Abstract
Introduction
Until now, a treatment protocol for Achilles tendon re-rupture (ATRR) occurring in the postoperative period 5–12 weeks following primary Achilles tendon repair has not been established. We refer to this time frame as the subacute postoperative phase, and the objective of this study was to assess the efficacy of conservative treatment for subacute ATRR in this phase.
Materials and methods
We conducted a retrospective review of 390 cases (385 patients) who had undergone primary Achilles tendon repair using the 4-strand Krachow method between January 2010 and August 2021. All patients were subjected to more than 12 months of follow-up and were categorized into two groups based on the presence of subacute ATRR: Group 1 comprised 370 cases without ATRR, while Group 2 comprised 20 cases with ATRR. Following confirmation of ATRR, we immediately applied a below-knee cast in an ankle plantar flexed position (25°–30°), followed by bracing according to the same rehabilitation plan used for the primary repair. After administering conservative treatment to the patients with ATRR, we compared several outcome parameters between the two groups, including isokinetic plantar flexion power measured using a dynamometer, time required for a single heel raise (t-SHR), time needed for ten repetitive SHRs (t-SHR10), Achilles Tendon Total Rupture Score (ATRS), and Foot and Ankle Ability Measure (FAAM) scores. The baseline timepoints for Groups 1 and 2 were the dates of the primary repair and the re-injury event.
Results
After primary Achilles tendon repair, subacute ATRR occurred in 5.1% of patients. There were no significant differences between the groups in terms of t-SHR and t-SHR10 (P = 0.281, 0.486). Similarly, the isokinetic dynamometer measurements revealed no significant differences in peak torque for plantar flexion at angular velocities of 30°/s and 120°/s, both in absolute values and as a percentage of the contralateral side, between the groups (P > 0.05 for each). However, ATRSs were significantly lower in Group 2 compared to Group 1 before 6 months (P < 0.05), as were FAAM-Activities of Daily Living scores at 6 months (P < 0.05). After 12 months, there were no significant differences in these scores between the two groups (both P > 0.05).
Conclusion
Conservative treatment for subacute ATRR following primary Achilles tendon repair yields clinical outcomes comparable to those without ATRR. Therefore, we recommend that surgeons consider relying on the patient’s natural healing capabilities rather than opting for aggressive surgical interventions, as expediting such operations may be unnecessary for subacute injuries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Achilles tendon rupture (ATR) caused by sudden dorsiflexion of the ankle is a common injury with a rising incidence [1], often resulting in significant morbidity. According to a recent nationwide study involving 16,830,532 individuals, the reported incidence rate was 2.40 per 10,000 person-years [2]. Various studies have examined and compared different operative and nonoperative treatment approaches. The consensus is that nonoperative treatment carries a higher risk of re-rupture compared to operative treatment but avoids potential surgical complications such as postoperative deep infection and sural nerve injury [3]. This perspective is supported by numerous systematic reviews and meta-analyses [4,5,6,7].
Achilles tendon re-rupture (ATRR) is characterized by the rupture of the previously repaired site, often associated with suture material failure due to an unexpected traumatic event. Specifically, we designate cases occurring between postoperative weeks 5 and 12 following primary Achilles tendon repair as “subacute ATRR.” Patients are particularly susceptible to ATRR during this time frame because the healing tendon has not fully regained strength, yet patients require rehabilitation, with or without a brace. Surgeons often ponder whether to opt for reoperation or simply monitor the patient, as there is a dearth of studies addressing ATRR during the subacute postoperative phase [8, 9]. Additionally, a standardized treatment protocol remains elusive. Consequently, this study delves into the effectiveness of conservative treatment for subacute ATRR, comparing objective and subjective outcomes in patients with and without this condition. Our hypothesis posits that reoperation may not be necessary, as conservative treatment can yield successful clinical results. Furthermore, we anticipate that most subacute ATRRs will manifest as incomplete injuries with residual repair-site continuity.
Materials and methods
Ethical considerations
This study was carried out in accordance with the principles outlined in the Declaration of Helsinki and received approval from our institution’s ethics review committee. Written informed consent was obtained from all enrolled patients.
Patient selection
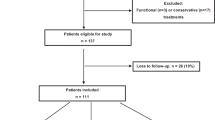
We conducted a retrospective analysis of the medical records of patients aged 15 years and older who underwent Achilles tendon repair (without a lengthening procedure, such as V–Y advancement or turn-down flap) for ATR between January 2010 and August 2021. Initially, we assessed 461 cases, involving 454 patients. Then, we excluded patients with an injury-to-operation duration of more than 4 weeks to include only patients who underwent operative treatment for acute ATR. Additionally, patients with a partial Achilles tendon rupture related to a skin laceration or Achilles tendon sleeve avulsions requiring Achilles tendon reattachment to the calcaneal tuberosity were excluded, as were those with less than 12 months follow-up after the primary repair or ATRR date. Further exclusions were made for patients who developed postoperative deep infections necessitating surgical debridement (five cases) and those who experienced late ATRR beyond 12 weeks postoperatively (four cases). Finally, 390 cases (385 patients) were included in this study.
The patients were divided into two groups based on the development of ATRR 5–12 weeks postoperatively: Group 1: without subacute ATRR; Group 2: with subacute ATRR. Figure 1 presents the patient selection algorithm.
Original postoperative rehabilitation protocol
Two orthopedic surgeons performed all primary Achilles tendon repairs. In all cases, a 4-strand Krachow stitch using no. 5 coated polyester sutures (Ethibond Excel®, Ethicon) was threaded through the tendon and tied to securely attach the ruptured free ends in an open manner. Subsequently, the paratenon was repaired with 2–0 polyglactin 910 sutures (Coated Vicryl®, Ethicon).
Postoperatively, the patients were prohibited from bearing weight for four weeks in below-knee casts with natural plantar flexed ankle positions (25°–30°). Afterward, the patients were instructed to use walking boots with three wedges (hereafter, brace) for the next four weeks. Each wedge was 1 cm in height, and the patients’ heels were lifted to 10°. During the first week, the patients were allowed to walk in the brace with all three wedges and were taught how to remove one wedge per week. During the fourth week, the patients remained in the brace without wedges. This technique was designed to enhance the patient’s walking ability with a gradual increase in ankle dorsiflexion. Subsequently, the patients performed double heel raise (DHR), range of motion, and one-leg standing exercises for proprioception. Single heel raise (SHR) exercises were started after the patients were proficient in DHRs. Sports activities were allowed when the patients could perform ten consecutive SHRs.
Subacute ATRR diagnosis and treatment
Initially, we conducted a thorough physical examination. The diagnostic criteria for ATRR encompassed the emergence of newly developed or exacerbated pain at the prior surgical site and the presence of skin dimpling subsequent to an unexpected traumatic incident. Unlike the physical examination for acute ATRs, reduced active ankle plantar flexion power and a positive Thompson squeeze test result could not help confirm ATRR since the gastrocnemius/soleus muscular atrophy was already developed from lack of use due to the preceding below-knee cast application. Therefore, a definitive diagnosis was established using magnetic resonance imaging (MRI) with a slice thickness of 3 mm and an interslice gap of 0.3 mm or ultrasound in all cases of suspected ATRR. Based on these imaging studies, we diagnosed ATRR by confirming the loss of continuity at the previous Achilles tendon repair site, and we determined whether it was a complete or incomplete rupture. Incomplete ATRR was defined as visible continuity of the repaired site on the consecutive two sagittal cuts by MRI. On ultrasound, incomplete re-rupture was diagnosed when the proximal part of the ATRR site moved with passive ankle dorsiflexion by the examiner.
Following the confirmation of ATRR, we promptly reapplied a below-knee cast with the ankle positioned in plantar flexion (25–30 degrees) for four weeks. Subsequent to the immobilization period, patients adhered to the same bracing regimen and rehabilitation plan as those undergoing primary repair.
Assessments
We collected demographic data from the patients, including age at operation, sex, body mass index (BMI), follow-up duration, smoking status, and any underlying diseases. Notably, for patients in Group 2, we conducted interviews to gather information regarding the circumstances of their reinjury, including when and how it occurred, along with the time elapsed between the date of subacute ATRR and the initiation of conservative treatment. Additionally, we assessed the findings from MRI or ultrasound examinations.
After surgery, it was a principle to have monthly follow-up appointments whenever possible for the patients without ATRR (Group 1). However, at the 3-month, 6-month, and 12-month post-surgery milestones, it was mandatory to visit the outpatient clinic. In cases where patients were unable to come in at other times, we encouraged them to call and make arrangements to attend the following month. This principle was similarly applied during the initial six months after developing ATRR for patients in Group 2 (Fig. 2). During these follow-up appointments, we collected data on the time required for SHR feasibility and the time taken to perform ten repetitive SHRs. For patients in Group 1, this data was recorded from the date of the primary repair. In Group 2, it was recorded from both the date of the primary repair and the date of ATRR. Additionally, we measured isokinetic plantar flexion power using a dynamometer [10, 11], specifically recording the peak torques at angular velocities of 30°/s and 120°/s once patients were capable of performing SHRs. This data included absolute values (Nm) and percentages relative to the contralateral side.
The clinical outcomes were measured using the Achilles tendon Total Rupture Score (ATRS) scale [12] and the Foot and Ankle Ability Measure (FAAM)-Activities of Daily Living (ADL) and Sports subscales [13, 14]. These parameters were measured 3, 6, and ≥ 12 months after the primary Achilles tendon repair date in Group 1 and after the ATRR date in Group 2. We also collected data about other postoperative complications during the follow-up period for patients in Group 2.
Statistical analyses
The means and standard deviations of all dependent parameters were calculated using SPSS version 21 (IBM Corp., Armonk, NY, USA). We assessed the data distributions using the Kolmogorov–Smirnov test. The Mann–Whitney U test was used to compare continuous numeric parameters between Groups 1 and 2, whereas the Fisher’s exact test was used to compare proportional parameters. Statistical significance was set at the 5% level.
Additionally, we calculated an appropriate effect size for the Mann–Whitney U test using the formula \(r=\frac{ |{\text{z}}|}{\sqrt{n}}\) (r, effect size; z, standardized test statistic; n, number of pairs).
Results
Demographic data
Table 1 presents the demographic data of the patients in our study. Out of a total of 390 cases, 20 (5.1%) experienced subacute ATRR. There were no significant differences in mean age at operation, BMI, and median follow-up duration between Groups 1 and 2 (P = 0.768, 0.562, and 0.175, respectively). Notably, Group 2 exclusively comprised male patients; in the overall patient population, women constituted 15.3% (P = 0.001). Additionally, the smoking rate was significantly higher in Group 2 compared to Group 1 (P = 0.001).
The patients in Group 2 sustained ATRR on average, 50.2 ± 11.5 days (range, 30–70) postoperatively. The most common circumstances leading to ATRR in this group were slipping while bathing without wearing the prescribed brace (14 out of 20 cases, 70.0%), followed by slipping without the brace during the period when brace use was still necessary (4 out of 20 cases, 20.0%), and an abrupt jumping motion after completing the brace protocol (2 out of 20 cases, 10.0%). On average, there was a delay of 6.9 ± 3.7 days (range 3–10) from the date of subacute ATRR to the initiation of cast immobilization. Interestingly, all patients in Group 2 exhibited incomplete ruptures as confirmed by either MRI or ultrasound imaging.
Outcome comparisons
The time elapsed from the primary operation date until patients were capable of performing SHRs was significantly longer in Group 2 compared to Group 1 (P = 0.001; see Table 2). However, the time required to perform SHRs from the primary repair date in Group 1 and the ATRR date in Group 2 did not exhibit a significant difference (P = 0.281). Likewise, the time interval from the primary operation date until patients could perform ten consecutive SHRs was notably longer in Group 2 (P = 0.001), but the duration measured from the primary repair date in Group 1 and the ATRR date in Group 2 to achieve ten repetitive SHRs did not show a significant difference (P = 0.486). Additionally, the results from isokinetic dynamometer testing revealed that the peak torques of plantar flexion at angular velocities of 30°/s and 120°/s, in both absolute values and as a percentage relative to the contralateral side, did not differ significantly between the two groups (all P > 0.05). Figure 3 illustrates the clinical scores obtained using the ATRS scale, as well as the FAAM-ADL and Sports subscales. The ATRS values at three and six months postoperatively were significantly lower in Group 2 compared to Group 1 (both P = 0.001), but there was no significant difference in the values after 12 months (P = 0.572). Similarly, the FAAM-ADL score at six months was lower in Group 2 (P = 0.01), but there were no significant differences in the values at three months or after 12 months between the two groups (both P > 0.05). Interestingly, the FAAM-Sports scores did not exhibit significant differences at any of the measured time points. Importantly, none of the patients in Group 2 experienced re–re-rupture again after the initial re-rupture, persistent loss of SHR, or deep infection requiring surgical debridement during the follow-up period.
Clinical score comparisons between patients with and without Achilles tendon re-rupture using the a ATRS scale, b FAAM-ADL and c FAAM-Sports subscales. The ATRS values were significantly lower in Group 2 than in Group 1 three and six months postoperatively (both P = 0.001); the values did not differ after 12 months (P = 0.572). The FAAM-ADL score was significantly lower at six months in Group 2 than in Group 1 (P = 0.01); the values at three months and 12 months did not differ (all P > 0.05). FAAM-Sports scores did not differ. *Significant difference between Group 1 and Group 2 (P < 0.05). ATRS Achilles tendon total rupture score, FAAM Foot and Ankle Ability Measure, ADL Activities of Daily Living
The effect size calculation for the difference in ΔATRS between the two groups was 0.706, implying a greater than medium effect size (> 0.5).
Discussion
This study found that conservative treatment was effective for incomplete subacute ATRR recovery; patients with subacute ATRR had inferior early clinical outcomes compared to those without ATRR, but the final clinical outcomes after 12 postoperative months did not differ between the two groups. These results are supported by the isokinetic dynamometer and SHR data obtained during serial follow-up. Accordingly, ATRR did not affect the peak torques of plantar flexion at 30°/s and 120°/s angular velocities if the patients could perform SHRs. Furthermore, the time from baseline to performing SHRs and ten repetitive SHRs did not differ between the two groups when considering each group’s specific baseline timepoint (i.e., Group 1: from the primary repair date; Group 2: from the re-injury date). However, when the baseline timepoint was set to the primary Achilles tendon repair date, the time to performing SHRs and ten repetitive SHRs was significantly longer in Group 2 than in Group 1. Consequently, it is advisable to establish a treatment plan with treatment restarting from the beginning when subacute ATRR occurs. Furthermore, all patients with subacute ATRR underwent imaging studies (MRI or ultrasound) to investigate the hypothesis that conservative treatment could yield satisfactory outcomes for ATRR. The results showed that all ATRRs were incomplete ruptures, which is a novel finding. Two possible theories were proposed to explain this outcome: (1) the injuries leading to ATRR may not have caused complete suture material failure, and (2) the repaired tendon in the remodeling phase might have sustained minor injuries, contributing to the occurrence of ATRR.
In this study, the terminology “subacute” ATRR was employed, deviating from the traditional classification that only distinguishes between acute and chronic ATR based on the 4-weeks post-injury timeframe [15]. We advocate for this distinct definition for ATRR due to its unique characteristics. Generally, the response of tendons to injury can be categorized into three overlapping stages [16, 17]: (1) the inflammatory stage (typically spanning a few days), (2) the proliferative or repair stage (beginning roughly two days into the injury response), and (3) the remodeling stage (beginning 1 to 2 months after injury). Throughout these healing stages, tendons undergo a series of structural modifications that significantly alter their mechanical properties. In animal models, the tensile strength of ruptured rabbit Achilles tendons was approximately five times lower at 3 weeks after repair compared to uninjured tendons, but it increased to nearly 80% of the uninjured value after 12 weeks [18]. Similarly, Hiramatsu et al. [19] observed an intratendinous hyperechoic area under ultrasound following operative treatment of ATR, which represented scar tissue. However, this hyperechoic area gradually diminished over time and was replaced by a fibrillar appearance by six months postoperatively. Our study chose to focus on re-injury occurring during the subacute postoperative phase because tendons that have undergone repair during this period might still be vulnerable to re-injury. Additionally, conducting revision surgeries during this phase is challenging due to the ongoing remodeling of scar tissues and the presence of inflammatory products. Therefore, the fundamental and practical question underlying our study was: Is it necessary to re-operate a subacute ATRR?
In clinical practice, surgeons may encounter patients with subacute ATRR relatively infrequently, as ATRR tends to be unpredictable and lacks specific patterns of occurrence. Notably, a recent study conducted by Maempel et al. [20] is noteworthy in shedding light on this issue. In their research, they reported an ATRR incidence rate of 1.16 per 100,000 individuals per year among those aged 18 years and older who had undergone mixed (nonoperative and operative) treatment for ATR. This ATRR incidence exhibited a male predominance and was more prevalent in patients from less socioeconomically deprived backgrounds. The study also found that younger age at the time of injury and initial nonoperative treatment involving traditional cast immobilization were independently associated with a higher risk of ATRR. Additionally, Jildeh et al. [21] reported an overall ATRR incidence rate of 1% among 423 patients who underwent operative treatment for ATR and suggested that longer operation and tourniquet times might be risk factors for ATRR. In our study, subacute ATRR exclusively affected male patients (20/20; 100%), and the incidence rate was relatively high at 5.1% (20 out of 390) compared to findings from some previous studies. It is also noteworthy that a significant proportion of ATRR patients in our study were smokers. Considering existing research indicating that smoking can impede tendon healing [22, 23], it may be prudent to consider prolonged immobilization as one potential option for preventing subacute ATRR, particularly in heavy smokers.
In our clinical experience, devising treatment plans for subacute ATRR based on scientific evidence presented several challenges. This was primarily due to the limited number of relevant studies available, and most of the existing studies encompassed heterogeneous patient populations that included individuals with both subacute and late (chronic) ATRRs (Table 3) [3, 8, 9, 20, 24,25,26,27]. Furthermore, some authors discussed operative treatment for ATRR following initial nonoperative treatment for Achilles tendon rupture [20, 26]. We contend that ATRR occurring after operative or nonoperative treatments should be distinguished and categorized differently, as the structure and components of the patient's tendon is not similar due to the influence of prior suture materials. Moreover, in the majority of the included studies [3, 8, 9, 20, 24,25,26], the authors reported outcomes of re-operation for ATRR without making direct comparisons to conservative treatment. This presentation could potentially lead readers to believe that operative treatment is a preferable option for subacute ATRR.
The current study had several limitations that should be acknowledged. Firstly, Group 2 had a relatively small sample size, which may have impacted the statistical power and generalizability of the findings. Secondly, the study's retrospective design could have introduced potential bias and limitations in data collection. Additionally, a substantial number of patients had to be excluded from the analysis, which might have influenced the study’s representativeness. Furthermore, it would have been more informative to compare early isokinetic dynamometer data with data from the final follow-up to provide a comprehensive assessment of patients’ progress over time. Additionally, the study did not include a comparison between the outcomes of conservative treatment for ATRR and operative treatment, which could have provided valuable insights into the most effective approach for managing this condition. Lastly, it is important to note that while the Korean version of the ATRS has been validated, the FAAM relied on a translated version without formal validation. Nonetheless, despite these limitations, this study presents a valuable contribution as the first to evaluate the effectiveness of conservative treatment for “subacute” ATRR by comparing various objective and subjective parameters to those without ATRR.
Practically, making the decision to perform a re-operation for patients with subacute ATRR is a complex and challenging task. By the time ATRR occurs in the subacute phase, the process of tendon healing has already commenced, leading to the formation of substantial scar tissue. Additionally, performing an end-to-end repair in these circumstances can be quite challenging, often requiring lengthening procedures or flexor hallucis longus transfers. As a recommendation, we advise surgeons to place trust in the inherent healing capacity of the patient’s body rather than resorting to more aggressive surgical interventions following the initial Achilles tendon repair. In many cases, there is no need to rush into these operations for subacute injuries, and allowing natural healing processes to take their course may lead to satisfactory outcomes.
In conclusion, following primary Achilles tendon repair, conservative treatment for patients with subacute ATRR yields clinical outcomes that are comparable to those observed in patients without ATRR. While patients with subacute ATRR exhibited initially poorer clinical outcomes than their counterparts without ATRR following the primary Achilles tendon repair, these differences diminished, and final clinical outcomes became comparable after 12 months of conservative treatment. We anticipate that this study will offer valuable scientific evidence to guide surgeons toward considering less invasive treatment approaches for patients with subacute ATRR.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Ganestam A, Kallemose T, Troelsen A, Barfod KW (2015) Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients. Knee Surg Sports Traumatol Arthrosc 24:3730–3737
Ahn HS, Kim HJ, Kang TU, Kazmi SZ, Suh JS, Choi JY (2021) Dyslipidemia is associated with increased risk of Achilles tendon disorders in underweight individuals to a greater extent than obese individuals: a Nationwide, population-based, longitudinal cohort study. Orthop J Sports Med 9(10):23259671211042600. https://doi.org/10.1177/23259671211042599
Pajala A, Kangas J, Ohtonen P, Leppilahti J (2002) Rerupture and deep infection following treatment of total Achilles tendon rupture. J Bone Joint Surg Am 85(11):2016–2021
Deng S, Sun Z, Zhang C, Chen G, Li J (2017) Surgical treatment versus conservative management for acute Achilles tendon rupture: a systematic review and meta-analysis of randomized controlled trials. J Foot Ankle Surg 56(6):1236–1243
Ochen Y, Beks RB, van Heiji M, Hietbrink F, Leenen LPH, van der Velde D et al (2019) Operative treatment versus nonoperative treatment of Achilles tendon rupture: systematic review and meta-analysis. BMJ 364:k5120
Reda Y, Farouk A, Abdelmonem I, El Shazly OA (2020) Surgical versus non-surgical treatment for acute Achilles’ tendon rupture. A systematic review of literature and meta-analysis. Foot Ankle Surg 26(3):280–288
Wilkins R, Bisson LJ (2012) Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am J Sports Med 40(9):2154–2160
Nilsson-Helander K, Swärd L, Silbernagel KG, Thomeé R, Eriksson BI, Karlsson J (2008) A new surgical method to treat chronic ruptures and reruptures of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc 16(6):614–620
Rettig AC, Liotta FJ, Klootwyk TE, Porter DA, Mieling P (2005) Potential risk of rerupture in primary Achilles tendon repair in Athletes younger than 30 years of age. Am J Sports Med 33(1):119–123
Chestor R, Costa ML, Shepstone L, Donell ST (2003) Reliability of isokinetic dynamometry in assessing plantarflexion torque following Achilles tendon rupture. Foot Ankle Int 24(12):909–915
Goren D, Ayalon M, Nyska M (2005) Isokinetic strength and endurance after percutaneous and open surgical repair of Achilles tendon ruptures. Foot Ankle Int 26(4):286–290
Park YH, Cho HW, Choi JW, Kim HJ (2021) Validation and cross-cultural adaptation of the Korean translation of the Achilles tendon Total Rupture Scoew. BMC Musculoskelet Disord 22:876
Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM (2005) Evidence of validity for the foot and ankle ability measure. Foot Ankle Int 26(11):968–983
Reb CW, Saini SS, Stenson JF, Albana AF, Pedowitz DI, Raikin SM et al (2018) Content relevance of the foot and ankle ability measure in patients with Achilles tendon disease. Foot Ankle Spec 11(3):217–222
Flint JH, Wade AM, Giuliani J, Rue JP (2014) Defining the terms acute and chronic in orthopaedic sports injuries: a systematic review. Am J Sports Med 42(1):235–241
Hope M, Saxby TS (2007) Tendon healing. Foot Ankle Clin 12(4):553–567
Voleti PB, Buckley MR, Soslowsky LJ (2012) Tendon healing: repair and regeneration. Annu Rev Biomed Eng 14:47–71
Nagasawa K, Noguchi M, Ikoma K, Kubo T (2008) Static and dynamic biomechanical properties of the regenerating rabbit Achilles tendon. Clin Biomech 23(6):832–838
Hiramatsu K, Tsujii A, Nakamura N, Mitsuoka T (2018) Ultrasonographic evaluation of the early healing process after Achilles tendon repair. Orthop J Sports Med 6(8):2325967118789883. https://doi.org/10.1177/2325967118789883
Maempel JF, White TO, Mackenzie SP, McCann C, Clement ND (2022) The epidemiology of Achilles tendon re-rupture and associated risk factors: male gender, younger age and traditional immobilizing rehabilitation are risk factors. Knee Surg Sports Traumatol Arthrosc 30(7):2457–2469
Jildeh TR, Okoroha KR, Marshall NE, Abdul-Hak A, Zeni F, Moutzouros V (2018) Infection and rerupture after surgical repair of Achilles tendons. Orthop J Sports Med 6(5):2325967118774302. https://doi.org/10.1177/2325967118774302
Kennedy P, Saloky K, Yadavalli A, Barlow E, Aynardi M, Garner M et al (2019) Comparison of Achilles tendon healing after exposure to combusted tobacco, vaping, and control in a rat model. Orthop J Sports Med 7(7 suppl5):2325967119S00328
Cheema AN, Newton JB, Boorman-Padgett JF, Weiss SN, Nuss CA, Gittings DJ et al (2019) Nicotine impairs intra-substance tendon healing after full thickness injury in a rat model. J Orthop Res 37(1):94–103
Metz R, van der Heijden GJ, Verleisdonk EJ, Andrlik M, van der Werken C (2011) Persistent disability despite sufficient calf muscle strength after rerupture of surgically treated acute Achilles tendon ruptures. Foot Ankle Spec 4(2):77–81
Pot JH, Frima H, Clevers GJ (2014) Clinical results of re-ruptures of the Achilles tendon. Foot Ankle Online J 7(3):5. https://doi.org/10.3827/faoj.2014.0703.0005
Scott WN, Inglis AE, Sculco TP (1979) Surgical treatment of reruptures of the tendoachilles following nonsurgical treatment. Clin Orthop Relat Res 140:175–177
Westin O, Helander KN, Silbernagel KG, Samuelsson K, Brorsson A, Karlsson J (2018) Patients with an Achilles tendon re-rupture have long-term functional deficits in function and worse patient-reported outcome than primary ruptures. Knee Surg Sports Traumatol Arthrosc 26(10):3063–3072
Funding
Authors have no other contributors and sources of funding.
Author information
Authors and Affiliations
Contributions
JY Choi wrote the article with organizing the data, participated in the design of the study, performed the statistical analysis and wrote the article. SK Choo involved in English editing. BH Kim sorted the involved patients with a review of medial record. JS Suh conceived of the study, and participated in its design and coordination and helped to draft the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Each author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical approval
This study was approved by Inje University Ilsan Paik hospital institutional ethics review committee and performed according to the tenets of the Declaration of Helsinki (IRB number 2022-11-014-001).
Informed consent to participate
Written informed consent was obtained from all enrolled patients.
Consent to publication
Authors agree to publication. This manuscript has not been published in any journals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, J.Y., Choo, S.K., Kim, B.H. et al. Conservative treatment outcome for Achilles tendon re-rupture occurring in the subacute phase after primary repair. Arch Orthop Trauma Surg 144, 1055–1063 (2024). https://doi.org/10.1007/s00402-023-05161-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-05161-w