined the relationship between PsO and CIMT to measure risk stratification for CAD [23]. Most of them observed that CIMT was greater in psoriatic patients compared with controls [24].Therefore, patients with PsO should be screened and followed up via CIMT monitoring due to the highest risk of cardiovascular events. In the present study, CIMT is considered to be the sole independent risk factor for PsO. This risk was not different from the type of treatment used. However, there is a possibility that deep fat is implicated in the development of atherosclerosis in patients with PsO, since a strong positive correlation was found between CIMT and VATc.
The present study has some limitations, including a small sample size, unequal gender distribution, and a much larger patient group than control group. As the study was performed at a single center, the sample may not be representative of the larger patient population. Lastly, causality could not be determined due to the study’s case–control design.
In conclusion, based on the present study’s findings PsO is associated with sd-LDL-C/LDL-C ratio, preperitoneal fat tissue, and CIMT. However, CIMT thickness was the sole risk factor for PsO in the present study. Based on these findings, screening psoriatic patients for CIMT and preperitoneal adipose tissue thickness, as well as careful monitoring for the traditional cardiovascular risk factors, so as to prevent ischemic comorbidities, might be considered highly warranted. Additional research is needed to further elucidate the relationship between the treatments for PsO and atherosclerosis.
References
Jung KJ, Kim TG, Lee JW, Oh J, Lee SE, Chang HJ et al (2019) Increased risk of atherosclerotic cardiovascular disease among patients with psoriasis in Korea: a 15-year nationwide population-based cohort study. J Dermatol 46(10):1–8
Han JH, Lee JH, Han KD, Kim HN, Bang CH, Park YM et al (2019) Increased risk of psoriasis in subjects with abdominal obesity: a nationwide population-based study. J Dermatol 46(8):695–701.
Jacob L, Kostev K (2017) Psoriasis risk in patients with type 2 diabetes in German primary care practices. Prim Care Diabetes 11(1):52–56
Kim HN, Han K, Song SW, Lee JH (2018) Hypertension and risk of psoriasis incidence: an 11-year nationwide population-based cohort study. PLoS ONE 13(8):e0202854
Snekvik I, Nilsen TIL, Romundstad PR, Saunes M (2019) Metabolic syndrome and risk of incident psoriasis: prospective data from the HUNT Study. Norway Br J Dermatol 180(1):94–99
Kobayashi H, Nakamura T, Miyaoka K, Nishida M, Funahashi T, Yamashita S et al (2001) Visceral fat accumulation contributes to insulin resistance, small-sized low-density lipoprotein, and progression of coronary artery disease in middle-aged non-obese Japanese men. Jpn Circ J 65(3):193–199
Nakamura T, Tokunaga K, Shimomura I, Nishida M, Yoshida S, Kotani K et al (1994) Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis 107(2):239–246
Balci A, Balci DD, Yonden Z, Korkmaz I, Yenin JZ, Celik E et al (2010) Increased amount of visceral fat in patients with psoriasis contributes to metabolic syndrome. Dermatology 220(1):32–37
Späh F (2008) Inflammation in atherosclerosis and psoriasis: common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol 159(2):10–17
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115(4):459–467
Evensen K, Slevolden E, Skagen K, Rønning OM, Brunborg C, Krogstad AL et al (2014) Increased subclinical atherosclerosis in patients with chronic plaque psoriasis. Atherosclerosis 237(2):499–503
Norata GD, Raselli S, Grigore L, Garlaschelli K, Vianello D, Bertocco S et al (2009) Small dense LDL and VLDL predict common carotid artery IMT and elicit an inflammatory response in peripheral blood mononuclear and endothelial cells. Atherosclerosis 206(2):556–562
Eknoyan G (2008) Adolphe Quetelet (1796-1874) the average man and indices of obesity. Nephrol Dial Transplant 23(1):47–51
World Health Organization (2008) Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva Available at: https://www.who.int/iris/handle/10665/44583. Accessed 20 Nov 2019
Hirooka M, Kumagi T, Kurose K, Nakanishi S, Michitaka K, Matsuura B et al (2005) A technique for the measurement of visceral fat by ultrasonography: comparison of measurements by ultrasonography and computed tomography. Intern Med 44(8):794–799
Sajja AP, Joshi AA, Teague HL, Dey AK, Mehta NN (2018) Potential immunological links between psoriasis and cardiovascular disease. Front Immunol 9:1234
Wong Y, Nakamizo S, Tan KJ, Kabashima K (2019) An update on the role of adipose tissues in psoriasis. Front Immunol 10:1507
Siegel D, Devaraj S, Mitra A, Raychaudhuri SP, Raychaudhuri SK, Jialal I (2013) Inflammation, atherosclerosis, and psoriasis. Clin Rev Allergy Immunol 44(2):194–204
Rivers JP, Powell-Wiley TM, Dey AK, Rodante JA, Chung JH, Joshi AA et al (2018) Visceral adiposity in psoriasis is associated with vascular inflammation by 18F-fluorodeoxyglucose positron-emission tomography/computed tomography beyond cardiometabolic disease risk factors in an observational cohort study. JACC Cardiovasc Imaging 11(2 Pt 2):349–357
Gentile M, Peluso R, Di Minno MND, Costa L, Caso F, de Simone B et al (2016) Association between small dense LDL and sub-clinical atherosclerosis in patients with psoriatic arthritis. Clin Rheumatol 35(8):2023–2029
Tadokoro N, Murano S, Nishide T, Suzuki R, Watanabe S, Murayama H et al (2000) Preperitoneal fat thickness determined by ultrasonography is correlated with coronary stenosis and lipid disorders in non-obese male subjects. Int J Obes Relat Metab Disord 24(4):502–507
Liu KH, Chan YL, Chan JC, Chan WB (2005) Association of carotid intima-media thickness with mesenteric, preperitoneal and subcutaneous fat thickness. Atherosclerosis 179(2):299–304
Jókai H, Szakonyi J, Kontár O, Marschalkó M, Szalai K, Kárpáti S et al (2013) Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol 69(4):523–529
Fang N, Jiang M, Fan Y (2016) Association between psoriasis and subclinical atherosclerosis: a meta-analysis. Medicine 95(20):e3576
Acknowledgements
Author Neslihan Akdogan, author Pinar Incel Uysal, author Murat Vural, author Ahmet Bokebatur Mendi, author Tuba Candar and author Basak Yalcin declare that they have no conflict of interest.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest—financial or otherwise—related to the material presented herein.
Ethical approval and informed consent
Patients provided informed consent and ethical approval was obtained from the University of Hacettepe Ethics Committee for Non-Interventional Studies (code E-17–1189, 11 October 2017).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akdogan, N., Uysal, P.I., Vural, M. et al. Thickness of carotid artery intima is an independent risk factor for psoriasis. Arch Dermatol Res 313, 147–154 (2021). https://doi.org/10.1007/s00403-020-02084-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-020-02084-z