Abstract
Heat is known as an environmental factor that causes significant skin pigmentation, but its effects on melanogenesis have been poorly studied. It has been shown that mitogen-activated protein kinase (MAPK) is involved in ultraviolet B (UVB) and stress-induced melanogenesis in melanocytes. In this study, we investigated the effects of heat and UVB, on melanocyte melanogenesis, differentiation, and MAPK phosphorylation. The results showed that heat (1 h at 40 °C for 5 days) increased cell dendrites, enlarged cell bodies, and induced extracellular signal-regulated kinases (ERK)/p38/MITF activation but did not influence melanogenesis of human epidermal melanocytes from skin phototype III. UVB irradiation (20 mJ/cm2 for 5 days) induced melanogenesis and c-jun N-terminal kinases (JNK)/p38/MITF/tyrosinase activation in melanocytes from skin phototype III. UVB combined with heat resulted in much more significant tyrosinase activation and melanogenesis as compared with UVB alone in melanocytes from skin phototype III. Furthermore, heat treatment and UVB irradiation induced JNK, ERK, and p38 activation but not melanogenic and morphological changes in melanocytes from skin phototype I. These findings suggested that heat promoted melanocyte differentiation, probably via heat-induced ERK/p38/MITF/activation. Furthermore, heat had an additive effect on the UVB-induced tyrosinase activation and melanogenesis. These results provide a new clue for dermatologists for the treatment of hypopigmented skin disease with heat combined with UVB irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin pigmentation, which results from the production and distribution of melanin in the epidermis, is the major physiological defense against solar irradiation [7]. The solar radiation that reaches the earth’s surface consists chiefly of the ultraviolet (UV, from 280 to 400 nm), the visible spectrum (from 400 to 760 nm) and the near infrared (from 760 to 1,000 nm) [5]. Most of the studies on sunlight-induced pigmentation of skin focused on UV radiation-induced pigmentation and ways to prevent it [4, 6, 8]. It has been shown that infrared radiation (or heat) and visible component of sunlight can also cause significant skin pigmentation [16, 19, 21]. Recently, we observed a striking repigmentation effect in some vitiligo patients treated with moxibustion (burning herbs to stimulate acupuncture points or vitiligo lesions). These findings suggested a melanogenic role of heat treatment.
Melanin synthesis is regulated by melanogenic enzyme tyrosinase, tyrosinase-related protein 1 (TRP-1) and tyrosinase-related protein 2 (TRP-2). Tyrosinase catalyzes the rate-limiting step of melanogenesis and TRP-2 and TRP-1 work at the downstream points in the melanin biosynthetic pathway [9, 10, 18]. These enzymes are transcriptionally regulated by microphthalmia-associated transcription factor (MITF), a pivotal regulator in melanocyte biology and development [23]. In addition, growing evidence has shown that MITF is activated by the mitogen-activated protein kinase (MAPK) signaling pathways, including extracellular signal-regulated kinases (ERK), c-jun N-terminal kinases (JNK) and p38 MAPK [1, 3, 14, 23, 27].
It has been documented that p38 MAPK cascade is involved in UV-induced MITF activation and melanogenesis [6, 8]. In contrast, decreased melanin synthesis and MAPK activation have been reported in heat-treated Mel-Ab cells (a mouse melanocyte cell line), which suggested that the imbalance between ERK and p38 activation may play a role in MITF degradation and hypopigmentation [13]. However, Nakazawa et al. [16] reported a melanogenic response induced by heat treatment, as well as enlarged cell bodies and increased dendrites in human epidermal melanocytes. The detailed mechanisms involved in heat- or UV-induced melanocyte biological changes have not been thoroughly studied.
In the present study, by comparing the effects of heat with those of UVB on human epidermal melanocytes from different skin phototype viability/morphology, melanogenesis and MAPK expression, we found that heat treatment promoted melanocyte differentiation but does not influence melanogenesis, which differs from the melanogenic effect induced by UVB. In addition, heat treatment had an additive effect on the UVB-induced tyrosinase activation and melanogenesis.
Materials and methods
Cell culture
Primary human epidermal melanocytes were isolated from the epidermis of donated human foreskin with skin phototype III. To compare the effects of heat treatment on melanocytes from different skin phototypes, the human epidermal melanocyte cell line HEM-l (ScienCell, Catalog # 2200, America) was also used in our study. Melanocytes were cultured in Medium 254 (Cascade Biologics; USA), supplemented with human melanocyte growth supplement (HMGS) (Cascade Biologics; USA) containing bovine pituitary extract (BPE), fetal bovine serum (FBS), bovine insulin, bovine transferrin, basic fibroblast growth factor, hydrocortisone, heparin, and phorbol 12-myristate 13-acetate (PMA). Antibiotics (penicillin and streptomycin) were usually added to the cell culture medium. Second passage melanocytes were used in the experiments.
Heat treatment and UVB irradiation
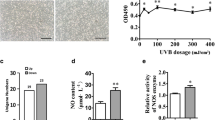
As heat and UVB are two major stimuli of solar light (Fig. 1a), UVB treated group was used as a positive control to investigate the effects of heat treatment and the interaction between the two solar stimuli. Heat treatment was performed in a separate incubator in 5 % CO2 for 1 h at 40, 42 and 44 °C (for 5 days) to determine the appropriate temperature that is non-cytotoxic to melanocytes. UVB irradiation was performed with phototherapy equipment (SS-01B-2 UV, Shanghai Sigma High Technology Co., LTD, Shanghai, China) with a light intensity of 11.2 mW/cm2 at 20 mJ/cm2 for 5 days. Cells were divided into four groups to examine the effects of heat and UVB: control group, heat-treated group (1 h at 40 °C for 5 days), UVB-irradiated group (20 mJ/cm2 for 5 days) and UVB-plus-heat-treated group [treated with UVB (20 mJ/cm2 once daily for 5 days) following the pretreatment with heat (1 h at 40 °C once daily for 5 days)].
a Solar radiation spectrum. b Effect of heat treatment on cell viability in human epidermal melanocytes was assessed by MTT assay. c Representative morphology changes in human epidermal melanocytes from skin phototype III after heat treatment (1 h at 40 °C for 5 days) were observed by a phase contrast microscope. The results shown are representative of three independent experiments. *p < 0.05, **p < 0.01, compared with the control
Cell viability assay and morphology observation
Cell viability was assessed using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT, Sigma) assay. The assay was performed according to the instructions of the manufacturer. Briefly, human epidermal melanocytes were cultured in 96-well plates; 20 μl of 5 mg/ml MTT labeling reagent was added to each well containing cells in 150 μl of medium, and the plate was incubated for 4 h in a humidified incubator at 37 °C to allow the MTT to be metabolized. The media were removed and cells were re-suspended in formazan (MTT metabolic product) in 200 μl dimethyl sulfoxide (DMSO). The plate was placed on a shaker for 5 min to thoroughly mix the formazan into the solvent. The absorbance of the samples was measured at a wavelength of 490 nm. The extent of MTT conversion in cells exposed to heat is expressed as a percentage of the control. Morphology observation was performed under a phase contrast microscope (Olympus Optical Co., Tokyo) and images were captured at 200× magnification using a CoolSNAP digital video camera system (Rope Scientific, Inc., Tucson, AZ).
Quantitative measure of morphological parameter
The cell body area was measured using the Image Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD) according to Uusisaari’s method for neuron morphometry analysis [26]. Cell body area was determined by outlining the perimeter of cell body. The transition from cell body to proximal dendrite may be arbitrarily determined but the same criteria were applied to all melanocytes. Number of dendrites was counted manually. In each group, three pictures were taken and 20 melanocytes per picture were randomly selected for image analysis. All pictures were obtained and processed for image analysis by two pathologists, each of whom was blinded to the examples.
Melanin content determination
The total amount of melanin content was measured using the sodium hydroxide solubilization method [25]. After treatment, cells were cultured for 2 days and collected. Cell pellets were then dissolved in 1 ml of 1 N NaOH at 100 °C for 30 min and centrifuged for 20 min at 16,000g. Optical densities of supernatants were measured at 400 nm using an ELISA reader. The absorbance was compared with a standard curve of synthetic melanin (Sigma, Poole, England).
MAPK inhibition assay
To investigate the role of MAPKs in melanocyte biology, the affected MAPKs subtypes (ERK and p38) were inhibited and the cells were divided into four groups: control group, heat-treated group (1 h at 40 °C for 5 days), heat + ERK inhibited group (treated with heat in the presence of pretreatment with PD98059 at 20 μM for 1 h) and heat + p38 inhibited group (treated with heat in the pretreatment with SB203580 at 20 μM for 1 h). The cells were obtained 2 days after the last heat treatment.
Western blot analysis
Cultured human epidermal melanocytes were homogenized using RIPA lysis buffer [50 mM Tris/HCl, pH 8.0, 250 mM NaCl, 1 % NP40, 0.5 % (w/v) sodium deoxycholate, 0.1 % sodium dodecylsulfate, complete mini-protease inhibitors (Roche)]; 50 μg of protein from each cell extract was separated on an SDS-polyacrylamide gel, and the fractionated proteins were then transferred from the gel onto the nitrocellulose membrane (Millipore Corporation, Massachusetts). The membranes were blocked overnight at room temperature with 10 % bovine serum albumin in blot wash buffer at 4 °C overnight, and then incubated with the primary Ab diluted in 10 % bovine serum albumin for 60 min. Blots were incubated with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies. Rabbit monoclonal antibodies to MITF (1:500, Neomarker, USA), tyrosinase (1:500, Neomarker, USA), ERK/phospho-ERK, JNK/phospho-JNK, and p38/phospho-p38 MAPK (1:1,000, 1:1,000, 1:1,000; Cell Signaling Technology, USA) were used as primary antibodies. Immunocomplexes were visualized using the enhanced chemiluminescence system (Amersham International, USA). The results were analyzed using the Image J software (NIH Image, National Institutes of Health, Bethesda, MD; online at: http://rsb.info.nih.gov/ij/).
Statistical analysis
Data were presented as mean ± standard deviation (SD) and analyzed by SPSS 15.0 software (SPSS). One-way analysis of variance (ANOVA) was used to compare the quantitative data. The differences were considered statistically significant at p < 0.05 among the groups: heat treated group, UVB irradiated group, UVB + heat treated group and the control.
Results
Heat treatment at 40 °C was non-cytotoxic to human epidermal melanocytes from skin phototype III
Firstly, cell viability was assessed to determine the appropriate temperature that is non-cytotoxic to melanocytes. The results showed that heat treatment exceed 42 °C decreased the cell viability in human epidermal melanocytes from skin phototype III. No significant difference in cell viability was observed between 40 °C treated group and the control, suggesting that 40 °C is non-cytotoxic to human epidermal melanocytes (Fig. 1b). Thus 40 °C was chosen as the temperature to perform heat treatment.
Heat treatment increased the dendrites and enlarged the cell bodies in human epidermal melanocytes from skin phototype III but not in skin phototype I
After 5 days of heat treatment (1 h at 40 °C), melanocytes from phototype III developed increased dendrites and enlarged cell bodies (Fig. 1c). Quantitative measurement of morphological parameters by image analysis revealed a significant elevation in cell body area and number of dendrites in the heat-treated group compared with the control, indicating a differential effect of heat treatment (Table 1). No morphologic changes were detected in the UVB treated group, as well as the UVB + heat treated group (Fig. 1c; Table 1). However, No morphological changes were observed in HEM-l after above stimuli (Table 1).
UVB but not heat treatment induced melanogenesis of melanocytes from skin phototype III
To investigate the effect of heat and UVB on melanogenesis, the melanin content of melanocytes from skin phototype III was examined after heat or/and UVB treatments. UVB induced a significant increase in melanin content compared with the control. In contrast, there was no remarkable change in melanin content following heat treatment. Compared with the UVB irradiated group, UVB + heat treated group showed much more significant increase in melanin content, suggesting that heat treatment had an additive effect on the UVB-induced melanin synthesis (Fig. 2a).
a Effect of heat or/and UVB treatment on melanin synthesis of melanocytes from skin phototype III. The sodium hydroxide solubilization method was used to determine the melanin content. b Protein expression levels were determined by Western blot using antibodies directed against phospho-ERK, phospho-JNK, phospho-p38, MITF and tyrosinase. GAPDH was included as a protein-loading control. c Quantification of phosphorylated protein levels of MAPKs normalized to GAPDH. Baseline phosphorylated protein level of the control was assigned a value of 1, and fold changes above baseline were presented as mean ± standard deviation. *p < 0.05, **p < 0.01 indicates a significant difference between the heat- or UVB-treated group and the control. † p < 0.05, ‡ p < 0.01 indicates a significant difference between the UVB + heat treated group and the UVB-irradiated group
Heat treatment induced ERK/p38/MITF activation whereas UVB induced JNK/p38/MITF and tyrosinase activation in melanocytes from skin phototype III
To elucidate the intracellular signaling pathways involved in UVB-induced melanogenesis and heat-induced morphological changes, we analyzed the phosphorylation levels of MAPKs in heat and UVB treated human epidermal melanocytes from skin phototype III. Western blot analysis revealed that phosphorylated JNK and p38 were significantly up-regulated in the UVB irradiated group, concomitant with the elevation of MITF/tyrosinase and melanin content (Fig. 2a–c). Among these MAPK subtypes, ERK signaling pathway was unaffected by UVB irradiation. In contrast, heat treatment induced significant activation of ERK, p38 and MITF, with no significant effect on JNK signaling pathway. Interestingly, p38 was activated following both heat treatment and UVB irradiation, indicating the pivotal role of p38 in stress-induced MITF/tyrosinase activation and melanogenesis. Compared with UVB irradiation, UVB + heat treatment induced much more significant phosphorylation of JNK, ERK and p38, concomitant with a significant elevation of MITF/tyrosinase and melanin content (Fig. 2a–c), which indicated the additive effects of heat treatment and UVB on MITF/tyrosinase activation and melanogenesis.
MAPK signaling pathways were involved in tyrosinase activation and melanin synthesis in melanocytes from skin phototype III
To further investigate the role of MAPKs in heat-induced MITF activation or morphological changes, we pre-incubated human epidermal melanocytes from skin phototype III with selective MAPK inhibitors before heat treatment. Pre-incubation with a selective inhibitor of p38, SB203580 (20 μM), significantly attenuated the heat-induced MITF activation and melanin production, indicating a melanogenic role of p38 (Fig. 3a–c). In contrast, pre-incubation with PD98059 (ERK inhibitor, 20 μM) markedly induced melanin content increase and MITF/tyrosinase activation, confirming the anti-melanogenic effect of ERK. Pre-incubation with SP600125 (JNK inhibitor, 20 μM) induced no melanogenesis or morphological changes (data not shown). These findings indicated a melanogenic role of p38 and an anti-melanogenic role of ERK, which may also contribute to the melanocyte differentiation.
a Effects of p38 and ERK inhibitor on MITF/tyrosinase activation and melanogenesis in melanocytes from skin phototype III After serum starvation for 24 h, 20 μM of PD98059 and SB203580 was pretreated for 1 h prior to heat treatment. The total amount of melanin content was measured using the sodium hydroxide solubilization method. b Protein expression levels were determined by Western blot using antibodies directed against phospho-ERK, phospho-JNK, phospho-p38, MITF and tyrosinase. GAPDH was included as a protein-loading control. c Quantification of phosphorylated protein levels of MAPKs normalized to GAPDH. Baseline phosphorylated protein level of control was assigned a value of 1, and fold changes above baseline were presented as mean ± standard deviation. *p < 0.05, **p < 0.01 indicates a significant difference between the heat + MAPKs inhibited group and the heat treated group
HEM-l melanocytes responded similar with the melanocytes from skin phototype III in melanogenesis signal pathway
To compare the effects of heat treatment on melanocytes from different skin phototypes, commercial melanocytes from skin phototype I, HEM-l were also used in this study. In HEM-1, heat treatment induced JNK, ERK, and p38 activation, which concords with the effect of heat treatment on melanocytes from skin phototype III (Fig. 4b, c). Inconsistently, there were no significant changes in MITF, tyrosinase, melanogenesis after heat treatment (Fig. 4a–c).
a Effects of UVB or/and heat treatment on melanin synthesis of melanocytes from skin phototype I. b Protein expression levels were determined by Western blot using antibodies directed against phospho-JNK, phospho-ERK, phospho-p38, MITF and tyrosinase. GAPDH was included as a protein-loading control. c Quantification of phosphorylated protein levels of MAPKs normalized to GAPDH. Baseline phosphorylated protein level of control was assigned a value of 1, and fold changes above baseline were presented as mean ± standard deviation. *p < 0.05, **p < 0.01 indicates a significant difference between the heat or UVB treated group and the control. † p < 0.05, ‡ p < 0.01 indicates a significant difference between the UVB + heat treated group and the UVB irradiated group
Discussion
Direct induction of melanogenesis by UV irradiation has been discussed in several types of cultured melanocytes [4, 15, 24]. Few studies have addressed the biological effects of heat on melanocytes though heat is one of the major environmental factors that affect the human skin [11, 12, 13, 16]. The first study investigating the relationship of melanocyte biology and heat treatment was done by Nakazawa et al. They demonstrated enlarged cell bodies, more dendrites and higher levels of melanin content in heat-treated human epidermal melanocytes (42 °C for 1 h for 5 days) [16]. Consistently, our results showed that heat treatment (40 °C for 1 h for 5 days) increased dendrites and enlarged cell bodies. Moreover, heat treatment induced significant activation of ERK and MITF, which play pivotal roles in melanocyte biology [20, 23]. ERK and MITF activation provided a clue to explain the increased dendrites and enlarged the cell bodies, which indicated the differentiation of melanocyte. However, heat treatment did not affect the melanin production, which seems to be contradictory to the findings of Nakazawa or Kim, who reported increase or decrease of melanin content in heat-treated melanocytes. The discrepancy may be due to the different duration and temperature of heat treatment. In this study, we found that the temperature 40 °C was non-cytotoxic to melanocytes, thus heat treatment was performed at 40 °C (1 h once daily, for 5 days), which differs from the temperature in previous studies [13, 16].
Growing evidence showed that MAPK signaling pathways play important roles in MITF modulation and melanogenesis [15, 22, 28]. It has been reported that p38 MAPK pathway was involved in the stress-induced melanogenesis [1, 3, 8]. Recently, Kim et al. [13] reported that both ERK and p38 were activated in the heat-treated melanocytes, suggesting that the balance between ERK and p38 may play a role in the degradation and hypopigmentation effect of MITF. In this study, both phosphorylated ERK and p38 were elevated after heat treatment, which is consistent with the results of Kim et al. Interestingly, the phosphorylated ERK and p38 had no effect on melanin content, suggesting that the imbalance between ERK and p38 in our study was insufficient to induce any change in melanin synthesis.
As UVB and heat are two major stimuli of solar radiation, the UVB treated group was set as a positive control to study the effect of heat treatment. It was found that heat treatment induced different expression pattern of MAPKs from UVB. Compared with the UVB-induced MITF/tyrosinase/melanin elevation, much more significant MITF/tyrosinase/melanin elevation associated with prominent p38 and JNK phosphorylation was shown in the UVB plus heat treated group. This finding indicated that heat had an additive effect on the UVB-induced tyrosinase activation and melanogenesis, probably though the significant p38 elevation. However, UVB plus heat induced no morphological changes in melanocytes. The remarkable JNK activation induced by UVB plus heat treatment and thus a probable inhibitory effect on cell differentiation may contribute to the unaffected morphology. However, the detailed mechanism still remains unclear.
Selective inhibition assay showed that p38 inhibitor decreased the MITF/tyrosinase activation and melanin content, suggesting a melanogenic role of p38, which is consistent with previous studies [2, 17]. On the contrary, ERK inhibitor increased the MITF/tyrosinase activation and melanin production, indicating that ERK had an anti-melanogenic effect, which supports the findings of Kim [11].
Skin phototypes of Chinese people are mainly composed of phototype III. And it is inconvenient for us to get melanocytes from skin phototype I people. In this study, melanocytes from skin phototype III were used to investigate the effects of heat treatment and UVB on melanocytes. In this study, different cell lines responded differently to heat treatment. Heat treatment and UVB irradiation induced JNK, ERK, and p38 activation but not melanogenic and morphological changes in HEM-l, and did not concord with the response of melanocytes from skin phototype III. The mechanism was unclear. Compared with melanocytes from dark-colored skin, melanocytes from light-colored skin may be less affected by heat treatment and UVB.
Taken together, heat treatment promoted melanocyte differentiation via the activation of ERK which played an anti-melanogenic role. In addition, heat had a synergetic effect on UVB-induced tyrosinase activation and melanogenesis. These findings provided a new clue for the physical treatment of hypopigmented skin disease.
References
Ahn JH, Jin SH, Kang HY (2008) LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch Dermatol Res 300:325–329
Ahn EH, Schroeder JJ (2006) Sphinganine causes early activation of JNK and p38 MAPK and inhibition of AKT activation in HT-29 human colon cancer cells. Anticancer Res 26:121–127
Bellei B, Maresca V, Flori E, Pitisci A, Larue L, Picardo M (2010) p38 regulates pigmentation via proteasomal degradation of tyrosinase. J Biol Chem 285:7288–7299
Bolognia J, Murray M, Pawelek J (1989) UVB-induced melanogenesis may be mediated through the MSH-receptor system. J Invest Dermatol 92:651–656
Christiaens FJ, Chardon A, Fourtanier A, Frederick JE (2005) Standard ultraviolet daylight for nonextreme exposure conditions. Photochem Photobiol 81:874–878
Corre S, Primot A, Sviderskaya E (2004) UV-induced expression of key component of the tanning process, the POMC and MC1R genes, is dependent on the p-38-activated upstream stimulating factor-1 (USF-1). J Biol Chem 279:51226–51233
Fitzpatrick TB, Seiji M, Mc GA (1961) Melanin pigmentation. N Engl J Med 265:430–434 concl
Galibert MD, Carreira S, Goding CR (2001) The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced Tyrosinase expression. EMBO J 20:5022–5031
Hearing VJ, Jimenez M (1987) Mammalian tyrosinase—the critical regulatory control point in melanocyte pigmentation. Int J Biochem 19:1141–1147
Kameyama K, Sakai C, Kuge S (1995) The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell Res 8:97–104
Kim DS, Park SH, Kwon SB, Na JI, Huh CH, Park KC (2007) Additive effects of heat and p38 MAPK inhibitor treatment on melanin synthesis. Arch Pharm Res 30:581–586
Kim DS, Park SH, Kwon SB (2003) Temperature regulates melanin synthesis in melanocytes. Arch Pharm Res 26:840–845
Kim DS, Park SH, Kwon SB, Youn SW, Park ES, Park KC (2005) Heat treatment decreases melanin synthesis via protein phosphatase 2A inactivation. Cell Signal 17:1023–1031
Levy C, Khaled M, Fisher DE (2006) MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 12:406–414
Mizutani Y, Hayashi N, Kawashima M, Imokawa G (2010) A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch Dermatol Res 302:283–294
Nakazawa K, Sahuc F, Damour O, Collombel C, Nakazawa H (1998) Regulatory effects of heat on normal human melanocyte growth and melanogenesis: comparative study with UVB. J Invest Dermatol 110:972–977
Park SY, Kim YH, Park G, Lee SJ (2010) Beta-carboline alkaloids harmaline and harmalol induce melanogenesis through p38 mitogen-activated protein kinase in B16F10 mouse melanoma cells. BMB Rep 43:824–829
Prota G (1993) Regulatory mechanisms of melanogenesis: beyond the tyrosinase concept. J Invest Dermatol 100:156S–161S
Ramasubramaniam R, Roy A, Sharma B, Nagalakshmi S (2011) Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochem Photobiol Sci 10:1887–1893
Saha B, Singh SK, Sarkar C (2006) Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res 19:595–605
Sklar LR, Almutawa F, Lim HW, Hamzavi I (2013) Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci 12:54–64
Smalley K, Eisen T (2000) The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. FEBS Lett 476:198–202
Tachibana M (2000) MITF: a stream flowing for pigment cells. Pigment Cell Res 13:230–240
Tada A, Pereira E, Beitner-Johnson D, Kavanagh R, Abdel-Malek ZA (2002) Mitogen- and ultraviolet-B-induced signaling pathways in normal human melanocytes. J Invest Dermatol 118:316–322
Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y (1998) Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res 11:275–282
Uusisaari M, Obata K, Knopfel T (2007) Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol 97:901–911
Yanase H, Ando H, Horikawa M, Watanabe M, Mori T, Matsuda N (2001) Possible involvement of ERK 1/2 in UVA-induced melanogenesis in cultured normal human epidermal melanocytes. Pigment Cell Res 14:103–109
Ye Y, Wang H, Chu JH, Chou GX, Yu ZL (2011) Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp Dermatol 20:755–757
Acknowledgments
This study was supported by the grant from the National Natural Science Foundation of China (No. 81271745 and 81271774), and the special financial support from the China Postdoctoral Science Foundation (2012T50876).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
W-J. Gu and H-J. Ma contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gu, WJ., Ma, HJ., Zhao, G. et al. Additive effect of heat on the UVB-induced tyrosinase activation and melanogenesis via ERK/p38/MITF pathway in human epidermal melanocytes. Arch Dermatol Res 306, 583–590 (2014). https://doi.org/10.1007/s00403-014-1461-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1461-y