Abstract
Autophagy, the major lysosomal pathway for degrading damaged or obsolete constituents, protects neurons by eliminating toxic organelles and peptides, restoring nutrient and energy homeostasis, and inhibiting apoptosis. These functions are especially vital in neurons, which are postmitotic and must survive for many decades while confronting mounting challenges of cell aging. Autophagy failure, especially related to the declining lysosomal (“phagy”) functions, heightens the neuron’s vulnerability to genetic and environmental factors underlying Alzheimer’s disease (AD) and other late-age onset neurodegenerative diseases. Components of the global autophagy–lysosomal pathway and the closely integrated endolysosomal system are increasingly implicated as primary targets of these disorders. In AD, an imbalance between heightened autophagy induction and diminished lysosomal function in highly vulnerable pyramidal neuron populations yields an intracellular lysosomal build-up of undegraded substrates, including APP-βCTF, an inhibitor of lysosomal acidification, and membrane-damaging Aβ peptide. In the most compromised of these neurons, β-amyloid accumulates intraneuronally in plaque-like aggregates that become extracellular senile plaques when these neurons die, reflecting an “inside-out” origin of amyloid plaques seen in human AD brain and in mouse models of AD pathology. In this review, the author describes the importance of lysosomal-dependent neuronal cell death in AD associated with uniquely extreme autophagy pathology (PANTHOS) which is described as triggered by lysosomal membrane permeability during the earliest “intraneuronal” stage of AD. Effectors of other cell death cascades, notably calcium-activated calpains and protein kinases, contribute to lysosomal injury that induces leakage of cathepsins and activation of additional death cascades. Subsequent events in AD, such as microglial invasion and neuroinflammation, induce further cytotoxicity. In major neurodegenerative disease models, neuronal death and ensuing neuropathologies are substantially remediable by reversing underlying primary lysosomal deficits, thus implicating lysosomal failure and autophagy dysfunction as primary triggers of lysosomal-dependent cell death and AD pathogenesis and as promising therapeutic targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autophagy comprises a network of cross-regulated pathways that engage and deliver potentially toxic and damaged organelles for degradation in lysosomes (Fig. 1). The cellular systems involved in its regulation offer powerful protection against a premature triggering of apoptosis when cells are stressed. This is a particularly critical function of autophagy for neurons—a cell type that cannot regenerate and must survive in some cases for more than a century in a long-lived individual despite these stresses. This review, part of a series on neuronal cell death, addresses the pathobiology in Alzheimer’s disease that overwhelms these survival mechanisms. It focuses especially on the converging disease factors that impede lysosomes from completing the autophagy clearance process and turn damaged lysosomes into triggers of cell death.
Major routes of substrate delivery to lysosomes. Macroautophagy is characterized by the formation of a double-membrane enveloping structure, the phagophore (Ph) and the sequestration of cytoplasmic constituents, including organelles, targeted for degradation into double-membrane vesicles called autophagosomes. Fusion with lysosomes introduces acid hydrolases and a proton pump (vATPase) that acidifies the lumen and activates an array of hydrolases that can fully digest most substrates to unit metabolites, which are recycled for energy or new synthesis. Import of chloride and fluxes of other ions balance the electrogenic gradient during proton import to facilitate acidification. An intermediate step particularly active in axons is autophagosome fusion with a rab7-positive late endosome to form an amphisome (AMP), which amplifies its retrograde motility [31, 133]. In chaperone-mediated autophagy (CMA), proteins carrying pentapeptide KFERQ-like sequences are recognized by Hsp70, which associates with the integral lysosome membrane protein LAMP-2A, triggering translocation of the bound protein into the lysosome interior. In microautophagy, cytoplasmic substrates are internalized into late endosomes/MVB by membrane invagination followed by release of the cargo by membrane scission into the lumen for degradation in lysosomes. Heterophagy involves the lysosomal degradation of plasma membrane components and exogenous substrates after they are internalized by receptor-mediated or bulk endocytosis. Selected proteins are sorted to different cellular destinations or recycled to the plasma membrane. Proteins targeted for degradation are trafficked to late endosomes/ multivesicular bodies (MVB), which mature to lysosomes to effect complete degradation [162]. Buildup of lipofuscin reflects declining clearance inefficiency as neurons age. Compaction of ineffective autolysosomes containing hydrolysis-resistant substrates reduces but does not eliminate the damaging impact on cell function. Lysosomes fully mature and concentrate within the soma of neurons and are scarce or absent in axons. Instead, anterogradely moving Golgi carrier vesicles deliver lysosomal components to the amphisomes and late endosomes moving toward the soma to facilitate their maturation
Autophagy regulators of protein quality control and metabolic homeostasis have been established as key determinants of species longevity [159], which in turn depends on the long-term survival of neurons [87] and their resilience to brain disorders. Capture and complete degradation of an autophagic substrate, termed “autophagy flux”, must be optimally maintained over the individual’s entire life. Although abnormal accumulation of autophagy-related compartments (autophagic vacuoles or AVs) is often the most striking feature of neurodegenerative disease, a primary failure of lysosomes is more often the basis rather than heightened substrate sequestration and self-digestion. Being the repository of dozens of activated hydrolytic enzymes, lysosomes are more than qualified to have been designated potential “suicide bags” by their discoverer, Christian DeDuve, to underscore a potential to release damaging hydrolases into the cytoplasm [66] while triggering other cell death routines [80, 235]. Either acute lysosome disruption or gradual leakage of enzymes from damaged were each recognized by DeDuve’s associates in the 1960s as primary effectors of neuronal cell death [54, 173]. However, the appreciation of lysosomes or autophagy in neuronal cell death adult neurodegenerative disorders escaped the attention of most investigators until this past decade, despite prior knowledge of > 50 congenital lysosomal storage disorders (LSDs), most featuring prominent neurodevelopmental or neurodegenerative phenotypes [172]. It is important to note that investigators often incorrectly equate “autophagy” with just the substrate sequestration steps of the pathway even though autophagy derives its name from its digestive ‘phagy’ (“eating”) lysosomal step. Despite the obvious redundancy, a useful convention is to refer to an “autophagy–lysosomal pathway”, or ALP, to underscore the crucial importance of the degradative step in autophagy and its outsized importance within the pathway as a target in neurodegenerative disease [169].
In this review, the author will discuss how ALP failure in AD evolves, leads to neuron death, and serves as a conceptual framework for explaining the emergence of hallmark neuropathological features in the disease. Relevance to other neurodegenerative disorders is briefly mentioned here and more broadly reviewed recently in Ref. [169]. Adult-onset neurodegenerative diseases have been commonly referred to as proteinopathies, emphasizing the toxic action(s) of a particular aggregation-prone pathogenic protein. Their toxicity, however, is often manifest as clinical disease only after these substrates accumulate in the failing lysosomes of neurons in the aging brain, underscoring the critical roles of lysosomes in precipitating disease. This review also describes a unique morphological pattern of extreme autophagy dysfunction recently identified in select neurons within the broader autophagy-compromised populations of highly vulnerable pyramidal neurons at early stages of AD. This select subpopulation of neurons undergoes a massive build-up of autophagic vacuoles laden with undegraded substrates, including Aβ, and form plaque-like β-amyloid fibrillar aggregates intraneuronally. Although it is only one of multiple possible cell death cascades operating in AD brain [224], autophagy-associated lysosomal-dependent neuron cell death is directly driven by the genes and risk factors responsible for AD. The unique pattern of extreme pathological autophagy offers a rare opportunity to characterize cell-autonomous neuronal death evolving during a disease stage preceding hallmark AD lesions and inflammation, which complicate distinguishing primary from secondary neurodegeneration events [130]. Emergence of the pattern at a “pre-pathology” stage of AD and its role in β-amyloid plaque formation highlights an exceptionally early intraneuronal phase of Alzheimer’s disease that has been largely unexplored, especially in relation to cell death (Fig. 2).

Adapted from Jack et al., 2010 [98] and 2013 [97], Leuzy et al., 2019 [134], and McDade and Bateman, 2018 [150]
Autophagy–lysosomal pathway (ALP) abnormalities progress during an exceptionally early “intraneuronal” stage of AD. A hypothetical timeline of pathological changes in Alzheimer’s disease (AD) is depicted. Beginning during the preclinical (“intraneuronal”) stage and continuing in later stages, primary lysosomal dysfunction initiates a cascade of autophagy failure, lysosomal membrane permeability, intraneuronal amyloid plaque formation, and death of select neurons, which instigates and propels extracellular AD neuropathology, as discussed further in the review. This intraneuronal disease stage is followed by the earliest detection by PET (or other imaging modalities) of sequential emergence of extracellular β-amyloid plaques, tau tangles, inflammation and accelerated neurodegeneration associated with more rapid cognitive decline. It should be noted, based on neuropathological studies [88], that the first tau lesions precede by age of occurrence the first amyloid-β plaques. A caveat to ordering definitively the sequence of appearance of different anomalies is the relative sensitivity of the detection methods applied. Biomarkers for soluble amyloid-β peptide and modified tau species in cerebrospinal fluid (not shown) become abnormal before amyloid-β and tau aggregates are detectable by PET or histologically. Elevated levels of these soluble biomarkers in sporadic AD may precede detectable extracellular lesions by 1–2 decades or more before symptoms [139] which could possibly overlap temporally with changes during the “intraneuronal” stage, although this has not been studied.
Autophagy in healthy and aging neurons
Brief overview of the autophagy–lysosomal network
In macroautophagy, the pathway of this network most crucial for neuron survival [118], cytoplasmic constituents including damaged or obsolete organelles are captured in double-membraned autophagosomes either constitutively at a bulk level or selectively by engaging members of a family of adaptor proteins [64, 166, 194] (Fig. 1). The same processes are induced further under stress conditions as a neuroprotective response [39, 164]. Substrate-laden autophagosomes mature to autolysosomes via direct fusion with lysosomes which introduce dozens of hydrolases capable of completely digesting most normal substrates completely to their unit components (amino acids, lipids, etc.) for reutilization in new synthesis or to generate energy (detailed reviews [64, 194]). A proton (H+) pump, the vacuolar ATPase (vATPase), is also introduced enabling intra-lysosomal acidification down to the pH range of 4.5–5.0 needed to optimally activate the “acid” hydrolases with varying acidic pH optima. Cross-dependencies also exist between the autophagy–lysosome pathway and the endolysosomal–lysosomal pathway [72, 198]. For example, an amphisome is created when an autophagosome fuses with an endosome [75]—a process especially important in neurons to enhance motility during delivery of sequestered cargoes from long neuronal processes to the soma where lysosomes are concentrated (Fig. 1).
In addition to macroautophagy, proteins containing a KFERQ targeting motif are delivered by chaperone-mediated autophagy directly to lysosomes after binding to the chaperone HSC70 and a LAMP2a complex that delivers the protein inside the lysosome [110]. By microautophagy, cytoplasmic substrates can also be introduced through invaginations of late endosomal membranes and delivered to lysosomes [18]. While not an autophagy route per se, the endocytic pathway delivers certain internalized extracellular materials and plasma membrane components to lysosomes if they are not sorted to other cellular destinations or recycled to the cell surface (Fig. 1). Within this network of systems for capturing substrates, lysosomes deserve special emphasis as the only degradative compartment shared by all autophagy and endocytosis-related substrate delivery routes.
Autophagy in healthy neurons confers resilience to aging and disease
Healthy neurons efficiently eliminate newly formed autophagosomes or amphisomes by rapidly fusing with lysosomes and degrading the content within autolysosomes. Even very high levels of autophagy induction via mTOR or AMPkinase and substrate sequestration do not cause autophagosomes in most neurons to build up. In fact, the ultrastructural detection of more than occasional autophagosomes with undegraded material in a cortical pyramidal cell body is rare [171] and likely a harbinger of a declining lysosomal efficiency rather than an over-active induction or autophagosome over-production. By contrast, even brief exposure of neurons to inhibitors of cathepsins or lysosome acidification prompts the rapid accretion of autophagy intermediates [13]. These observations imply that healthy neurons can have a relatively high rate of constitutive autophagy induction but also normally have a lysosomal clearance system with enough reserve capacity to prevent temporary surges of induction from causing dangerous substrate build-up [13].
Additional responses in healthy neurons confer resilience to an increase of damaged proteins and organelles that can threaten survival. Upregulation of transcription factors (e.g., TFEB, TFE3) controlling the “CLEAR Network” of genes encoding autophagy and especially lysosomal biogenesis constitutes a successful strategy to delay effects of cell aging or extend longevity in vivo in aging models [179] and to slow or prevent neurodegenerative disease progression in mouse models [149]. In addition, in some neural cell types, failing lysosomes can jettison accumulated lysosomal cargoes by exocytosis [93, 178, 243]. Over-burdening lysosomes with cargoes from the endocytic pathway can also be attenuated by a default release via exosomes that are formed via late endosome membrane invaginations that capture cytoplasmic materials into vesicles [135]. These vesicles are released when an endosome fuses with the plasma membrane. In addition, endosome cargoes can enter the autophagy pathway by fusing with autophagosomes that can expel their contents by exocytosis—a process so far documented mainly in non-neuronal cells [153]. These various “unconventional secretory” pathways may be constitutive but are upregulated as default pathways when lysosomal efficiency declines in aging and disease [3]. In a final adaptation to cope with poorly degraded substrates in autolysosomes, ineffective congested autolysosomes can fuse and undergo further compaction via residual hydrolysis and chemical modification, which yields the aging-related lipopigment, lipofuscin, a relatively but not completely inert family of lipo-proteinaceous granules [162].
Neuronal aging: the gateway to lysosomal failure and neuron death in AD
Disease emergence in aged adults coincides temporally with waning neuronal proteostasis, especially in autophagy involving declines in autophagosome biogenesis [183, 230], waste trafficking and degradation [230], and translocation of CMA substrates into lysosomes [49, 50]. Particularly influential to the autophagy pathway decline in aging cells of lower species [30] and likely also mammals [24] is progressive lysosomal dysfunction tied to failing lumenal acidification [230]. This is shown, at least in part, to be due to ROS and aldehydes (e.g., 4-hydroxynonenal) from oxidized lipids [190] causing oxidative damage to vATPase complex subunits and lysosomal enzymes [189, 192, 253].
Proteostasis protects against pathogenic protein accumulation for decades until aging-related autophagy impairments [26, 60, 188, 191] trigger a rise in levels of toxic substrates, boosting oxidative stress [199] and mitochondrial damage that yield calcium dyshomeostasis and calpain activation, known to mediate varied effects of cell aging [197, 228]. The long-term protection against these challenges until late age explains why even individuals with autosomal dominant mutations in a pathogenic protein are functionally normal until sufficient lysosomal dysfunction emerges. Aging’s role in precipitating neurodegenerative disease involving lysosomal mechanisms can be appreciated from genes that, in homozygote mutant form, cause childhood lysosomal storage diseases but, even in heterozygote form, increase risk for a late-age onset neurodegenerative disorder, such as Parkinson’s disease (GBA) or frontotemporal dementia (CLN11) [233].
Lysosomal membrane permeabilization (LMP) to a variable extent (Fig. 3) is the inevitable outcome of these cumulative aging-related insults to lysosomes and is often the harbinger of neurodegeneration [79]. LMP is defined as the selective destabilization of the lysosomal membrane, which allows certain lysosomal contents to be released into the cytoplasm. Instigating factors include free radicals, membrane incorporation of damaged proteins and oxidized lipids, osmotic shifts due to ion flux changes [121, 176], and changes in membrane lipid composition, such as increased lyso-phosphatidylcholine and ceramide [12]. Calpain activities actually increase in aging [147, 170] and can potentially act upstream of, or coincident with, pH dysregulation [151, 158] to damage organelles and cytoskeleton, adding to LMP and necrotic damage in AD [197, 228]. Aging-related declines in the endogenous inhibitor of calpains, calpastatin [170, 197], also lie upstream of cathepsin release during lysosomal-associated cell death [234].
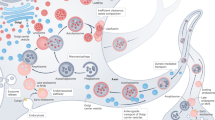
Lysosomal membrane permeability (LMP) and lysosomal neuronal death cascade in AD. This diagram of the lysosome illustrates a crucial inciting decline in vATPase activity and lysosome acidification, which is a primary target of AD causative genes/gene products (PSEN1 mutations, APP, and genetic and environmental risk factors (e.g., ROS, neuronal aging, and high cholesterol), as discussed further in the text. Counter-productive induction of autophagy when lysosomal dysfunction is advanced may also exacerbate waste storage, ROS, and deacidification, thus promoting LMP. Also depicted is the cascade of further lysosome disruptions and lysosome- associated processes affecting mitochondria and innate immune function. The diagram further illustrates how LMP is connected to other pathways of cell death that can be upstream contributors (e.g., calpain activation and Hsp70), coincident exacerbating factors (e.g., ferroptosis), or downstream consequences of the release of cathepsins during LMP or lysosomal cell death (cathepsins, caspases, calpains, and Cdk5/p25), which facilitate end stages of cell death. Collective injury to the lysosomal limiting membrane from the depicted sources induces lysosomal membrane permeability (LMP)—a state of injured membranes that allows relatively small proteins like cathepsins to pass through into the cytoplasm from an otherwise intact lysosome. Some hydrolases commonly released during LMP, such as cathepsins B and D, remain partially active at neutral pH and their potential cytotoxicity is buffered by cystatin C, an endogenous cysteine protease inhibitor in the cytosol and lysosomes [217]. The release of cathepsin B is linked to IL-1 activation, NLRP3 inflammasome activation, and neuroinflammation and can activate multiple cell death cascades, including caspases that trigger apoptosis. Cathepsin D release by LMP promotes necroptosis [143, 257]. Impaired ferritin degradation invokes features of the cell death pattern seen in ferroptosis [201]. Calcium release via TRPML1 and TPC2 channels activates calpains and calcium-dependent protein kinases promoting hyperphosphorylation of pathogenic proteins like tau and activation of RIPK1, which initiates necrosis-associated neurodegeneration. TRPML1- and TPC2-mediated calcium release is linked to mitochondrial dysfunction, and mTORC1 activation
These effects of aging are countered by the chaperone Hsp70, which binds to the endolysosomal anionic phospholipid BMP [116], a co-factor that enhances acid sphingomyelinase activity. Under the vATPase deficient conditions that develop during aging [46, 127], heightened lysosomal calcium release into the cytosol activates calpains and protein kinases that have significant pathogenic consequences discussed below. Mitochondrial deterioration, a major contributor to cell aging activates neuronal mitophagy [22, 188] represents another LMP instigator [107, 231]. A strong surge of cytosolic calcium and calpain activation is sufficient to induce necrotic cell death [170]. Collectively, aging-related changes precede and add to lysosomal insults imposed by causal and risk disease genes discussed below.
How cell death evolves in the first neurons to die in AD
Faulty lysosomal acidification and autophagy flux failure arise exceptionally early
As early as Braak stage II, pyramidal neurons in prefrontal cortex of the late-onset AD (LOAD) brain exhibit increased numbers of pro-cathepsin D and cathepsin D-positive lysosomes, elevated cathepsin D mRNA transcription, and mannose-6-phosphate receptor (MPR) trafficking of lysosomal enzymes to endosomes [32, 33], all implying upregulated lysosomal biogenesis. Microarray analysis of laser-captured CA1 hippocampal neurons in LOAD (Braak III and V) further demonstrate upregulated lysosomal gene transcripts [16] and proteomic analysis of the large ROSMAP [106, 129], and Banner [239] AD cohorts indicate early and sustained inhibition of mTOR [129]. These data suggest that, surprisingly, elevated autophagy induction persists [16, 141] even as rising lysosome levels of LC3 and inactive catD document a decline of lysosomal clearance [129]. Dual-immunolabeling with antibodies to catD and LC3 by Braak III stage confirm build-up of grossly enlarged catD/LC3-positive autolysosomes and depletion of CatD-positive lysosomes [129]. Collectively, these analyses indicate that autolysosome maturation to lysosomes (i.e., substrate digestion) stalls early in AD and worsens with disease progression. Proteomic analyses revealing declines in levels of most vATPase subunits in AD brain [106, 129], which together with complementary evidence in mouse AD models discussed below, strongly suggest that a lysosomal acidification deficit underlies impaired autolysosome maturation. Sustained autophagy induction compounds autophagic stress under these conditions—a seemingly maladaptive neuroprotective response that over-burdens failing lysosomes by delivering even more substrates [16, 129, 225].
An autophagy–lysosomal pattern similar to that in late-onset AD brain has been extensively detailed in neurons of different mouse AD models ranging from late disease onset in mice expressing human wt APP or mutant APP, to early onset in mice expressing mutant forms of both APP and Presenilin 1 (PSEN 1) [130, 140]. To track autophagy changes in neurons in vivo, a probe of autophagy and vesicle pH, the mRFP-eGFP-LC3 (“TRGL”) construct, was stably expressed selectively in neurons (Fig. 4). As in AD brain, autophagy becomes dysregulated broadly in vulnerable populations of cortical and hippocampal neurons well before β-amyloid or glial reactive responses are detectable. Most of these neurons accumulate enlarged poorly acidified autolysosomes filled with undegraded substrates. Lysosomes isolated from these brains have significantly lowered vATPase activity (Fig. 5). The basis for the defect is impaired autolysosome acidification stemming from deficient lysosomal vATPase activity related to APP-βCTF [95] which accumulates together with Aβ selectively in these poorly acidified autolysosomes [95] (Figs. 3, 5). More generally, lysosomal pH dysregulation originating from causative mutations of one of multiple ion channels is an emerging common theme in adult neurodegenerative diseases, which may potentially disrupt pH in either direction [169].
A transgenic ratiometric autophagy probe in neurons enables interrogation of brain autophagy in vivo. a Schematic representation of the dual fluorescence autophagy sensor, mRFPeGFP-LC3 (tfLC3) with a Thy1 promoter (“TRGL mice”). Transgene founders were identified with a forward primer in Thy1 and a reverse primer in the RFP gene. b The schematic illustrates changes in fluorescence signals during the progression of autophagy in TRGL mouse neurons. Yellow puncta indicate autophagosomes, orange puncta indicate incompletely acidified autolysosomes, which may in the process of maturing or are autolysosomes pathologically deficient in acidification. Red (mRFP only) puncta identifies autolysosomes that are normally acidified to a pH level that quenches GFP fluorescence after AP-LY fusion, indicating fully acidified ALs with detectable ongoing LC3 digestion. The distinction between a normal maturing autolysosome and an abnormal poorly acidified autolysosome, both appearing yellow/orange, is achieved by IHC co-labeling with a lysosomal marker (CTSD). In the triple fluorescence condition, acidified autolysosomes are purple, white puncta are autolysosomes that are poorly acidified (eGFP not quenched), and lysosomes are blue. Net fluorescence “color” is objectively quantified ratiometrically as hue angle yielding a measure of relative pH. c Changes of morphology and pH of autophagy pathway organelles are illustrated in the somas of neocortical neurons in TRGL mice. A vehicle control condition is compared with conditions where lysosomal pH is abnormally elevated (see Ref. [130] for further details). Scale bar: 20 μm
Extreme autophagy–lysosomal dysfunction (“PANTHOS”), LMP, and loss of highly vulnerable pyramidal neurons in AD: an exceptionally early unique pattern of progressive autophagic stress, autolysosome pH deficits and plasma membrane blebbing (“PANTHOS”) is detected in varied mouse models of AD pathology (only Tg2576 model shown here) expressing a dual-fluorescent RFP—eGFP—LC3 autophagy reporter in neurons. Especially when the nucleus is DAPI-stained, the pattern, termed PANTHOS—p-anthos or “poisonous flower”, resembles brightly colored flower blossoms and was referring to the slow but inevitable death of these neurons (see also Fig. 6). Appropriate fluorescent antibody markers have recently detected a similar autophagy dysfunction and PANTHOS lesions in human late-onset (LOAD) brain [68] (Fig. 7). a Representative tfLC3 fluorescence images of 10-month-old Tg2576/TRGL mouse brain depicting neurons at three different stages toward PANTHOS state: i: early pH change in autolysosomes (CSTD-positive in c.); ii: focal plasma membrane bulges as poorly acidified (yellow) autolysosomes (pa-ALs) enlarge and proliferate (arrowhead); iii: full PANTHOS pattern (arrow). b Lower magnification image of cortex highlights the flower-like pattern of PANTHOS, its frequency at a mild/moderate stage of disease, and the preponderance of yellow (poorly acidified autolysosomes in the blebs—see also e). c Staining of PANTHOS neurons using nuclear marker (DAPI) in 10-month-old Tg2576/TRGL mouse brain. Scale bar, 10 μm. Over 90% of amyloid lesions are PANTHOS (detectable central DAPI nucleus) at early-stage disease and > 65% at later stage [130]. d Autolysosome acidification deficits develop early in AD model mice and progress with age. Poorly acidified autolysosome number in 5-month-old Tg2576/TRGL is elevated and acidified autolysosome number is lowered compared to neurons in TRGL littermates. Scale bar, 20 μm. Lysosomal vATPase activity is also decreased at 6-months [130]. e A third fluorophore (cathepsin D IHC) reveals that most autophagic vacuoles in the blebs are autolysosomes that have fused with lysosomes but fail to acidify (appearing as white puncta reflecting presence of all 3 fluorescence labels). f Lysosomal membrane permeability develops early in mouse models. Lysosomal enzyme distribution (ratio in cytosol vs membrane/vesicle fraction) is normal at 2.7 months of age but significantly abnormal by 6-months in 5xFAD versus WT mice. (Panels reproduced from Nature Neuroscience [130] with permission)
In a select small subpopulation neurons that are most affected, poorly acidified autolysosomes build-up so massively that these fluorescent autophagic vacuoles bulge the plasma membrane outward, forming rosettes of large petal-shaped blebs—a flower-like pattern referred to as PANTHOS (p-ANTHOS or poisonous flower), which is seen in mouse and human AD brain [129] (Fig. 6). Within the soma, proliferated autophagosomes are frequently seen fused with endoplasmic reticulum (ER) tubules, suggesting that they have stalled in completing the normally highly active turnover of ER by autophagy (“ER-phagy”) (Fig. 7b). Proteins, including amyloidogenic metabolites of APP, build up in the ER [130], as they can in other abnormal states [85, 174], and, in a mechanism still not fully understood, form fibrillar aggregates of β-amyloid intracellularly within the ER membrane tubular network that rings the nucleus [186]. A neuron at this stage of PANTHOS, which is still fully intact by confocal and 3D ultrastructural analysis, is likely to be misclassified as an extracellular amyloid plaque when examined only using β-amyloid immunocytochemistry or silver staining (Figs. 6, 7).
Evolution of autophagy–lysosome dysfunction in Alzheimer’s disease leading to neurodegeneration. a A primary effect of APP-βCTF elevation in AD brain [2, 74, 91, 101, 104, 187, 193, 219, 227, 231] is the inhibition of vATPase activity, which disrupts lysosomal acidification [95, 102, 130]. Coupled with multiple genetic, environmental, and cell aging factors [19, 37, 113, 122, 125, 126], APP-βCTF begins to corrupt lysosomal function at the earliest stage of the disease and before hallmark neuropathology appears. b Increased autophagy induction, an early neuroprotective cellular stress response, becomes counter-productive as degradative compartments progressively fail to clear the growing waste burden. Ensuing build-up of autophagic vacuoles (AVs), mainly poorly acidified autolysosomes, causes a unique pattern of extreme perikaryal membrane blebbing and trafficking deficits of retrograde moving amphisomes and late endosomes that produce swellings along axons (dystrophic neurites-DN. Within the perikaryon, Aβ accumulation in autolysosomes accelerates, and aggregates of fibrillar β-amyloid form within endoplasmic reticulum (ER) tubules. Reflecting an apparent stalling of ER-phagy—a normally highly active constitutive process of ER turnover by autophagy. c This intracellular pathobiology evolving within still intact neurons, precedes lysosomal membrane permeability and the lysosomal-associated neuronal cell death and replacement of each dying neuron with a senile (“amyloid”) plaque (SP). An inflammatory response to the extracellular β-amyloid involves the recruitment of reactive astrocytes (A) and phagocytic microglia (M) to the disintegrating neuron and release of damaging cytokines and hydrolases that gradually clear the extracellular debris. Bystander neurotoxicity in nearby neurons and further glial invasion expands some senile plaques (not shown in the diagram). PANTHOS-like centrifugal blebs projecting from the central area of a senile plaque represent the important contribution of somal AV-filled blebs to the neuritic appearance of senile plaques in human AD brain drawn by Oskar Fischer [71] interpreted mainly as dystrophic neurites by later investigators. Diagrams are adapted from [130, 169]
Comparable PANTHOS-pattern autophagic pathology and intraneuronal β-amyloid in a mouse AD models and b early-stage human AD brain. IHF labeling using 4 fluorescence labels (TRGL 5xFAD mouse model, DAPI nuclear stain, and amyloid immunolabeling (4G8) demonstrate similar intraneuronal Aβ accumulation surrounding a visible DAPI-positive nucleus within a PANTHOS neuron in both human and mouse brains. Scale bar, 10 μm. c Immunohistochemistry image of the ROI (box) used for serial SEM imaging of a 2.7-month-old 5xFAD/TRGL mouse brain. Scale bar, 40 μm. d z-stacked serial SEM image of the ROI area, serial sections 370–430 through the PANTHOS depicted in the ROI. Scale bar, 40 μm. Arrow indicates the PANTHOS of interest. e 3D reconstruction of 573 serial sections through the indicated PANTHOS neuron demonstrating its integrity as a neuron and the abundance of AVs in blebs with narrow necks (colored pink) projecting from the somal PM. The central nucleus is colorized blue. f Confocal image of a PANTHOS state of a neuron from a 5xFAD mouse model highlighting the protrusions of AV-filled blebs with narrow necks originating from the somal plasma membrane and resembling similar plaque lesions in human AD brain depicted initially by Oskar Fischer [71] in panel g (panels a, c-f adapted from Lee et al. 2022 [130] with permission)
Balancing lysosome membrane permeability (LMP) against lysosomal repair, lysophagy, and other defenses
In the mouse AD models studies above, LMP detected at the initial stage of AD using the classical assay devised by Christian DeDuve, the discover of lysosomes, coincides with impaired lysosomal acidification, an established cause of LMP [21,22,23]. Other likely routes contributing to LMP [176] in AD are damage from free radicals, including especially oxidative stress-induced Hsp70.1 carbonylation and calcium- and calpain-mediated cleavage [244]. Lysosomal repair is a critical physiological/homeostatic defense against worsening LMP that leads to catastrophic disruption and irreversible LCD via release of hydrolases and calcium. Repair is assisted by Hsp70, which binds to bis(monoacylglycerol)phosphate (BMP) and increases acid sphingomyelinase activity, which helps to rebalance lysosomal membrane lipid composition [180] (Fig. 3). Recruitment of the endosomal sorting complexes required for transport (ESCRT) machinery plays a key role [195, 211, 256], leading to restoration of lysosomal acidity. The upregulation and acetylation of Hsp70 also induces an autophagic induction response [184] and attenuates the likelihood of caspase-independent and caspase-dependent cell death by inhibiting Apaf1 and AIF [156]. Autophagy upregulation is anti-apoptotic in several additional ways. Beclin1, a key component and regulator of autophagy, binds and suppresses the pro-apoptotic molecule Bcl2 [11, 57]. Pro- versus anti-apoptotic balance plays out differently in a given neuron population. Although Beclin 1 levels were reported to be lowered in AD brain, subsequent analyses have measured normal levels [106, 129]. In PS1/APP mouse neocortex, rare neurons undergoing classical apoptosis are seen within the larger population exhibiting various degrees of PANTHOS-pattern autophagy–lysosomal dysfunction. Notably, a third population exhibits clusters of autophagic vacuoles containing activated caspase 3, suggesting another way by which autophagy can neutralize an apoptosis threat [247]. As a second line of defense when LMP in a lysosome becomes overwhelming, lysophagy eliminates the lysosome. In this process, galectins enter the lysosome, bind to intralumenal β-galactosides resulting in their surface exposure and LC3-mediated engulfment into autophagosomes for clearance by intact lysosomes [180].
Neurons exhibiting PANTHOS form intracellular β-amyloid plaques and transform into extracellular senile plaques when they die
Despite the massive autophagic pathology in intact PANTHOS neurons, they are not significantly invaded by microglia for multiple weeks in the mouse models of amyloidosis and not until they appear to have lost structural integrity. The prolonged “agonal” state of PANTHOS neurons and their significantly delayed recognition by microglia as being irreversibly compromised confirms their persistent viability even in this compromised state. The absence of caspase 3 activation [130] and the slow removal of PANTHOS neuron corpses mediated by invading glial cells [130] further distinguishes this form of cell death from apoptosis. It further suggests that even the clearly maladaptive sustained induction of autophagy in the face of lysosome failure is a lasting albeit futile attempt to maintain viability rather than it being a stimulus to enter an autophagic or apoptotic cell death program. PANTHOS is ultimately accompanied by worsening LMP and autophagic-lysosomal death followed by microglial and astrocytic invasion of the PANTHOS corpse which further matures the extracellular senile (“amyloid”) plaque into lesions of diverse morphologies [8, 14, 52, 73, 94, 102, 124, 126, 130, 163].
The PANTHOS morphology of a still intact neuron in an FAD mouse model of ß-amyloidosis (Fig. 7a) closely matches a corresponding PANTHOS profile in human AD brain at Braak III stage (PFC) (Fig. 7b). Aβ immunoreactivity is present in the Aβ-positive autolysosomes contained within plasma membrane blebs, similar to that described in mouse models [130]. An advanced stage of PANTHOS may also exhibit perinuclear amyloid accumulation or can remain more dispersed and assume a coarse-grained plaque morphology at the early Braak stages. As AD pathology progresses in neurons expressing the neuron-specific autophagy reporter, there is greater diversity of plaque morphologies [15, 61, 229] reflecting the evolution of morphological changes that result from the progressive proteolytic clearance and bystander neurodegeneration [169]. The complexity of pathology in later-stage AD, coupled with the fact that glia strongly immunolabel with lysosomal markers, impedes an unequivocal characterization of plaque origins. The ability in TRGL-expressing mice to track plaque development from a single pyramidal neuron population as it transforms into a senile plaque and further mature provides important new insight into the possibility that a sizeable proportion of the plaque diversity derives from the sequence of neuronal death, neighbor recruitment, and slow progressive clearance by glia.
The evolution of PANTHOS in some highly vulnerable neuron populations supports an “inside-out” origin of neuron cell death and plaque development in AD [51, 82], emphasizing that significantly deleterious Aβ actions are exacted intraneuronally on lysosomal compartments and ultimately initiate death of the neuron, yielding a senile plaque [130], which then can trigger additional secondary responses that accelerate disease. In these later phases of disease, it is possible that (possibly defective) microglia dying after taking up amyloid and related plaque debris can also develop PANTHOS-like states that transform into a classic plaque via a similar inside-out mechanism [8]. The additional possibilities of neuritic dystrophy [115] or synaptic degeneration seeding diffuse plaques or the expansion of plaques from extracellular amyloid cannot be excluded. However, in mouse AD models, amyloid plaques grow mainly by corrupting neighboring neurons to accelerate PANTHOS and forming the larger plaques [130]. The foregoing sequence of events is consistent with data showing that neuronal cell death is prominent in mouse models of AD pathology that exhibit greatest intraneuronal accumulation of Aβ and/or APP-βCTF [29, 67, 81, 109, 130, 160], whether or not they develop extracellular plaques [240] or whether ß-amyloid experimentally is redistributed from extracellular to intracellular locations [160]. Neuron loss is a robust feature in a variety of single and multiple transgenic lines: pyramidal neuron loss especially appears related to intraneuronal Aβ accumulation [41].
The genetic basis of primary lysosomal pH dysregulation in AD
That lysosomal acidification impairment is among the earliest pathogenic deficits known in AD brain is consistent with evidence that lysosomal APP-βCTF is a major triggering factor [95], acting together with possible further lysosome damage from other accumulating lysosomal oxidized substrates, including Aβ. Acidification is mediated mainly by the ATP-dependent proton pump, vacuolar H + -ATPase (vATPase) [45, 47, 161], a 14 subunit complex regulated mainly by the extent of reversible association of a cytoplasmic subcomplex (V1) and a membrane associated V0 subcomplex (Fig. 3, [169]). Crucial to pathogenesis of AD and likely additional neurodegenerative diseases [46], the a1 subunit of the V0 subcomplex (V0a1) is responsible for mediating the association between the V1 and V0 subcomplexes that regulates vATPase activity [48]. APP-βCTF binds selectively to the V0a1 subunit and inhibits this association [94], thus linking its elevation in the lysosomes of AD neurons to the early decline in acidification [102, 130] and extreme autolysosomal and lysosomal pathology [94, 102, 124, 126, 130, 163]. The negative impact of APP-βCTF on lysosomes adds to its other pathogenic actions in triggering endosome dysfunction linked to cholinergic neurodegeneration at early stages of AD [100,101,102, 123, 220] and in dysregulating mitochondria-associated ER membrane interactions (MAMs) that disturb calcium and lipid homeostasis [5] and elevate reactive oxygen species (ROS) levels [5] (Figs. 1, 3).
Evidence tying causative AD genes to lysosomal pH dysregulation first arose from studies of Presenilin 1 (PSEN1) mutations, the most common cause of early-onset familial AD [131]. Most notably as the catalytic subunit of the γ-secretase complex [55, 84], an intra-membrane endoproteinase acting on many endolysosomal substrates, PSEN1 cleaves APP-βCTF to yield amyloid-β (Aβ) peptide. As importantly, PSEN1 by itself in its uncleaved holoprotein form has varied functions [92, 99, 103, 216] including acting as a chaperone in the ER and Golgi to facilitate the folding and glycosylation of the crucial V0a1 subunit [131], thereby stabilizing it against premature ERAD proteolysis before it is delivered to lysosomes for assembly of the vATPase complex [7, 95, 127, 131, 209, 241]. PSEN1 is, therefore, required for adequate lysosomal acidification. Its deletion sharply reduces lysosomal vATPase activity and clearance of substrates, including APP-βCTF and Aβ [95, 130].
Restoration or enhancement of lysosomal function attenuates autophagic stress, neuronal cell death, and amyloid plaque formation in AD models
An essential criterion for lysosomal cell death is its inhibition or significant delay by restoring lysosomal functionality. The primary or central role of the autophagy–lysosomal pathway and its closely networked endosomal-lysosomal pathway in driving diverse pathological and pathophysiological features of AD is strongly supported by the broad disease amelioration achieved in AD models by enhancing lysosomal function and autophagy flux [68, 148, 214, 227, 249]. Rescue of deficits ranging from failed waste clearance [17, 45, 127, 236], amyloid and tau pathologies and synaptic and cognitive deficits [89, 117], has been repeatedly confirmed using widely varying approaches sharing the property of enhancing flux through the entire autophagy pathway [28, 148, 214, 250]. Notably, similar levels of rescue are achieved when lysosomal proteolytic efficiency is specifically enhanced including elevating cathepsin activities [25, 157] by genetic manipulation of lysosomal protease inhibitors [217, 250], increasing lysosomal biogenesis and transcription of vATPase subunits [56, 148, 213], or pharmacologically re-acidifying lysosome, including using lysosomal-targeted acidic nanoparticles [17, 45, 127]. Pharmacological restoration of lysosomal acidity has been shown in preliminary studies to substantially block death of neurons exhibiting PANTHOS and commensurately lowering senile plaque number.
Autophagy and the concept of lysosomal-dependent cell death in AD
“Heterogeneity” of neuron cell death patterns in AD
The modes of death reported for different cell types in the AD brain vary widely in different pathological contexts. Scattered cortical neurons of the PS1/APP mouse AD model undergo unequivocal apoptosis without the substantial antecedent autophagy pathology evident in most other neocortical cells [247]. In the prolonged neuronal survival battle that is ongoing in neurodegenerative diseases like AD, multiple cascades are activated as disease advances. When a biomarker selective for a single cell death pathway detects its activation at one stage of the demise, other participating modes of cell death may frequently be overlooked. The complexity of cell death analysis in AD brain is compounded as phagocytic, neuroinflammatory, circuit disconnections, and neurotrophic failures superimpose triggers of additional death cascades over the primary initiator. One may ask, for example, whether the demonstrated activation of an apoptotic program should be considered the defining mode of cell death in a disease if lysosomal or necrosis-related triggers have already rendered the neuron irreversibly on a path to death? These are key considerations in deciding whether events leading to death are triggers or executioners or both. PANTHOS associated lysosomal-dependent neuron death evolves during a disease stage preceding superimposition of many complicating disease accelerants (e.g., inflammation and glial proteases).
Roles of lysosomes in the execution of neuronal cell death in AD
An acute massive disruption of lysosomes is sufficient to induce and partially execute death as was classically illustrated by the rapid cell death induced by the uptake of large particles, such as silica, into lysosomes which can be delayed or substantially slowed by cathepsin inhibitors [181]. Severe LMP that overwhelms repair mechanisms and lysophagy can be driven by converging AD-related factors, including inhibition of vATPase [105], APP-βCTF and cholesterol, calcium-mediated activation of calpains, and ROS-generation from oxidized lipids, proteins, and Aβ, which are elevated early in vulnerable neurons in AD brain [20]. Even under extreme conditions of lysosomal disruption, however, other cell death cascades are inevitably activated, resulting in end-stage necrosis and varying levels of caspase activation. Such a chain reaction of cell death executioners is documented from studies of acute and gradually progressive LMP [79, 23]. Various cathepsins such as aspartic cathepsin D and cysteine cathepsins B, C, F, H, K, L, O, S, V, W, and X are considered effectors in apoptotic cell death [156]. The contribution of cathepsins, however, should not be underestimated, given that restoring acidification of autolysosomes substantially blocks neuron loss and amyloid plaque production.
In addition to promoting LMP and the release of cytotoxic hydrolases into the cytosol, declining lysosomal acidification broadly inhibits intraluminal hydrolase activity and especially the most acidic cathepsins like cathepsin D, which is optimally active at pH 4.0–4.5. Lowered neuronal cathepsin D activity is an outcome of lysosomal pH rise in essentially all mouse models of AD pathology as well as in late-onset human AD [129, 223, 233, 257]. Cat D deficiency alone, causing an autophagy–lysosomal phenotype reminiscent of PANTHOS in mice, induces cell death without a dominant involvement of apoptosis [208].
The foregoing discussion implies that even at the terminal stages of neuronal compromise, autophagy induction is playing a neuroprotective role in prolonging neuron survival by suppressing apoptosis and attempting waste clear despite extreme lysosomal inefficiency that impedes autophagy flux. This pattern is distinct from classical autophagic cell death [44], a death process associated with over-exuberant autophagy induction and preserved or possibly enhanced lysosomal function, enabling the cell to be consumed from within, ultimately killing it by eliminating cellular constituents essential for survival. Autophagy-dependent cell death is executed by the autophagy machinery [58] in the absence of apoptosis [4, 11] and requires evidence that death can be blocked or substantially delayed by selectively inhibiting autophagy. Programmed autophagic death of an entire cell population is seen in some lower invertebrates to achieve organ/tissue reorganization [164]. Over-activated autophagy may also contribute to the death of neurons injured by acute injuries, such as hypoxia/ischemia and trauma [164] and involves enzymes from lysosomes [58]. Beyond these situations, however, lysosome failure is a far more common cause of neuron death than is a defect in upstream autophagy. In AD models, enhancing induction of autophagy experimentally (e.g., rapamycin, TFEB activation) is somewhat effective in attenuating pathology because it can upregulate lysosomal efficiency and autophagy flux by stimulating lysosomal biogenesis and the synthesis of subunits of the vATPase proton pump [114, 185].
Autophagy-associated lysosomal-dependent neuron death in AD in the context of other late-onset neurodegenerative diseases
The lysosome or its related degradative compartments, the autolysosome and endolysosome, are the primary or secondary targets of an expanding list of genes causing late-age onset neurodegenerative diseases besides AD [169]. The point of mechanistic convergence in many cases is dysregulation of ion balance and pH, resulting in signaling and hydrolytic failure. Lysosome dysfunction, and particularly acidification, are being increasingly appreciated as targets of pathogenic proteins causing other neurodegenerative diseases [46]. Channels on the lysosomal membrane regulating the import of chloride (ClC7, CLC5), potassium/H+ balance (TMEM175), calcium efflux/influx/proton leak (TRPML1 and TPC2) among other ions, greatly influence physiological proton content and pH but can also mitigate pathological changes in pH (detailed review: [169]). These ion fluxes influence baseline proton content and pH and can rebalance lysosomal pH under certain pathological conditions. The modulators of lysosomal ion balance and signaling have themselves been implicated as causative for diseases, such as Parkinson’s disease, e.g., SCNA and LRRK2), frontotemporal lobar dementia, and several less common diseases [169]. In particular, lysosomal calcium exchanges with the ER and mitochondria maintain the large lysosomal calcium store that influences pH balance [42, 43, 83, 127] as well as the local calcium signaling that regulates varied steps in autophagy endolysosome trafficking and fusion events. Regulated calcium efflux from lysosomes mediated by phosphoinositides and other cell signals modulates lysosome motility, fusion with other organelles, exocytosis, and local signaling controlling autophagy flux [70, 144, 221], including transcription of genes encoding autophagy and lysosomal biogenesis [152]. By contrast, uncontrolled calcium efflux caused by a sustained rise of lysosomal pH in AD models dangerously elevates cytosolic calcium levels, which activates calpains and varied pathogenic kinases that collectively are capable of triggering vesicular trafficking deficits, tauopathy, and cell death. One consequence discussed below is AD-like neuritic dystrophy in aged PSEN1 FAD knock-in mice. Reduced lysosomal calcium levels, likely from excessive efflux, are also seen in LRRK2-related PD [112] and are attributable to enhanced activity or responsivity of Two Pore Channel 2 (TPC2), a suspected target of LRRK2 phosphorylation.
Although primary lysosomal dysfunction is shared among multiple major neurodegenerative diseases, the PANTHOS pattern of autophagy pathology seen in AD is not present to this magnitude in other adult neurodegenerative diseases so far reported or studied in vivo with the TRGL autophagy probe (e.g., ALS-SOD1 mutation; HD-HTT mutation [10, 215]. Critical differences between AD and these other diseases are the importance of APP, specifically the direct inhibitory action of APP-βCTF on lysosomal acidification, contributory roles of other genes corrupting lysosomes, such as presenilin mutations and APOE4, and, importantly, the persistence of a high level of autophagy induction throughout the course of AD. APP over-expression per se is not an explanation for PANTHOS given that late-onset human AD [129] exhibit PANTHOS and accumulate higher levels of APP-βCTF. Potentially instructive in this regard is the childhood lysosomal disorder Niemann–Pick type C, which exhibits APP miss-metabolism, AD-like endosome enlargement, modest β-amyloid deposits, and striking autophagic vacuole build-up. Like AD, autophagy induction is sustained and exacerbates the autophagy phenotype because when autophagy induction is experimentally suppressed, the build-up of storage material is attenuated [69, 146, 248]. Moreover, in contrast to AD and NPC, autophagy induction is not increased and may, in fact, diminish during the course of PD and FTLD [108, 182], which is expected to reduce the burden on lysosomes.
Further consequences of lysosomal dysfunction for AD pathological development
Tauopathy
Hallmark neurofibrillary tangles of AD, composed mainly of tau protein, are abundant in AD brain and in a few uncommon aging-related degenerative diseases caused by rare tau mutations, including one form of frontotemporal dementia. An underlying mechanism involving the lysosome is suggested by observations that NFTs are also present in the lysosomal disorders, NPC1 and mucopolysaccharidosis type IIB [175, 203, 204]. Despite its notoriety as a neuropathological AD hallmark, however, NFT accumulation in AD has been proposed to be a marker of resilience [53] compared to the neurons that accumulate abeta [29] and other observations that neurons may live for decades with neurofibrillary tangles [90, 155]. Other work has suggested that accumulation of pTau may not impair neuronal function, at least initially [120] and may instead facilitate neurons escape from apoptosis [136, 242]. Collectively, these studies raise the possibility that NFT formation may not be a primary driver of neuronal death in AD but rather may be a marker of resilience [29].
Abnormal hyperphosphorylation [63, 132], truncation of tau [38, 177], and cathepsin D suppression [223] are each considered to promote tauopathy-related neuronal dysfunctions in AD, whether or not they are associated with tangle formation [63, 77]. Each is also an outcome of lysosomal deacidification and associated with TRPML1 activation [127]. These findings, together with evidence that tau is an autophagy substrate [9], suggest mechanistic links between tauopathy and lysosomal dysfunction, including calcium release from TRPML1 channels which may be regulated in a combined manner by pH, Ca2+, phosphoinositides, and LAMTOR1 subunit of the Ragulator complex [127, 140, 207, 218, 222]. The cytosolic calcium rise in PSEN1 AD cell models via lysosomal TRPML1 activates calpains [127, 140] which generates truncated tau prone to aggregation [38] and the p25 cleavage product of cdk5 that hyper-phosphorylates tau [132] in AD and in FTD models [196] while promoting cell death [132, 165]. Notably, inhibition of calpains specifically by over-expressing its endogenous inhibitor calpastatin markedly reduces tauopathy and neuron loss in tau P301L and P301S tauopathy mouse models [142, 196].
Tau is metabolized by both the ubiquitin proteasome system and autophagy and contains a motif that targets it for chaperone-mediated autophagy [27]. Incomplete chaperone-mediated autophagy of tau generates fragments that aggregate and are cleared by macroautophagy [237]. Moreover, autophagy preferentially degrades a caspase-cleaved fragment of tau implicated in tau neurotoxicity [62]. Consistent with these findings, autophagy induction reduces tau pathology in the triple transgenic AD mouse model [28]. Conversely, autophagic-lysosomal dysfunction amplifies pathology and neurotoxicity of tau in other AD models [86, 111]. In addition, asparagine endopeptidase, a lysosomal cysteine proteinase upregulated during aging and activated in AD [254], is released in neurons injured by brain ischemia and hypoxia [254]. This enzyme and I2PP2A translocate from neuronal lysosomes and the nucleus, respectively, to the cytoplasm where they interact and lead to tau hyperphosphorylation [9] rescued by asparagine endopeptidase inhibition [254].
Neuritic dystrophy of AD: a selective autophagy-related trafficking deficit
Another hallmark AD lesion linked to autophagy–lysosomal impairment is neuritic dystrophy, referring to focal axonal swellings filled nearly exclusively with autophagic vacuoles and commonly present within senile plaques (or “neuritic plaques”) but more widely distributed. As in PANTHOS, amyloid-β, BACE1 and βCTF are enriched in AVs providing for potential release from affected dystrophic regions and formation of diffuse β-amyloid in the local vicinity. However, AD-like dystrophic neurites also form in the absence of β-amyloid as shown in aged PSEN1 knock-in FAD model mice [140] and GGA3-deleted mice that express high levels of BACE1 [115, 145]. In the former model, local calcium release from late endosome/amphisome compartments via TRPML1 activates JNK phosphorylation of dynein intermediate chains, impedes retrograde motility of autophagic/amphisomal vesicles [140] and induces their selective accumulation within focal axon swellings [140] recapitulating AD neuritic dystrophy. Restoring proper lysosome acidity blocks these events [140]. Earlier studies showed that pharmacologically inhibiting vATPase in primary neurons selectively slows the retrograde motility of vesicles tagged with LC3 or LAMP1 (i.e., autophagic vacuoles) and also induces their selective accumulation within focal axon swellings [133]. Involvement of calcium release from compromised lysosomes [138] is suggested by the PSEN1 FAD knock-in mouse model study [140] in vivo and in vitro, where the lysosomal calcium release activates c-jun N-terminal kinase and phosphorylates dynein thereby interfering with retrograde axonal transport of endolysosome compartments.
APP-βCTF is also linked to the lysosomal pH dysregulation associated with impaired AV retrograde transport and accelerated neuritic dystrophy in individuals carrying a polymorphism of the AD risk gene, phospholipase D3 (PLD3) encoding a lysosomal protein abundant in neurons [232, 251]. As discussed above under “Tauopathy”, the local release of calcium from poorly acidified endolysosomes likely disrupts dynein-mediated retrograde axonal motility [140].
Ferroptosis
In the face of heightened oxidative stress, genes involved in iron homeostasis are essential for neuron survival along with the genes for superoxide dismutases, endolysosomal function, autophagy, and cholesterol handling [226], all of which are widely implicated in neurodegenerative diseases. Rising oxidative stress in aging brain and neurodegenerative disease especially from oxidized lipid accumulations in lysosomes elevate Fe2+ entry into cells [96, 252] through the endolysosomal system [6, 210], which helps to further drive oxidative stress via the Fenton reaction [206, 212] and promotes lysosomal deacidification [245]. Iron release from endolysosomes to cytoplasm requires low acidic pH and is key to maintaining appropriate cytoplasmic iron levels. When lysosomal acidification is defective, Fe3+ derived from ferritinophagy, mitophagy or transferrin endocytosis cannot be reduced to Fe2+ thereby mpeding iron export and causing a deficiency of iron in the cytoplasm and in mitochondria [122]. Iron deficiency activates a pseudo-hypoxia response, loss of mitochondrial function, and non-apoptotic cell death [245]. Moreover, iron deficiency is sufficient to trigger inflammatory cell-autonomous inflammatory gene expression in cultured neurons and in vivo, triggering non-apoptotic cell death known to accompany sterile inflammatory responses [200, 238]. Importantly, inhibition of lysosomal vATPase is sufficient to initiate this cascade in vivo, which can be ablated by repleting iron through the diet [245]. Furthering the link between ferroptosis, LMP, and lysosomal cell death is evidence that ferroptosis requires cathepsin B expression, which can be mediated by STAT3, a positive regulator of ferroptosis in some cell lines and also a promoter of lysosomal-mediated cell death in mice during mammary gland involution [119, 205]. Inhibiting lysosome-dependent cell death by pharmacological blockade of cathepsin activity or vATPase limits erastin-induced ferroptosis [76].
Consistent with the discussion that lysosome dysfunction catalyzes varied cell death cascades, a significant contribution by ferroptosis in AD has been increasingly suggested to be a secondary consequence of lysosomal dysfunction although the mechanism remains unclear and the relevance to human AD is, therefore, circumstantial [78, 130]. Alterations in the three primary factors in this process—iron export [65], thiols (reverse transporter system xc− and glutathione (GSH)/glutathione peroxidase 4) and lipids, are considered contributors to AD pathogenesis. Collectively, these alterations lead to iron dyshomeostasis [59, 154] and iron-dependent lipid peroxide elevations, which are also seen in the brain in AD [1, 246] and are associated ultimately with cell death [255]. In conditions of high iron, such as AD, APP translation is increased [137], which can contribute to elevations of APP-βCTF and Aβ [40, 122, 202], thus accelerating lysosomal dysfunction.
Selected methods for autophagy–lysosomal evaluation in human brain
Striking neuronal autophagy–lysosomal abnormalities were initially appreciated in early AD brain using cathepsin D immunocytochemistry [34,35,36, 167, 168]. It was proposed at the time that the presence of active neuronal lysosomal cathepsins in extracellular plaques reflected a primary origin of plaques from dying neurons [35], although the integrity of these grossly distorted neurons and their relationship to amyloid plaques was difficult to establish using a single marker with or without markers of amyloid. The neuron-specific LC3 autophagy probe selectively expressed in neurons of mouse AD models, however, enabled a visualization of the true extent of autophagy pathology culminating in the unique PANTHOS morphological pattern to be appreciated together with intraneuronal amyloid lesions and their transformation into extracellular plaques. With the PANTHOS evolution having been characterized, detection of this unique autophagy pattern and antecedent autophagic abnormalities in human AD brain can now also be achieved using appropriate pairs of antibodies and nuclear histochemistry [129]. The additional use of axonal markers and confocal imaging in the z-plane dimension facilitates distinguishing PANTHOS ongoing in neurons from the cells that have already transformed into plaques. It is important to note, however, that studies of neurons using TRGL mice do not exclude the possibility that microglial cells engorging amyloid-containing debris at later stages of AD may also exhibit a variant of inside-out plaque formation when they die. Conducting analyses in cortical regions of brain at early Braak stages (II–III) before the later-stage inflammatory response greatly simplifies the recognition of individual neurons undergoing lysosomal cell death.
Conclusions
Compromise of vulnerable neurons culminating in their death in AD is frequently an indolent process reflecting continuous mobilization of repair and other mechanisms of neuroprotection, including autophagy, to counter aging-related and disease-promoting obstacles. Evidence suggests that cell death modes may differ for individual types of neurons and other cells in brain. Moreover, depending on the pathological context, neurons even within the same population may activate different death mechanisms at varying stages. The characterization of neuronal cell death in AD is fraught with challenges reflecting the possible multiplicity of participating death subroutines, superimposed secondary toxicities from the brain’s responses to an initial insult, and technical difficulties of monitoring changes in the multiple cell death pathways that may be involved as neurons progress toward death.
The opportunity to characterize the entire course of an exceptionally early cell death of vulnerable neocortical pyramidal neurons in AD brain and mouse models of AD pathology by probing neuronal autophagy dysfunction prior to conventional neuropathological lesions is fortuitous. While ELA dysfunction emerges early in most layer III and V neocortical neurons, a select subset of these neurons develops unique states of worsening autophagic stress, LMP, abeta aggregation, and lysosomal-dependent death beginning during this poorly understood “intraneuronal” stage of AD and leaving extracellular amyloid plaques in their wake.
The early emergence of LMP beginning as the brain ages evolves to more fulminant AD-related lysosomal dysfunction driven by genetic and environmental factors many of which converge on a mechanism of disrupted vATPase activity causing intralumenal pH to rise. These events leading to cell death are triggered and partly executed by dysregulated lysosomes given that genetic and pharmacological remediation of lysosomal deficits attenuate neuron cell death and ensuing extracellular amyloid deposition and rescue synaptic and cognitive impairments. These observations do not exclude participation by other cell death subroutines at end stages when the fate of the neuron may possibly have already been determined. The foregoing weight of evidence, however, establishes autophagy-associated lysosome-dependent cell death as one cascade in AD having numerous implications for the further progression of the disease. The findings to date encourage investigation of LMP/LCD modulation and therapeutic strategies targeting lysosome dysfunction to defeat AD at its earliest stage.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- AVs:
-
Autophagic Vacuoles
- ER-phagy:
-
Autophagy of ER
- BMP:
-
Bis (monoacylglycerol) phosphate
- CMA:
-
Chaperone-mediated autophagy
- ELA:
-
Endosomal-lysosomal-autophagy
- ER :
-
Endoplasmic reticulum
- ERAD:
-
Endoplasmic reticulum-associated degradation
- ESCRT:
-
Endosomal sorting complexes required for transport
- fAD:
-
Familial AD
- Fe2+ :
-
Ferrous iron ions
- FTD:
-
Frontotemporal dementia
- FTLD:
-
Frontotemporal lobar degeneration
- HD:
-
Huntington’s disease
- LMP:
-
Lysosomal membrane permeabilization
- LSDs:
-
Lysosomal storage disorders
- LCD:
-
Lysosome-dependent cell death
- MPR:
-
Mannose-6-phosphate receptor
- MAMs:
-
Mitochondria-associated ER membrane interactions
- MMP:
-
Mitochondrial membrane potential
- NFTs:
-
Neurofibrillary tangles
- PANTHOS :
-
p-anthos or poisonous flower
- PD:
-
Parkinson’s disease
- Ph:
-
Phagophore
- PSEN 1:
-
Presenilin 1
- ROS:
-
Reactive oxygen species
- TFEB, TFE3:
-
Transcription factors
- TPC2:
-
Two-pore channel 2
- vATPase:
-
Vacuolar ATPase
References
Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12:125–169. https://doi.org/10.1089/ars.2009.2668
Ahmed RR, Holler CJ, Webb RL, Li F, Beckett TL, Murphy MP (2010) BACE1 and BACE2 enzymatic activities in Alzheimer’s disease. J Neurochem 112:1045–1053. https://doi.org/10.1111/j.1471-4159.2009.06528.x
Annunziata I, Patterson A, Helton D, Hu H, Moshiach S, Gomero E et al (2013) Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-beta secretion via deregulated lysosomal exocytosis. Nat Commun 4:2734. https://doi.org/10.1038/ncomms3734
Arakawa S, Tsujioka M, Yoshida T, Tajima-Sakurai H, Nishida Y, Matsuoka Y et al (2017) Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ 24:1598–1608. https://doi.org/10.1038/cdd.2017.84
Area-Gomez E, de Groof A, Bonilla E, Montesinos J, Tanji K, Boldogh I et al (2018) A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis 9:335. https://doi.org/10.1038/s41419-017-0215-0
Ashraf A, Clark M, So PW (2018) The aging of iron man. Front Aging Neurosci 10:65. https://doi.org/10.3389/fnagi.2018.00065
Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H (2013) Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J Biol Chem 288:1295–1306. https://doi.org/10.1074/jbc.M112.409250
Baik SH, Kang S, Son SM, Mook-Jung I (2016) Microglia contributes to plaque growth by cell death due to uptake of amyloid β in the brain of Alzheimer’s disease mouse model. Glia 64:2274–2290. https://doi.org/10.1002/glia.23074
Basurto-Islas G, Grundke-Iqbal I, Tung YC, Liu F, Iqbal K (2013) Activation of asparaginyl endopeptidase leads to Tau hyperphosphorylation in Alzheimer disease. J Biol Chem 288:17495–17507. https://doi.org/10.1074/jbc.M112.446070
Berg MJ, Veeranna, Rosa CM, Kumar A, Mohan PS, Stavrides P et al. (2024) Pathobiology of the autophagy-lysosomal pathway in the Huntington's disease brain. bioRxiv: https://doi.org/10.1101/2024.05.29.596470
Bialik S, Dasari SK, Kimchi A (2018) Autophagy-dependent cell death: where, how and why a cell eats itself to death. J Cell Sci. https://doi.org/10.1242/jcs.215152
Blom T, Li S, Dichlberger A, Back N, Kim YA, Loizides-Mangold U et al (2015) LAPTM4B facilitates late endosomal ceramide export to control cell death pathways. Nat Chem Biol 11:799–806. https://doi.org/10.1038/nchembio.1889
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH et al (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci 28:6926–6937. https://doi.org/10.1523/JNEUROSCI.0800-08.2008
Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE et al (2008) Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci 28:4283–4292. https://doi.org/10.1523/jneurosci.4814-07.2008
Boon BDC, Bulk M, Jonker AJ, Morrema THJ, van den Berg E, Popovic M et al (2020) The coarse-grained plaque: a divergent Aβ plaque-type in early-onset Alzheimer’s disease. Acta Neuropathol 140:811–830. https://doi.org/10.1007/s00401-020-02198-8
Bordi M, Berg MJ, Mohan PS, Peterhoff CM, Alldred MJ, Che S et al (2016) Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 12:2467–2483. https://doi.org/10.1080/15548627.2016.1239003
Bourdenx M, Daniel J, Genin E, Soria FN, Blanchard-Desce M, Bezard E et al (2016) Nanoparticles restore lysosomal acidification defects: implication for Parkinson and other lysosomal-related diseases. Autophagy 12:472–483. https://doi.org/10.1080/15548627.2015.1136769
Bourdenx M, Martin-Segura A, Scrivo A, Rodriguez-Navarro JA, Kaushik S, Tasset I et al (2021) Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell 184:2696-2714.e2625. https://doi.org/10.1016/j.cell.2021.03.048
Bourgeois A, Lauritzen I, Lorivel T, Bauer C, Checler F, Pardossi-Piquard R (2018) Intraneuronal accumulation of C99 contributes to synaptic alterations, apathy-like behavior, and spatial learning deficits in 3xTgAD and 2xTgAD mice. Neurobiol Aging 71:21–31. https://doi.org/10.1016/j.neurobiolaging.2018.06.038
Boya P (2012) Lysosomal function and dysfunction: mechanism and disease. Antioxid Redox Signal 17:766–774. https://doi.org/10.1089/ars.2011.4405
Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D et al (2003) Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med 197:1323–1334. https://doi.org/10.1084/jem.20021952
Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T et al (2003) Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22:3927–3936. https://doi.org/10.1038/sj.onc.1206622
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27:6434–6451. https://doi.org/10.1038/onc.2008.310
Burrinha T, Cunha C, Hall MJ, Lopes-da-Silva M, Seabra MC, Guimas Almeida C (2023) Deacidification of endolysosomes by neuronal aging drives synapse loss. Traffic 24:334–354. https://doi.org/10.1111/tra.12889
Butler D, Hwang J, Estick C, Nishiyama A, Kumar SS, Baveghems C et al (2011) Protective effects of positive lysosomal modulation in Alzheimer’s disease transgenic mouse models. PLoS ONE. https://doi.org/10.1371/journal.pone.0020501
Butterfield DA (2023) Oxidative stress in brain in amnestic mild cognitive impairment. Antioxidants (Basel). https://doi.org/10.3390/antiox12020462
Caballero B, Bourdenx M, Luengo E, Diaz A, Sohn PD, Chen X et al (2021) Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice. Nat Commun 12:2238. https://doi.org/10.1038/s41467-021-22501-9
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 285:13107–13120. https://doi.org/10.1074/jbc.M110.100420
Caramello A, Fancy N, Tournerie C, Eklund M, Chau V, Adair E, et al. (2023) Intra-cellular accumulation of amyloid is a marker of selective neuronal vulnerability in Alzheimer’s disease. medRxiv: 2023.2011.2023.23298911 https://doi.org/10.1101/2023.11.23.23298911
Carmona-Gutierrez D, Hughes AL, Madeo F, Ruckenstuhl C (2016) The crucial impact of lysosomes in aging and longevity. Ageing Res Rev 32:2–12. https://doi.org/10.1016/j.arr.2016.04.009
Cason SE, Carman PJ, Van Duyne C, Goldsmith J, Dominguez R, Holzbaur ELF (2021) Sequential dynein effectors regulate axonal autophagosome motility in a maturation-dependent pathway. J Cell Biol. https://doi.org/10.1083/jcb.202010179
Cataldo AM, Barnett JL, Berman SA, Li J, Quarless S, Bursztajn S et al (1995) Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron 14:671–680
Cataldo AM, Barnett JL, Mann DM, Nixon RA (1996) Colocalization of lysosomal hydrolase and beta-amyloid in diffuse plaques of the cerebellum and striatum in Alzheimer’s disease and Down’s syndrome. J Neuropathol Exp Neurol 55:704–715. https://doi.org/10.1097/00005072-199606000-00004
Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA (1996) Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer’s disease. J Neurosci 16:186–199
Cataldo AM, Hamilton DJ, Nixon RA (1994) Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res 640:68–80. https://doi.org/10.1016/0006-8993(94)91858-9
Cataldo AM, Nixon RA (1990) Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci USA 87:3861–3865. https://doi.org/10.1073/pnas.87.10.3861
Checler F, Afram E, Pardossi-Piquard R, Lauritzen I (2021) Is γ-secretase a beneficial inactivating enzyme of the toxic APP C-terminal fragment C99? J Biol Chem. https://doi.org/10.1016/j.jbc.2021.100489
Chen HH, Liu P, Auger P, Lee SH, Adolfsson O, Rey-Bellet L et al (2018) Calpain-mediated tau fragmentation is altered in Alzheimer’s disease progression. Sci Rep 8:16725. https://doi.org/10.1038/s41598-018-35130-y
Cherra SJ 3rd, Chu CT (2008) Autophagy in neuroprotection and neurodegeneration: a question of balance. Future Neurol 3:309–323. https://doi.org/10.2217/14796708.3.3.309
Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X et al (2010) Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem 285:31217–31232. https://doi.org/10.1074/jbc.M110.149161
Christensen DZ, Kraus SL, Flohr A, Cotel MC, Wirths O, Bayer TA (2008) Transient intraneuronal A beta rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol 116:647–655. https://doi.org/10.1007/s00401-008-0451-6
Christensen KA, Myers JT, Swanson JA (2002) pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci 115:599–607
Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A (2002) NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111:703–708. https://doi.org/10.1016/s0092-8674(02)01082-6
Clarke PG (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 181:195–213. https://doi.org/10.1007/bf00174615
Coffey EE, Beckel JM, Laties AM, Mitchell CH (2014) Lysosomal alkalization and dysfunction in human fibroblasts with the Alzheimer’s disease-linked presenilin 1 A246E mutation can be reversed with cAMP. Neuroscience 263:111–124. https://doi.org/10.1016/j.neuroscience.2014.01.001
Colacurcio DJ, Nixon RA (2016) Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev 32:75–88. https://doi.org/10.1016/j.arr.2016.05.004
Collins MP, Forgac M (2020) Regulation and function of V-ATPases in physiology and disease. Biochim Biophys Acta Biomembr. https://doi.org/10.1016/j.bbamem.2020.183341
Cotter K, Stransky L, McGuire C, Forgac M (2015) Recent insights into the structure, regulation, and function of the V-ATPases. Trends Biochem Sci 40:611–622. https://doi.org/10.1016/j.tibs.2015.08.005
Cuervo AM, Dice JF (2000) Regulation of lamp2a levels in the lysosomal membrane. Traffic 1:570–583. https://doi.org/10.1034/j.1600-0854.2000.010707.x
Cuervo AM, Dice JF (2000) Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci 113(Pt 24):4441–4450
D’Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH (2001) Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology 38:120–134. https://doi.org/10.1046/j.1365-2559.2001.01082.x
d’Errico P, Ziegler-Waldkirch S, Aires V, Hoffmann P, Mezö C, Erny D et al (2022) Microglia contribute to the propagation of Aβ into unaffected brain tissue. Nat Neurosci 25:20–25. https://doi.org/10.1038/s41593-021-00951-0
de Calignon A, Spires-Jones TL, Pitstick R, Carlson GA, Hyman BT (2009) Tangle-bearing neurons survive despite disruption of membrane integrity in a mouse model of tauopathy. J Neuropathol Exp Neurol 68:757–761. https://doi.org/10.1097/NEN.0b013e3181a9fc66
de Duve C (2005) The lysosome turns fifty. Nat Cell Biol 7:847–849. https://doi.org/10.1038/ncb0905-847
De Strooper B, Iwatsubo T, Wolfe MS (2012) Presenilins and gamma-secretase: structure, function, and role in Alzheimer Disease. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a006304
Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci USA 110:E1817-1826. https://doi.org/10.1073/pnas.1305623110
Decuypere JP, Parys JB, Bultynck G (2012) Regulation of the autophagic bcl-2/beclin 1 interaction. Cells 1:284–312. https://doi.org/10.3390/cells1030284
Denton D, Kumar S (2019) Autophagy-dependent cell death. Cell Death Differ 26:605–616. https://doi.org/10.1038/s41418-018-0252-y
Derry PJ, Hegde ML, Jackson GR, Kayed R, Tour JM, Tsai AL et al (2020) Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog Neurobiol. https://doi.org/10.1016/j.pneurobio.2019.101716
Di Domenico F, Coccia R, Cocciolo A, Murphy MP, Cenini G, Head E et al (2013) Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer’s disease neuropathology: redox proteomics analysis of human brain. Biochim Biophys Acta 1832:1249–1259. https://doi.org/10.1016/j.bbadis.2013.04.013
Dickson TC, Vickers JC (2001) The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience 105:99–107. https://doi.org/10.1016/s0306-4522(01)00169-5
Dolan PJ, Johnson GV (2010) A caspase cleaved form of tau is preferentially degraded through the autophagy pathway. J Biol Chem 285:21978–21987. https://doi.org/10.1074/jbc.M110.110940
Dong Y, Yu H, Li X, Bian K, Zheng Y, Dai M et al (2022) Hyperphosphorylated tau mediates neuronal death by inducing necroptosis and inflammation in Alzheimer’s disease. J Neuroinflammation 19:205. https://doi.org/10.1186/s12974-022-02567-y
Dooley HC, Razi M, Polson HE, Girardin SE, Wilson MI, Tooze SA (2014) WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol Cell 55:238–252. https://doi.org/10.1016/j.molcel.2014.05.021
Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K et al (2010) Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142:857–867. https://doi.org/10.1016/j.cell.2010.08.014
Duve Cd (1959) Lysosomes, a new group of cytoplasmic particle. The Ronald Press Co., City
Eimer WA, Vassar R (2013) Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Mol Neurodegener 8:2. https://doi.org/10.1186/1750-1326-8-2
El Far O, Seagar M (2011) A role for V-ATPase subunits in synaptic vesicle fusion? J Neurochem 117:603–612. https://doi.org/10.1111/j.1471-4159.2011.07234.x
Elrick MJ, Yu T, Chung C, Lieberman AP (2012) Impaired proteolysis underlies autophagic dysfunction in Niemann-Pick type C disease. Hum Mol Genet 21:4876–4887. https://doi.org/10.1093/hmg/dds324
Feng X, Yang J (2016) Lysosomal calcium in neurodegeneration. Messenger (Los Angel) 5:56–66. https://doi.org/10.1166/msr.2016.1055
Fischer P-DD, Oskar (1907) Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmässige Veränderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiatr Neurol 22:361–372. https://doi.org/10.1159/000211873
Fraser J, Simpson J, Fontana R, Kishi-Itakura C, Ktistakis NT, Gammoh N (2019) Targeting of early endosomes by autophagy facilitates EGFR recycling and signalling. EMBO Rep. https://doi.org/10.15252/embr.201947734
Fruhwürth S, Zetterberg H, Paludan SR (2024) Microglia and amyloid plaque formation in Alzheimer’s disease: evidence, possible mechanisms, and future challenges. J Neuroimmunol. https://doi.org/10.1016/j.jneuroim.2024.578342
Fukumoto H, Cheung BS, Hyman BT, Irizarry MC (2002) Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol 59:1381–1389
Ganesan D, Cai Q (2021) Understanding amphisomes. Biochem J 478:1959–1976. https://doi.org/10.1042/bcj20200917
Gao H, Bai Y, Jia Y, Zhao Y, Kang R, Tang D et al (2018) Ferroptosis is a lysosomal cell death process. Biochem Biophys Res Commun 503:1550–1556. https://doi.org/10.1016/j.bbrc.2018.07.078
Gendron TF, Petrucelli L (2009) The role of tau in neurodegeneration. Mol Neurodegener 4:13. https://doi.org/10.1186/1750-1326-4-13
Gleason A, Bush AI (2021) Iron and ferroptosis as therapeutic targets in Alzheimer’s disease. Neurotherapeutics 18:252–264. https://doi.org/10.1007/s13311-020-00954-y
Gomez-Sintes R, Ledesma MD, Boya P (2016) Lysosomal cell death mechanisms in aging. Ageing Res Rev 32:150–168. https://doi.org/10.1016/j.arr.2016.02.009
Gorman AM (2008) Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med 12:2263–2280. https://doi.org/10.1111/j.1582-4934.2008.00402.x
Gouras GK, Almeida CG, Takahashi RH (2005) Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiol Aging 26:1235–1244. https://doi.org/10.1016/j.neurobiolaging.2005.05.022
Gouras GK, Willen K, Faideau M (2014) The inside-out amyloid hypothesis and synapse pathology in Alzheimer’s disease. Neurodegener Dis 13:142–146. https://doi.org/10.1159/000354776
Guse AH, Lee HC (2008) NAADP: a universal Ca2+ trigger. Sci Signal 1:re10. https://doi.org/10.1126/scisignal.144re10
Haapasalo A, Kovacs DM (2011) The many substrates of presenilin/gamma-secretase. J Alzheimers Dis 25:3–28. https://doi.org/10.3233/JAD-2011-101065
Haeri M, Knox BE (2012) Endoplasmic reticulum stress and unfolded protein response pathways: potential for treating age-related retinal degeneration. J Ophthalmic Vis Res 7:45–59
Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C et al (2008) Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci 27:1119–1130. https://doi.org/10.1111/j.1460-9568.2008.06084.x
Hansen M, Rubinsztein DC, Walker DW (2018) Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 19:579–593. https://doi.org/10.1038/s41580-018-0033-y
Hansson O (2021) Biomarkers for neurodegenerative diseases. Nat Med 27:954–963. https://doi.org/10.1038/s41591-021-01382-x
Higashida H, Yokoyama S, Tsuji C, Muramatsu SI (2017) Neurotransmitter release: vacuolar ATPase V0 sector c-subunits in possible gene or cell therapies for Parkinson’s, Alzheimer’s, and psychiatric diseases. J Physiol Sci JPS 67:11–17. https://doi.org/10.1007/s12576-016-0462-3
Hof PR, Bussière T, Gold G, Kövari E, Giannakopoulos P, Bouras C et al (2003) Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J Neuropathol Exp Neurol 62:55–67. https://doi.org/10.1093/jnen/62.1.55
Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G (2002) Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol 51:783–786. https://doi.org/10.1002/ana.10208
Honarnejad K, Jung CK, Lammich S, Arzberger T, Kretzschmar H, Herms J (2013) Involvement of presenilin holoprotein upregulation in calcium dyshomeostasis of Alzheimer’s disease. J Cell Mol Med 17:293–302. https://doi.org/10.1111/jcmm.12008
Ibata K, Yuzaki M (2021) Destroy the old to build the new: activity-dependent lysosomal exocytosis in neurons. Neurosci Res 167:38–46. https://doi.org/10.1016/j.neures.2021.03.011
Im E, Jiang Y, Lee J-H, Nixon RA (2019) APP-βCTF regulates vATPase-mediated lysosomal acidification. Poster Session presented at the AD/PD The 14th International Conference on Alzheimer’s and Parkinson’s Diseases, City
Im E, Jiang Y, Stavrides P, Darji S, Erdjument-Bromage H, Neubert TA et al (2023) Lysosomal dysfunction in Down Syndrome and Alzheimer mouse models is caused by selective v-ATPase inhibition by Tyr682 phosphorylated APP βCTF. Sci Adv. https://doi.org/10.1126/sciadv.adg1925
JS (2019) Does high iron push a person with pathology into dementia? https://www.alzforum.org/news/research-news/does-high-iron-push-person-pathology-dementia
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. https://doi.org/10.1016/s1474-4422(12)70291-0
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW et al (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128. https://doi.org/10.1016/s1474-4422(09)70299-6
Jayne T, Newman M, Verdile G, Sutherland G, Munch G, Musgrave I et al (2016) Evidence for and against a pathogenic role of reduced gamma-secretase activity in familial Alzheimer’s Disease. J Alzheimers Dis 52:781–799
Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A et al (2010) Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci USA 107:1630–1635. https://doi.org/10.1073/pnas.0908953107
Jiang Y, Rigoglioso A, Peterhoff CM, Pawlik M, Sato Y, Bleiwas C et al (2016) Partial BACE1 reduction in a Down syndrome mouse model blocks Alzheimer-related endosomal anomalies and cholinergic neurodegeneration: role of APP-CTF. Neurobiol Aging 39:90–98. https://doi.org/10.1016/j.neurobiolaging.2015.11.013
Jiang Y, Sato Y, Im E, Berg M, Bordi M, Darji S et al (2019) Lysosomal dysfunction in Down Syndrome Is APP-dependent and mediated by APP-betaCTF (C99). J Neurosci 39:5255–5268. https://doi.org/10.1523/JNEUROSCI.0578-19.2019
Jin H, Sanjo N, Uchihara T, Watabe K, St George-Hyslop P, Fraser PE et al (2010) Presenilin-1 holoprotein is an interacting partner of sarco endoplasmic reticulum calcium-ATPase and confers resistance to endoplasmic reticulum stress. J Alzheimers Dis 20:261–273. https://doi.org/10.3233/jad-2010-1360
Jin LW, Shie FS, Maezawa I, Vincent I, Bird T (2004) Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol 164:975–985. https://doi.org/10.1016/s0002-9440(10)63185-9
Johansson AC, Appelqvist H, Nilsson C, Kagedal K, Roberg K, Ollinger K (2010) Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 15:527–540. https://doi.org/10.1007/s10495-009-0452-5
Johnson ECB, Carter EK, Dammer EB, Duong DM, Gerasimov ES, Liu Y et al (2022) Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat Neurosci 25:213–225. https://doi.org/10.1038/s41593-021-00999-y
Kallergi E, Siva Sankar D, Matera A, Kolaxi A, Paolicelli RC, Dengjel J et al (2023) Profiling of purified autophagic vesicle degradome in the maturing and aging brain. Neuron. https://doi.org/10.1016/j.neuron.2023.05.011
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18:571–580. https://doi.org/10.1038/cdd.2010.191
Kaur G, Pawlik M, Gandy SE, Ehrlich ME, Smiley JF, Levy E (2017) Lysosomal dysfunction in the brain of a mouse model with intraneuronal accumulation of carboxyl terminal fragments of the amyloid precursor protein. Mol Psychiatry 22:981–989. https://doi.org/10.1038/mp.2016.189
Kaushik S, Cuervo AM (2018) The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 19:365–381. https://doi.org/10.1038/s41580-018-0001-6
Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, Mulkearns E et al (2010) Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet. https://doi.org/10.1371/journal.pgen.1001026
Kilpatrick BS, Yates E, Grimm C, Schapira AH, Patel S (2016) Endo-lysosomal TRP mucolipin-1 channels trigger global ER Ca2+ release and Ca2+ influx. J Cell Sci 129:3859–3867. https://doi.org/10.1242/jcs.190322
Kim HS, Park CH, Cha SH, Lee JH, Lee S, Kim Y et al (2000) Carboxyl-terminal fragment of Alzheimer’s APP destabilizes calcium homeostasis and renders neuronal cells vulnerable to excitotoxicity. FASEB J 14:1508–1517
Kim KR, Park SE, Hong JY, Koh JY, Cho DH, Hwang JJ et al (2022) Zinc enhances autophagic flux and lysosomal function through transcription factor EB activation and V-ATPase assembly. Front Cell Neurosci. https://doi.org/10.3389/fncel.2022.895750
Kim W, Ma L, Lomoio S, Willen R, Lombardo S, Dong J et al (2018) BACE1 elevation engendered by GGA3 deletion increases β-amyloid pathology in association with APP elevation and decreased CHL1 processing in 5XFAD mice. Mol Neurodegener 13:6. https://doi.org/10.1186/s13024-018-0239-7
Kirkegaard T, Roth AG, Petersen NH, Mahalka AK, Olsen OD, Moilanen I et al (2010) Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463:549–553. https://doi.org/10.1038/nature08710
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12: 1–222 https://doi.org/10.1080/15548627.2015.1100356
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884. https://doi.org/10.1038/nature04723
Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J et al (2011) Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol 13:303–309. https://doi.org/10.1038/ncb2171
Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML et al (2014) Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci USA 111:510–514. https://doi.org/10.1073/pnas.1318807111
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113(Pt 20):3613–3622
Lardelli M (2023) An alternative view of familial Alzheimer’s disease genetics. J Alzheimers Dis 96:13–39. https://doi.org/10.3233/jad-230313
Lauritzen I, Becot A, Bourgeois A, Pardossi-Piquard R, Biferi MG, Barkats M et al (2019) Targeting gamma-secretase triggers the selective enrichment of oligomeric APP-CTFs in brain extracellular vesicles from Alzheimer cell and mouse models. Translat Neurodegen 8:35. https://doi.org/10.1186/s40035-019-0176-6
Lauritzen I, Pardossi-Piquard R, Bauer C, Brigham E, Abraham JD, Ranaldi S et al (2012) The beta-secretase-derived C-terminal fragment of betaAPP, C99, but not Abeta, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J Neurosci 32:16243–16255a. https://doi.org/10.1523/JNEUROSCI.2775-12.2012
Lauritzen I, Pardossi-Piquard R, Bourgeois A, Bécot A, Checler F (2019) Does intraneuronal accumulation of carboxyl-terminal fragments of the amyloid precursor protein trigger early neurotoxicity in Alzheimer’s Disease? Curr Alzheimer Res 16:453–457. https://doi.org/10.2174/1567205016666190325092841
Lauritzen I, Pardossi-Piquard R, Bourgeois A, Pagnotta S, Biferi MG, Barkats M et al (2016) Intraneuronal aggregation of the beta-CTF fragment of APP (C99) induces Abeta-independent lysosomal-autophagic pathology. Acta Neuropathol 132:257–276. https://doi.org/10.1007/s00401-016-1577-6
Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y et al (2015) Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep 12:1430–1444. https://doi.org/10.1016/j.celrep.2015.07.050
Lee JH, Rao MV, Yang DS, Stavrides P, Im E, Pensalfini A et al (2019) Transgenic expression of a ratiometric autophagy probe specifically in neurons enables the interrogation of brain autophagy in vivo. Autophagy 15:543–557. https://doi.org/10.1080/15548627.2018.1528812
Lee JH, Stavrides P, Darji S, Berg M, Goulbourne CN, PS M, et al. (2024) A preclinical intraneuronal stage of autophagy-lysosomal dysfunction, amyloidosis, and neuron death yields senile plaques in human late-onset Alzheimer’s Disease. In process
Lee JH, Yang DS, Goulbourne CN, Im E, Stavrides P, Pensalfini A et al (2022) Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat Neurosci 25:688–701. https://doi.org/10.1038/s41593-022-01084-8
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM et al (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141:1146–1158. https://doi.org/10.1016/j.cell.2010.05.008
Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405:360–364. https://doi.org/10.1038/35012636
Lee S, Sato Y, Nixon RA (2011) Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci 31:7817–7830. https://doi.org/10.1523/JNEUROSCI.6412-10.2011
Leuzy A, Chiotis K, Lemoine L, Gillberg PG, Almkvist O, Rodriguez-Vieitez E et al (2019) Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol Psychiatry 24:1112–1134. https://doi.org/10.1038/s41380-018-0342-8
Levy E (2017) Exosomes in the diseased brain: first insights from in vivo studies. Front Neurosci 11:142. https://doi.org/10.3389/fnins.2017.00142
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q et al (2007) Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A 104:3591–3596. https://doi.org/10.1073/pnas.0609303104
Li X, Liu Y, Zheng Q, Yao G, Cheng P, Bu G et al (2013) Ferritin light chain interacts with PEN-2 and affects γ-secretase activity. Neurosci Lett 548:90–94. https://doi.org/10.1016/j.neulet.2013.05.018
Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W et al (2016) A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol 18:404–417. https://doi.org/10.1038/ncb3324
Li Y, Yen D, Hendrix RD, Gordon BA, Dlamini S, Barthélemy NR et al (2024) Timing of biomarker changes in sporadic Alzheimer’s Disease in estimated years from symptom onset. Ann Neurol 95:951–965. https://doi.org/10.1002/ana.26891
Lie PPY, Yoo L, Goulbourne CN, Berg MJ, Stavrides P, Huo C et al (2022) Axonal transport of late endosomes and amphisomes is selectively modulated by local Ca(2+) efflux and disrupted by PSEN1 loss of function. Sci Adv. https://doi.org/10.1126/sciadv.abj5716
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A et al (2010) Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A 107:14164–14169. https://doi.org/10.1073/pnas.1009485107
Liu M, Wang L, Gao J, Dong Q, Perry G, Ma X et al (2019) Inhibition of calpain protects against tauopathy in transgenic P301S Tau mice. J Alzheimers Dis 69:1077–1087. https://doi.org/10.3233/jad-190281
Liu S, Li Y, Choi HMC, Sarkar C, Koh EY, Wu J et al (2018) Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death Dis 9:476. https://doi.org/10.1038/s41419-018-0469-1
Lloyd-Evans E, Waller-Evans H (2020) Lysosomal Ca(2+) homeostasis and signaling in health and disease. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a035311
Lomoio S, Willen R, Kim W, Ho KZ, Robinson EK, Prokopenko D et al (2020) Gga3 deletion and a GGA3 rare variant associated with late onset Alzheimer’s disease trigger BACE1 accumulation in axonal swellings. Sci Translat Med. https://doi.org/10.1126/scitranslmed.aba1871
MacLeod CM, Yousufzai FAK, Spencer LT, Kim S, Rivera-Rosario LA, Barrera ZD et al (2023) Trehalose enhances mitochondria deficits in human NPC1 mutant fibroblasts but disrupts mouse Purkinje cell dendritic growth ex vivo. PLoS ONE. https://doi.org/10.1371/journal.pone.0294312
Mahaman YAR, Huang F, Kessete Afewerky H, Maibouge TMS, Ghose B, Wang X (2019) Involvement of calpain in the neuropathogenesis of Alzheimer’s disease. Med Res Rev 39:608–630. https://doi.org/10.1002/med.21534
Majumder S, Richardson A, Strong R, Oddo S (2011) Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS ONE. https://doi.org/10.1371/journal.pone.0025416
Martini-Stoica H, Xu Y, Ballabio A, Zheng H (2016) The autophagy-lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci 39:221–234. https://doi.org/10.1016/j.tins.2016.02.002
McDade E, Bateman RJ (2018) Tau positron emission tomography in autosomal dominant Alzheimer Disease: small windows, big picture. JAMA Neurol 75:536–538. https://doi.org/10.1001/jamaneurol.2017.4026
McDaid J, Mustaly-Kalimi S, Stutzmann GE (2020) Ca(2+) dyshomeostasis disrupts neuronal and synaptic function in Alzheimer’s Disease. Cells. https://doi.org/10.3390/cells9122655
Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R et al (2015) Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17:288–299. https://doi.org/10.1038/ncb3114
Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C et al (2011) Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell 21:421–430. https://doi.org/10.1016/j.devcel.2011.07.016
Mills E, Dong XP, Wang F, Xu H (2010) Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med Chem 2:51–64. https://doi.org/10.4155/fmc.09.140
Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol 58:188–197. https://doi.org/10.1097/00005072-199902000-00008
Mrschtik M, Ryan KM (2015) Lysosomal proteins in cell death and autophagy. Febs j 282:1858–1870. https://doi.org/10.1111/febs.13253
Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J et al (2006) Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron 51:703–714
Mustaly-Kalimi S, Gallegos W, Marr RA, Gilman-Sachs A, Peterson DA, Sekler I et al (2022) Protein mishandling and impaired lysosomal proteolysis generated through calcium dysregulation in Alzheimer’s disease. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2211999119
Nakamura S, Oba M, Suzuki M, Takahashi A, Yamamuro T, Fujiwara M et al (2019) Suppression of autophagic activity by Rubicon is a signature of aging. Nat Commun 10:847. https://doi.org/10.1038/s41467-019-08729-6
Nilsson P, Loganathan K, Sekiguchi M, Matsuba Y, Hui K, Tsubuki S et al (2013) Abeta secretion and plaque formation depend on autophagy. Cell Rep 5:61–69. https://doi.org/10.1016/j.celrep.2013.08.042
Nishi T, Forgac M (2002) The vacuolar (H+)-ATPases–nature’s most versatile proton pumps. Nat Rev Mol Cell Biol 3:94–103. https://doi.org/10.1038/nrm729
Nixon RA (2020) The aging lysosome: an essential catalyst for late-onset neurodegenerative diseases. Biochim Biophys Acta. https://doi.org/10.1016/j.bbapap.2020.140443
Nixon RA (2017) Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease. FASEB J 31:2729–2743. https://doi.org/10.1096/fj.201700359
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29:528–535. https://doi.org/10.1016/j.tins.2006.07.003
Nixon RA (2000) A “protease activation cascade” in the pathogenesis of Alzheimer’s disease. Ann N Y Acad Sci 924:117–131
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19:983–997. https://doi.org/10.1038/nm.3232
Nixon RA, Cataldo AM (1993) The lysosomal system in neuronal cell death: a review. Ann N Y Acad Sci 679:87–109
Nixon RA, Cataldo AM, Paskevich PA, Hamilton DJ, Wheelock TR, Kanaley-Andrews L (1992) The lysosomal system in neurons. involvement at multiple stages of Alzheimer’s disease pathogenesis. Ann N Y Acad Sci 674:65–88
Nixon RA, Rubinsztein DC (2024) Mechanisms of autophagy-lysosome dysfunction in neurodegenerative diseases. Nat Rev Mol Cell Biol. https://doi.org/10.1038/s41580-024-00757-5
Nixon RA, Saito KI, Grynspan F, Griffin WR, Katayama S, Honda T et al (1994) Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer’s disease. Ann N Y Acad Sci 747:77–91
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A et al (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64:113–122. https://doi.org/10.1093/jnen/64.2.113
Nixon RA, Yang DS, Lee JH (2008) Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy 4:590–599. https://doi.org/10.4161/auto.6259
Novikoff AB, Beaufay H, De Duve C (1956) Electron microscopy of lysosomerich fractions from rat liver. J Biophys Biochem Cytol 2:179–184
Oakes SA, Papa FR (2015) The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10:173–194. https://doi.org/10.1146/annurev-pathol-012513-104649
Ohmi K, Kudo LC, Ryazantsev S, Zhao HZ, Karsten SL, Neufeld EF (2009) Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc Natl Acad Sci U S A 106:8332–8337. https://doi.org/10.1073/pnas.0903223106
Ono K, Kim SO, Han J (2003) Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor alpha-induced cell death. Mol Cell Biol 23:665–676. https://doi.org/10.1128/mcb.23.2.665-676.2003
Ozcelik S, Sprenger F, Skachokova Z, Fraser G, Abramowski D, Clavaguera F et al (2016) Co-expression of truncated and full-length tau induces severe neurotoxicity. Mol Psychiatry 21:1790–1798. https://doi.org/10.1038/mp.2015.228
Padamsey Z, McGuinness L, Bardo SJ, Reinhart M, Tong R, Hedegaard A et al (2017) Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron 93:132–146. https://doi.org/10.1016/j.neuron.2016.11.013
Palmieri M, Impey S, Kang H, di Ronza A, Pelz C, Sardiello M et al (2011) Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet 20:3852–3866. https://doi.org/10.1093/hmg/ddr306
Papadopoulos C, Kravic B, Meyer H (2020) Repair or lysophagy: dealing with damaged lysosomes. J Mol Biol 432:231–239. https://doi.org/10.1016/j.jmb.2019.08.010
Papadopoulos C, Meyer H (2017) Detection and clearance of damaged lysosomes by the endo-lysosomal damage response and lysophagy. Curr Biol 27:R1330-r1341. https://doi.org/10.1016/j.cub.2017.11.012
Park H, Kang JH, Lee S (2020) Autophagy in neurodegenerative diseases: a hunter for aggregates. Int J Mol Sci. https://doi.org/10.3390/ijms21093369
Park SJ, Frake RA, Karabiyik C, Son SM, Siddiqi FH, Bento CF et al (2022) Vinexin contributes to autophagic decline in brain ageing across species. Cell Death Differ 29:1055–1070. https://doi.org/10.1038/s41418-021-00903-y
Park YH, Seo JH, Park JH, Lee HS, Kim KW (2017) Hsp70 acetylation prevents caspase-dependent/independent apoptosis and autophagic cell death in cancer cells. Int J Oncol 51:573–578. https://doi.org/10.3892/ijo.2017.4039
Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L et al (2011) Regulation of TFEB and V-ATPases by mTORC1. EMBO J 30:3242–3258. https://doi.org/10.1038/emboj.2011.257
Pensalfini A, Albay R 3rd, Rasool S, Wu JW, Hatami A, Arai H et al (2014) Intracellular amyloid and the neuronal origin of Alzheimer neuritic plaques. Neurobiol Dis 71:53–61. https://doi.org/10.1016/j.nbd.2014.07.011
Pera M, Montesinos J, Larrea D, Agrawal RR, Velasco KR, Stavrovskaya IG et al (2020) MAM and C99, key players in the pathogenesis of Alzheimer’s disease. Int Rev Neurobiol 154:235–278. https://doi.org/10.1016/bs.irn.2020.03.016
Perluigi M, Di Domenico F, Giorgi A, Schinina ME, Coccia R, Cini C et al (2010) Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J Neurosci Res 88:3498–3507. https://doi.org/10.1002/jnr.22500
Perluigi M, Domenico FD, Butterfield DA (2024) Oxidative damage in neurodegeneration: roles in the pathogenesis and progression of Alzheimer disease. Physiol Rev 104:103–197. https://doi.org/10.1152/physrev.00030.2022
Phadatare P, Debnath J (2023) Lysosomal lipid peroxidation mediates immunogenic cell death. J Clin Invest. https://doi.org/10.1172/jci169240
Plascencia-Villa G, Perry G (2023) Roles of oxidative stress in synaptic dysfunction and neuronal cell death in Alzheimer’s Disease. Antioxidants (Basel). https://doi.org/10.3390/antiox12081628
Pontrello CG, McWhirt JM, Glabe CG, Brewer GJ (2022) Age-related oxidative redox and metabolic changes precede intraneuronal Amyloid-β accumulation and plaque deposition in a transgenic Alzheimer’s Disease mouse model. J Alzheimers Dis 90:1501–1521. https://doi.org/10.3233/jad-220824
Pulina MV, Hopkins M, Haroutunian V, Greengard P, Bustos V (2020) C99 selectively accumulates in vulnerable neurons in Alzheimer’s disease. Alzheimers Dement 16:273–282. https://doi.org/10.1016/j.jalz.2019.09.002
Puri C, Gratian MJ, Rubinsztein DC (2023) Mammalian autophagosomes form from finger-like phagophores. Dev Cell. https://doi.org/10.1016/j.devcel.2023.08.016
Radulovic M, Schink KO, Wenzel EM, Nähse V, Bongiovanni A, Lafont F, et al. (2018) ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. Embo J https://doi.org/10.15252/embj.201899753
Rao MV, McBrayer MK, Campbell J, Kumar A, Hashim A, Sershen H et al (2014) Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J Neurosci 34:9222–9234. https://doi.org/10.1523/JNEUROSCI.1132-14.2014
Rao MV, Mohan PS, Peterhoff CM, Yang DS, Schmidt SD, Stavrides PH et al (2008) Marked calpastatin (CAST) depletion in Alzheimer’s disease accelerates cytoskeleton disruption and neurodegeneration: neuroprotection by CAST overexpression. J Neurosci 28:12241–12254. https://doi.org/10.1523/JNEUROSCI.4119-08.2008
Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC (2010) Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol 12:747–757. https://doi.org/10.1038/ncb2078
Reed TT, Pierce WM Jr, Turner DM, Markesbery WR, Allan Butterfield D (2009) Proteomic identification of nitrated brain proteins in early Alzheimer’s disease inferior parietal lobule. J Cell Mol Med 13:2019–2029. https://doi.org/10.1111/j.1582-4934.2008.00478.x
Rock KL, Kono H (2008) The inflammatory response to cell death. Annu Rev Pathol 3:99–126. https://doi.org/10.1146/annurev.pathmechdis.3.121806.151456
Rogers JT, Cahill CM (2023) Iron responsiveness to lysosomal disruption: a novel pathway to Alzheimer’s Disease. J Alzheimers Dis 96:41–45. https://doi.org/10.3233/jad-230953
Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H et al (2002) An iron-responsive element type II in the 5’-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem 277:45518–45528. https://doi.org/10.1074/jbc.M207435200
Ryazantsev S, Yu WH, Zhao HZ, Neufeld EF, Ohmi K (2007) Lysosomal accumulation of SCMAS (subunit c of mitochondrial ATP synthase) in neurons of the mouse model of mucopolysaccharidosis III B. Mol Genet Metab 90:393–401
Salvalaio M, D’Avanzo F, Rigon L, Zanetti A, D’Angelo M, Valle G et al (2017) Brain RNA-Seq profiling of the mucopolysaccharidosis type II mouse model. Int J Mol Sci. https://doi.org/10.3390/ijms18051072
Sargeant TJ, Lloyd-Lewis B, Resemann HK, Ramos-Montoya A, Skepper J, Watson CJ (2014) Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat Cell Biol 16:1057–1068. https://doi.org/10.1038/ncb3043
Schubert D, Chevion M (1995) The role of iron in beta amyloid toxicity. Biochem Biophys Res Commun 216:702–707. https://doi.org/10.1006/bbrc.1995.2678
Scotto Rosato A, Montefusco S, Soldati C, Di Paola S, Capuozzo A, Monfregola J et al (2019) TRPML1 links lysosomal calcium to autophagosome biogenesis through the activation of the CaMKKβ/VPS34 pathway. Nat Commun 10:5630. https://doi.org/10.1038/s41467-019-13572-w
Shacka JJ, Klocke BJ, Young C, Shibata M, Olney JW, Uchiyama Y et al (2007) Cathepsin D deficiency induces persistent neurodegeneration in the absence of Bax-dependent apoptosis. J Neurosci 27:2081–2090. https://doi.org/10.1523/jneurosci.5577-06.2007
Sharma D, Otto G, Warren EC, Beesley P, King JS, Williams RSB (2019) Gamma secretase orthologs are required for lysosomal activity and autophagic degradation in Dictyostelium discoideum, independent of PSEN (presenilin) proteolytic function. Autophagy 15:1407–1418. https://doi.org/10.1080/15548627.2019.1586245
Sheftel AD, Zhang AS, Brown C, Shirihai OS, Ponka P (2007) Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 110:125–132. https://doi.org/10.1182/blood-2007-01-068148
Skowyra ML, Schlesinger PH, Naismith TV, Hanson PI (2018) Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science. https://doi.org/10.1126/science.aar5078
Smith MA, Perry G (1995) Free radical damage, iron, and Alzheimer’s disease. J Neurol Sci 134(Suppl):92–94. https://doi.org/10.1016/0022-510x(95)00213-l
Spampanato C, Feeney E, Li L, Cardone M, Lim JA, Annunziata F et al (2013) Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med 5:691–706. https://doi.org/10.1002/emmm.201202176
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D et al (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE. https://doi.org/10.1371/journal.pone.0009979
Stavrides P, Goulbourne CN, Peddy J, Huo C, Rao M, Khetarpal V et al. (2024) mTOR inhibition in Q175 Huntington's disease model mice facilitates neuronal autophagy and mutant huntingtin clearance. bioRxiv: https://doi.org/10.1101/2024.05.29.596471
Suga K, Tomiyama T, Mori H, Akagawa K (2004) Syntaxin 5 interacts with presenilin holoproteins, but not with their N- or C-terminal fragments, and affects β-amyloid peptide production. Biochemical Journal 381:619–628. https://doi.org/10.1042/bj20040618
Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S et al (2008) Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron 60:247–257. https://doi.org/10.1016/j.neuron.2008.10.001
Sun J, Liu Y, Hao X, Lin W, Su W, Chiang E et al (2022) LAMTOR1 inhibition of TRPML1-dependent lysosomal calcium release regulates dendritic lysosome trafficking and hippocampal neuronal function. EMBO J. https://doi.org/10.15252/embj.2021108119
Takasugi N, Komai M, Kaneshiro N, Ikeda A, Kamikubo Y, Uehara T (2023) The pursuit of the “Inside” of the amyloid hypothesis-is C99 a promising therapeutic target for Alzheimer’s Disease? Cells. https://doi.org/10.3390/cells12030454
Tamayev R, Matsuda S, Arancio O, D’Adamio L (2012) beta- but not gamma-secretase proteolysis of APP causes synaptic and memory deficits in a mouse model of dementia. EMBO Mol Med 4:171–179. https://doi.org/10.1002/emmm.201100195
Tedeschi V, Secondo A (2022) Emerging role of lysosomal calcium store as a hub of neuroprotection. Neural Regen Res 17:1259–1260. https://doi.org/10.4103/1673-5374.327340
Tedeschi V, Sisalli MJ, Petrozziello T, Canzoniero LMT, Secondo A (2021) Lysosomal calcium is modulated by STIM1/TRPML1 interaction which participates to neuronal survival during ischemic preconditioning. Faseb J. https://doi.org/10.1096/fj.202001886R
Terron HM, Parikh SJ, Abdul-Hay SO, Sahara T, Kang D, Dickson DW et al (2024) Prominent tauopathy and intracellular β-amyloid accumulation triggered by genetic deletion of cathepsin D: implications for Alzheimer disease pathogenesis. Alzheimers Res Ther 16:70. https://doi.org/10.1186/s13195-024-01443-6
Thal DR, Gawor K, Moonen S (2024) Regulated cell death and its role in Alzheimer’s disease and amyotrophic lateral sclerosis. Acta Neuropathol 147:69. https://doi.org/10.1007/s00401-024-02722-0
Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM (2012) A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485:109–113. https://doi.org/10.1038/nature11083
Tian R, Abarientos A, Hong J, Hashemi SH, Yan R, Dräger N et al (2021) Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat Neurosci 24:1020–1034. https://doi.org/10.1038/s41593-021-00862-0
Tian Y, Bustos V, Flajolet M, Greengard P (2011) A small-molecule enhancer of autophagy decreases levels of A{beta} and APP-CTF via Atg5-dependent autophagy pathway. FASEB J 25:1934–1942. https://doi.org/10.1096/fj.10-175158
Trinchese F, Fa M, Liu S, Zhang H, Hidalgo A, Schmidt SD et al (2008) Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest 118:2796–2807. https://doi.org/10.1172/JCI34254
Tsering W, Prokop S (2024) Neuritic plaques: gateways to understanding Alzheimer’s Disease. Mol Neurobiol 61:2808–2821. https://doi.org/10.1007/s12035-023-03736-7
Tsong H, Holzbaur EL, Stavoe AK (2023) Aging differentially affects axonal autophagosome formation and maturation. Autophagy. https://doi.org/10.1080/15548627.2023.2236485
Vaillant-Beuchot L, Mary A, Pardossi-Piquard R, Bourgeois A, Lauritzen I, Eysert F et al (2021) Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer’s disease models and human brains. Acta Neuropathol 141:39–65. https://doi.org/10.1007/s00401-020-02234-7
Van Acker ZP, Perdok A, Hellemans R, North K, Vorsters I, Cappel C et al (2023) Phospholipase D3 degrades mitochondrial DNA to regulate nucleotide signaling and APP metabolism. Nat Commun 14:2847. https://doi.org/10.1038/s41467-023-38501-w
Vidoni C, Follo C, Savino M, Melone MA, Isidoro C (2016) The role of cathepsin d in the pathogenesis of human neurodegenerative disorders. Med Res Rev 36:845–870. https://doi.org/10.1002/med.21394
Villalpando Rodriguez GE, Torriglia A (2013) Calpain 1 induce lysosomal permeabilization by cleavage of lysosomal associated membrane protein 2. Biochim Biophys Acta 1833:2244–2253. https://doi.org/10.1016/j.bbamcr.2013.05.019
Vitale I, Pietrocola F, Guilbaud E, Aaronson SA, Abrams JM, Adam D et al (2023) Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ 30:1097–1154. https://doi.org/10.1038/s41418-023-01153-w
Wang Y, Floor E (1998) Hydrogen peroxide inhibits the vacuolar H+-ATPase in brain synaptic vesicles at micromolar concentrations. J Neurochem 70:646–652. https://doi.org/10.1046/j.1471-4159.1998.70020646.x
Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM et al (2009) Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet 18:4153–4170. https://doi.org/10.1093/hmg/ddp367
Weinlich R, Oberst A, Beere HM, Green DR (2017) Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18:127–136. https://doi.org/10.1038/nrm.2016.149
Wingo AP, Dammer EB, Breen MS, Logsdon BA, Duong DM, Troncosco JC et al (2019) Large-scale proteomic analysis of human brain identifies proteins associated with cognitive trajectory in advanced age. Nat Commun 10:1619. https://doi.org/10.1038/s41467-019-09613-z
Wirths O, Zampar S (2020) Neuron loss in Alzheimer’s disease: translation in transgenic mouse models. Int J Mol Sci. https://doi.org/10.3390/ijms21218144
Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA (2013) Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci 37:1949–1961. https://doi.org/10.1111/ejn.12169
Wu M, Chen Z, Jiang M, Bao B, Li D, Yin X et al (2023) Friend or foe: role of pathological tau in neuronal death. Mol Psychiatry 28:2215–2227. https://doi.org/10.1038/s41380-023-02024-z
Xie YX, Naseri NN, Fels J, Kharel P, Na Y, Lane D et al (2022) Lysosomal exocytosis releases pathogenic α-synuclein species from neurons in synucleinopathy models. Nat Commun 13:4918. https://doi.org/10.1038/s41467-022-32625-1
Yamashima T (2016) Can “calpain-cathepsin hypothesis” explain Alzheimer neuronal death? Ageing Res Rev 32:169–179. https://doi.org/10.1016/j.arr.2016.05.008
Yambire KF, Rostosky C, Watanabe T, Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A et al (2019) Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife. https://doi.org/10.7554/eLife.51031
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA et al (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6:49. https://doi.org/10.1038/s41392-020-00428-9
Yang DS, Kumar A, Stavrides P, Peterson J, Peterhoff CM, Pawlik M et al (2008) Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer’s disease. Am J Pathol 173:665–681. https://doi.org/10.2353/ajpath.2008.071176
Yang DS, Stavrides P, Kumar A, Jiang Y, Mohan PS, Ohno M et al (2017) Cyclodextrin has conflicting actions on autophagy flux in vivo in brains of normal and Alzheimer model mice. Hum Mol Genet 26:843–859. https://doi.org/10.1093/hmg/ddx001
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M et al (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain 134:258–277. https://doi.org/10.1093/brain/awq341
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M et al (2011) Therapeutic effects of remediating autophagy failure in a mouse model of Alzheimer disease by enhancing lysosomal proteolysis. Autophagy 7:788–789
Yuan P, Zhang M, Tong L, Morse TM, McDougal RA, Ding H et al (2022) PLD3 affects axonal spheroids and network defects in Alzheimer’s disease. Nature 612:328–337. https://doi.org/10.1038/s41586-022-05491-6
Zhang G, Zhang Y, Shen Y, Wang Y, Zhao M, Sun L (2021) The potential role of ferroptosis in Alzheimer’s Disease. J Alzheimers Dis 80:907–925. https://doi.org/10.3233/jad-201369
Zhang S, Eitan E, Mattson MP (2017) Early involvement of lysosome dysfunction in the degeneration of cerebral cortical neurons caused by the lipid peroxidation product 4-hydroxynonenal. J Neurochem 140:941–954. https://doi.org/10.1111/jnc.13957
Zhang Z, Song M, Liu X, Kang SS, Kwon IS, Duong DM et al (2014) Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat Med 20:1254–1262. https://doi.org/10.1038/nm.3700
Zhao D, Yang K, Guo H, Zeng J, Wang S, Xu H et al (2023) Mechanisms of ferroptosis in Alzheimer’s disease and therapeutic effects of natural plant products: a review. Biomed Pharm Biomede Pharm. https://doi.org/10.1016/j.biopha.2023.114312
Zhen Y, Radulovic M, Vietri M, Stenmark H (2021) Sealing holes in cellular membranes. Embo J. https://doi.org/10.15252/embj.2020106922
Zou J, Kawai T, Tsuchida T, Kozaki T, Tanaka H, Shin KS et al (2013) Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity 38:717–728. https://doi.org/10.1016/j.immuni.2012.12.007
Acknowledgements
Research in the Nixon laboratory has been supported by grants (most recently P01 AG017617; R01 AG062376) from the National Institute on Aging (NIH), New Vision Research Foundation (Leonard Litwin Scholar award), Cure Huntington’s Disease Initiative (CHDI) foundation, Takeda Corp, and Johnson & Johnson. The author gratefully acknowledges the expert assistance of Swati Jain in preparing the figures and manuscript for this publication. Additional assistance from Rosemarie LoFaro and the contributions of the author’s lab members over 4 decades to the published work on the subjects reviewed. The author also appreciates the research contributions made to the subjects reviewed that regretfully could not be cited due to space and reference limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nixon, R.A. Autophagy–lysosomal-associated neuronal death in neurodegenerative disease. Acta Neuropathol 148, 42 (2024). https://doi.org/10.1007/s00401-024-02799-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00401-024-02799-7