Abstract
α-synuclein (αSyn) is an intrinsically disordered protein which can undergo structural transformations, resulting in the formation of stable, insoluble fibrils. αSyn amyloid-type nucleation can be induced by misfolded ‘seeds’ serving as a conformational template, tantamount to the prion-like mechanism. Accumulation of αSyn inclusions is a key feature of dementia with Lewy bodies (DLB) and multiple system atrophy (MSA), and are found as additional pathology in Alzheimer’s disease (AD) such as AD with amygdala predominant Lewy bodies (AD/ALB). While these disorders accumulate the same pathological protein, they exhibit heterogeneity in clinical and histological features; however, the mechanism(s) underlying this variability remains elusive. Accruing data from human autopsy studies, animal inoculation modeling, and in vitro characterization experiments, have lent credence to the hypothesis that conformational polymorphism of the αSyn amyloid-type fibril structure results in distinct “strains” with categorical infectivity traits. Herein, we directly compare the seeding abilities and outcome of human brain lysates from these diseases, as well as recombinant preformed human αSyn fibrils by the intracerebral inoculation of transgenic mice overexpressing either human wild-type αSyn or human αSyn with the familial A53T mutation. Our study has revealed that the initiating inoculum heavily dictates the phenotypic and pathological course of disease. Interestingly, we have also established relevant host-dependent distinctions between propagation profiles, including burden and spread of inclusion pathology throughout the neuroaxis, as well as severity of neurological symptoms. These findings provide compelling evidence supporting the hypothesis that diverse prion-type conformers may explain the variability seen in synucleinopathies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyloids are classically defined as insoluble protein aggregates formed from long, unbranched strands of misfolded polypeptides, stacked into antiparallel, β-pleated sheets and characteristically display a cross-β X-ray diffraction pattern [15, 18, 22]. Highly organized hydrogen bonds, a key feature of amyloids, provide the fibril strands with immense stability, allowing them to remain and accumulate further within cells or tissues. This longevity facilitates amyloids to serve as nucleation sites, wherein, through a process termed conformational templating, proteins with the same, or highly homologous, primary amino acid sequences, are converted from their native, functional state into β-sheet amyloid structures [38]. However, polypeptides with identical primary amino acid sequences can result in phenotypically distinct variants, or polymorphs, depending on which side chains interdigitate to form the amyloid protofilament spine [44, 70, 82]. These polymers may have distinct aggregration kinetics, transmissibility and disease characteristics despite deriving from the same protein. The biophysical characteristics of amyloids are thought to be the molecular mechanism of prion-like strains, wherein the resultant protein aggregates may have unique, stable aggregation and biochemical properties that can be passaged or “transmitted”.
The accumulation of amyloidogenic α-synuclein (αSyn) inclusions is an established feature of many human diseases [10] and are especially insidious in neurodegenerative diseases, such as dementia with Lewy bodies (DLB), multiple system atrophy (MSA) [77], and Alzheimer’s Disease with amygdala predominant Lewy bodies (AD/ALB) [54, 74]. In these diseases, hyperphosphorylated, misfolded αSyn polymerizes into amyloids to form pathological inclusions [21, 79]. αSyn, a small, predominantly intrinsically disordered protein [81], composed of only 140 amino acids, is highly expressed in the brain, accounting for ~ 0.5% of all cystolic proteins in a neuron [37], and is primarily located at presynaptic terminals [37], where it assists in vesicle recycling and synaptic transmission. Although its native form is disordered, αSyn takes on an alpha-helix conformation when in contact with highly curved lipid membranes, such as vesicles, due to its apolipoprotein-like amino-terminus which consists of four imperfect KTKEGV repeat domains, and are critical for alpha-helix formation [23, 37]. Two additional imperfect KTKEGV repeat domains reside in a region referred to as nonamyloid component (NAC) [32], creating the hydrophobic core of αSyn that is essential for fibril assembly [25].
There is increasing evidence that pathological αSyn adopts prion-like conformational templating as a key mechanism of intercellular propagation associated with disease progression [28, 31, 76, 77]. Indeed, many studies have shown that, like prions [1, 18], exogenous pathological αSyn can induce conformational alterations in endogenous αSyn and promote integration into aggregates. This notion has been demonstrated in vitro [49, 67, 80], as well as in vivo, using intracerebral [35, 48, 64, 72] and peripheral [2, 8, 56, 66, 75] αSyn preformed fibrils (PFFs) injections, providing a possible molecular mechanism for the progressive nature of synucleinopathies. Here, we have used αSyn derived from diverse synucleinopathy-patient samples, as well as recombinant PFFs, to directly compare variances between αSyn proteoforms and elucidate the extent that PFF-induced pathology emulates that which is induced by disease-derived proteoforms. We have found that seeding studies in different αSyn transgenic mouse lines can induce αSyn pathology that mirrors key characteristics of prion strain biology with heterogeneity that exemplifies synucleinopathies. Furthermore, the resultant neurological and histological outcomes rely on both strain-derivation and host genotype.
Materials and methods
Autopsy case material
Human brain tissue was obtained through the University of Florida Neuromedicine Human Brain Tissue Bank in accordance with institutional review board approval. Post-mortem neuro-pathological staging and diagnoses were made according to the current guidelines and criteria proposed by the National Institute of Aging-Alzheimer’s Association, the Dementia with Lewy Bodies Consortium [36, 52], and the Neuropathology Working Group on MSA [27]. Frozen, unfixed samples of amygdala were obtained from three DLB cases, two AD/ALB, and one aging control. Additional frozen, unfixed samples of cerebellum were obtained from two MSA cases and one aging control. For all cases, formalin fixed sections from the contralateral amygdala or cerebellar hemisphere were also obtained for immunohistochemical characterization. A summary of all case demographics and pathologic characteristics is presented in (Table 1).
Mouse lines
All animal experimental procedures were performed in accordance to University of Florida Institutional Animal Care and Use Committee regulatory policies following approval. Mice were housed in a stable environment with a 12 h light/dark cycle and access to food and water ad libitum. In total, 88 animals were included in this study (Table 2). 45 mice were hemizygous M83 transgenic (Tg) mice, which overexpress human αSyn harboring the A53T mutation, driven by the mouse PrP promoter (MoPrP.Xho). These were generated by crossing homozygous M83 transgenic mice [24] with non-transgenic (nTg) C3H/BL6 (Charles River) mice. These mice abbreviated hereinafter as TgM83+/−, accumulate pathologic inclusions that can spread throughout most of the neuro-axis after 18 months of age [24]. The other 43 mice were transgenic line M20 mice, hemizygous for wild-type (WT) human αSyn, driven by the mouse PrP promoter (MoPrP.Xho) [24]; they were maintained on a C57BL/C3H background by mating with nTg C3H/BL6 (Charles River) mice. These mice abbreviated hereinafter as TgM20+/−, do not intrinsically develop pathology or a phenotype, but can be exogenously induced to form widespread αSyn pathology via intracerebral pre-formed αSyn fibril injection [24, 65].
Biochemical fractionation
Amygdalae from DLB, AD/ALB and control individuals or cerebellar white matter of MSA and control individuals were dissected from coronal brain sections (Table 1). For all cases, ~ 250 mg of these tissues were homogenized by mild probe sonication 3 mL/g tissue high-salt (HS) buffer (50 mM Tris–HCl, pH 7.5, 0.75 M NaCl, 2 mM EDTA, 50 mM NaF with a cocktail of protease inhibitors: 1 mM phenylmethylsulphonyl fluoride and 1 μg/ml each of pepstatin, leupeptin, N-tosyl-l-phenylalanyl chloromethyl ketone, N-tosyl-lysine chloromethyl ketone, and soybean trypsin inhibitor) and sedimented at 100,000 × g for 30 min at 4 °C. The HS supernatant was collected (HS fraction) and insoluble material was re-suspended in 3 mL/g tissue HS buffer/1% Triton X-100 with protease inhibitors. Re-suspended material was again sedimented at 100,000 × g for 30 min at 4 °C and the supernatant was collected (HS/T soluble fraction). Pellets were homogenized in 3 mL/g tissue HS buffer/1 M sucrose with protease inhibitors and sedimented at 100,000 × g for 30 min at 4 °C in order to remove myelin, which was discarded in the supernatant. The insoluble material was homogenized by probe sonication in ~ 150 µL PBS and stored as the Triton-insoluble fractions. The concentration of each fraction was determined using bicinchoninic acid assay (BCA) with bovine serum albumin (BSA) as the standard. SDS sample buffer (1% SDS, 10 mM Tris, pH 6.8, 1 mM EDTA, 40 mM DTT, 0.005% bromophenol blue, and 0.0025% pyronin yellow, 10% sucrose) was added to some portion of the sequential fractions that were further heated to 100 °C for 10 min for immunoblotting analysis. Samples were aliquoted and stored at − 80 °C until used.
Western blot analysis
For biochemical characterization of fractionated human brain tissue, 5 µg of lysate from either the HS soluble or Triton X-100 insoluble fractions were loaded onto 15% polyacrylamide gels and resolved by SDS-PAGE, followed by electrophoretic transfer onto 0.2 μm pore size nitrocellulose membranes (Bio-Rad, Hercules, CA) in carbonate transfer buffer (10 mM NaHCO3, 3 mM Na2CO3, pH 9.9, 20% methanol) [17]. Membranes were blocked in 5% dry milk/Tris buffered saline (TBS) and incubated overnight at 4 °C with anti-αSyn antibody 3H11 [14] diluted in block solution. After washing in TBS, membranes were incubated with goat anti-mouse secondary antibody conjugated to horseradish peroxidase (Jackson Immuno Research Labs, Westgrove, PA) diluted in 5% dry milk/TBS for 1 h at room temperature; immunocomplexes were detected using Western Lightning-Plus ECL reagents (PerkinElmer, Waltham, MA) followed by chemiluminescence imaging (PXi, Syngene, Frederick, MD).
Recombinant human αSyn expression, purification, and fibril formation
The pRK172 bacterial expression vector containing the cDNA encoding WT human αSyn was transformed into BL21 (DE3)/RIL Escherichia coli (E. coli; New England BioLabs Inc) to express recombinant αSyn; this was then purified using size exclusion chromatography, followed by anion exchange, as previously described [25, 29]. Protein concentrations were determined by BCA using BSA as the protein standard. To generate PFFs for injection, recombinant human αSyn protein [5 mg/ml in sterile phosphate buffered saline (PBS)] was incubated at 37 °C with constant shaking at 1050 RPM (Thermomixer R, Eppendorf) for > 48 h. Fibril formation was monitored by K114 [(trans, trans)-1-bromo-2,5-bis-(4-hydroxy) styrylbenzene] fluorometry as previously described [12]. Fibrils were diluted to 2 mg/ml in sterile PBS and sonicated in a water bath for two hours. Sonicated fibrils were then aliquoted, stored at − 80 °C and thawed when required. Each experiment in this study was performed using PFFs from the same preparation, to limit batch to batch variation.
Stereotaxic intra-hippocampal injections
For comparison of αSyn prion-like seeding properties between DLB, AD/ALB, MSA, control human tissue lysates and human αSyn preformed fibrils (PFFs), cohorts of 4–5 mice for each inoculum (Table 2) were stereotaxically injected, unilaterally, into the right hippocampus (coordinates from Bregma: anterior/posterior − 2.2 mm, lateral − 1.6 mm, dorsal/ventral − 1.2 mm) at ~ 2 months of age as previously described [63]. For injection, 2 µL of solution (sterile PBS) containing 4 µg of PFFs or 20 µg of insoluble fraction lysate, from each respective case, were used. At 6 months post-surgery, or upon onset of fatal motor symptoms, animals were sacrificed for histological analysis [65, 72].
Antibody creation
Antibody 3H19 is a new mouse monoclonal antibody that was generated, as previously described [33], by immunizing female BALB/c mice using a synthetic peptide, corresponding to amino acid residues 110–119 in human αSyn, conjugated to Imject maleimide-activated mariculture keyhole limpet hemocyanin (mcKLH; Thermo Scientific, Waltham, MA) (Supplementary Fig. 1, online resource).
Tissue processing and histopathological analysis
Mice were euthanized with CO2 and perfused with a heparin/PBS solution. For histopathological analysis, brains and spinal cords of mice were harvested and fixed in 70% EtOH/150 mM NaCl, paraffin embedded, and sectioned, as previously described [75]. Immunostaining of both mouse and human sections was performed using established methods [16]. Briefly, for mouse tissue sections, slides were deparaffinized with xylenes, then rehydrated in graded 100–70% ethanol steps, followed with heat-induced epitope retrieval (HIER) in a steam bath for 60 min in water with 0.05% Tween-20, unless otherwise indicated. After antigen retrieval, sections were washed in running deionized H2O for 15 min. Endogenous peroxidase was quenched by incubating sections in 1.5% hydrogen peroxide/0.005% Triton-X-100 diluted in PBS, pH 7.4 for 10 min. Sections were then rinsed in running deionized H2O for 15 min, washed three times for five minutes in 0.1 M Tris, pH 7.6, and then blocked in 2% fetal bovine serum (FBS)/0.1 M Tris, pH 7.6 solution for five minutes. Slides were incubated with primary antibodies diluted in blocking solution and stored overnight in 4 °C. Primary antibodies and dilution factors are listed in (Supplementary Table 1, online resource). After overnight incubation, primary antibody was removed from slides with a quick rinse with deionized H2O, then incubated with agitation for five minutes in 0.1 M Tris, pH 7.6, three times. Tissue sections were incubated for one hour with either goat anti-rabbit or anti-mouse biotinylated IgG (Vector Laboratories; Burlingame, CA) in 0.1 M Tris, pH 7.6/2% FBS (1:3000) at room temperature. Secondary antibody was rinsed three times with 0.1 M Tris, pH 7.6 for five minutes. Sections were then incubated with an avidin–biotin complex (ABC) solution (Vectastain ABC Elite kit; Vector Laboratories, Burlingame, CA) for one hour at room temperature, then rinsed again, three times, with 0.1 M Tris, pH 7.6, for five minutes. Sections were developed using chromogen 3,3′-diaminobenzidine (DAB kit; KPL, Gaithersburg, MD) and counterstained using hematoxylin (Sigma Aldrich, St. Louis, MO). Summary of antibodies used and corresponding retrieval methods are summarized in (Supplementary Table 1, online resource).
Semi-quantification and digital analysis of pathology
All IHC sections were digitally scanned using an Aperio ScanScope CS instrument (40 × magnification; Aperio Technologies Inc., Vista, CA, USA), and images of representative areas of pathology were captured using the ImageScope software (40 × magnification; Aperio Technologies Inc.). Tissue sections were analyzed using Aperio ImageScope. Regions of interest (ROIs) were selected and quantified separately using a modified version of ImageScope’s Color Deconvolution algorithm v9, tailored to each staining, and slides were scored based on the quantified optical density (OD) analysis of the immunoreactive area (IRA) for the DAB color channel. For analysis, scores were normalized by setting the highest score from either all cohorts or cohorts within specified analysis as the maximum value. Representative images were adjusted for white values; brightness/contrast corrections were applied identically on captured images within each figure using Adobe Photoshop CS3 (Adobe Systems, San Jose, CA, USA). All raw files and algorithms are available upon request.
Statistical analysis
Paralysis curves were generated in GraphPad Prism software, and both the Log-rank test and Gehan-Breslow-Wilcoxon were used to individually compare various cohorts to detect significant differences in disease onset after inoculation. For IHC analysis, data was tested for normality using D’Agostino-Pearson test. A Two-Way ANOVA was used to compare quantified IHC results of each inoculum individually, between each cohort, for each quantified antibody; Holm-Sidak test was used to correct for multiple comparisons and each P value was multiplicity adjusted. Family-wise significance was set at 0.05. Statistical analysis was performed using Prism software (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± SEM, and level of significance was set at p < 0.05.
Results
Generation and injection of human brain αSyn inoculums for comparative induction of pathology in transgenic mice expressing WT or A53T human αSyn
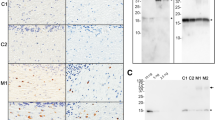
To assess the propensity of αSyn proteoforms to produce discrete disease phenotypic and neuropathological outcomes, we inoculated (stereotactic hippocampus injections) TgM83+/− and TgM20+/− mice, at 2 months of age, with brain extracts from the amygdala of control, DLB or AD/ALB brains and the cerebellum (white matter) of control or MSA brains (Fig. 1a). The ability of these human lysates to induce αSyn pathology was also compared to WT human αSyn PFFs (Fig. 1a). We designed these studies to do parallel injections in TgM83+/− mice, which express human αSyn with the PD-associated mutation, A53T, and are more primed to seeding that can result in a severe motor impairment [24], compared to TgM20+/− mice, which express WT human αSyn and do not intrinsically develop αSyn inclusions during their lifespan [19, 24]. These mice express similar levels of human αSyn but it is slightly higher in TgM20+/− mice. The brain tissues selected for DLB and AD/ALB were the amygdalae, which are rich in neuronal Lewy bodies and Lewy neurites, versus MSA cerebellar white matter, which predominantly contain abundant glial cytoplasmic inclusions as shown by IHC (Fig. 1c). The frozen brain tissues from the other hemisphere were biochemically fractionated and assessed by immunoblotting (Fig. 1b). αSyn was present in the HS fraction for all cases, although it is less abundant in cerebellar white matter. αSyn was also present in the Triton X-100 insoluble fractions from diseased brains, but in DLB and AD/ALB, compared to MSA, it appears to be highly modified or resistant to complete SDS-denaturation, as evidenced by the predominant altered migration as higher molecular weight species on the SDS–polyacrylamide gel (Fig. 1b). The insoluble fractions from cases DLB-1, DLB-3, AD/ALB-1, AD/ALB-2, MSA-1, MSA-2 and a control were used for inoculation of both TgM83+/− and TgM20+/− mice (Table 2).
Study design and characterization of brain tissues used for inoculations a Schematic of experimental design and types of brain inoculations in hemizygous TgM83+/− and TgM20+/− mice. b Immunoblots with anti-αSyn antibody 3H11 of high-salt (HS) soluble and Triton X-100 insoluble fractions derived from human brain homogenates with either DLB, AD/ALB, MSA or control amygdala or white matter cerebellum as described in Materials and Methods. The relative mobility of molecular mass markers is indicated on the left of the blots. c Representative images of αSyn immunostaining in tissue sections from the amygdala or cerebellum white matter from control, DLB, AD/ALB and MSA patients, (as indicated at the top right), with αSyn antibody, 3H11. Sections were counterstained with hematoxylin. Scale bar = 150 µm; insert = 10 µm. AD/ALB Alzheimer’s disease with amygdala predominant Lewy bodies, AMY amygdala, CB cerebellum, Ct control, DLB dementia with Lewy bodies, MSA multiple system atrophy, PFFs preformed fibrils
Transgenic mice expressing A53T human αSyn are more vulnerable to MSA and PFFs seeding induced paralysis than WT human αSyn transgenic mice
Following hippocampal inoculation with brain lysates or PFFs, induction of neurological illness was dependent on the transgenic model, as well as the derivation of the injected αSyn proteoform. Within the pre-established experimentally designed endpoint of 6 months post brain injection, none of the TgM20+/− mice developed any overt observable phenotypes, regardless of inoculation, although a few mice were found dead of unknown cause (see Table 2). By contrast, TgM83+/− mice injected with PFFs or MSA lysates portrayed a clinical phenotype of severe motor impairment and paralysis requiring euthanasia (Fig. 2a). TgM83+/− mice injected with control, DLB, or AD/ALB brain lysates did not present this phenotype by the study endpoint (Fig. 2a). Histological analysis of post-mortem tissue from the CNS of TgM83+/− and TgM20+/− mice revealed inclusions positive for αSyn phosphorylated at Ser129 (pSer129), a marker of pathological αSyn aggregation [21, 79] in mice injected with PFFs and MSA, DLB and AD/ALB lysates, but not in mice injected with the control lysates (Figs. 2b, 3). The induced inclusions presented p62 (sequestrome-1) accumulation (Figs. 2b, 3) which can be a marker of dysregulation in autophagic processing [40, 42]. Interestingly, histological analysis using 5G4, an antibody targeted for aggregation specific αSyn [41], showed abundant positive staining in TgM20+/−, but not TgM83+/− mice, despite extensive pSer129-positive αSyn pathology (Fig. 3, data not shown for TgM83+/−). Further investigation, using immunoblot analysis, revealed that 5G4 is selective for human WT αSyn, and does not readily react with mouse αSyn, which differs from human αSyn with the A53T amino acid substitution, and its immunoreaction is compromised by the H50Q, G51D, A53E and A53T mutations in human αSyn (Supplementary Fig. 2, online resource). This is likely due to the amino acid sequence of this antibody’s epitope, 46–53, EGVVHGVA, with alteration of the Ala53 disrupting antibody recognition. This suggests that the αSyn-positive inclusions identified in these mice are formed de novo, recruiting endogenous αSyn, and are not simply the result of uncleared injectate. Together, these findings demonstrate that αSyn proteoforms derived from DLB, AD/ALB, and MSA brains are capable of inducing αSyn pathology in both mouse models, but with MSA derived seeds and PFFs being the most prionogenic in inducing severe phenotypical dysfunction in the TgM83+/− mice.
Pathological outcome and histological pathology in TgM83+/− mice induced by inoculation a Kaplan–Meier paralysis curves of TgM83+/− mice inoculated with brain extract from the amygdala of control, DLB or AD/ALB brains or the cerebellum of control or MSA brains, or WT human αSyn PFFs. b Representative immunohistochemistry images of pathological αSyn deposition in TgM83+/− mice using antibodies specific for αSyn phosphorylated on Ser129 (81A), for αSyn (3H19), and p62/sequestrasome-1. Regions from the hippocampus are shown for DLB, AD/ALB and control amygdala lysates and PFF injected mice, while regions from the midbrain are depicted for MSA and control lysates injected mice to accurately represent relative pathology. Scale bars = 200 μm. AD/ALB Alzheimer’s disease with amygdala predominant Lewy bodies, AMY amygdala, CB cerebellum, DLB dementia with Lewy bodies, MSA multiple system atrophy, PFFs preformed fibrils
Characterization of histological αSyn pathology in TgM20+/− mice after inoculation Representative IHC images of pathological αSyn deposition in TgM20+/− mice using antibodies specific for αSyn phosphorylated on Ser129 (81A), for αSyn (3H19), p62/sequestrasome-1, and for aggregated αSyn (5G4). Regions from the hippocampus are shown. Scale bars = 200 μm. AD/ALB Alzheimer’s disease with amygdala predominant Lewy bodies, DLB dementia with Lewy bodies, MSA multiple system atrophy, PFFs preformed fibrils
Transmission pattern of αSyn pathology modulated by αSyn proteoform and αSyn transgene expressed by host
To investigate plausible molecular motives for the variation in neurological severity between mouse models and inoculums, the distribution and abundance of αSyn inclusion deposition throughout the CNS was investigated. Hippocampal inoculation with DLB or AD/ALB lysate produced αSyn pathology that was generally restricted to the region of injection, in both TgM83+/− and TgM20+/− mice (Figs. 4, 5; Supplementary Fig. 3, online resource). Analysis of bilateral distribution of pathology in TgM83+/− mice revealed that there was no significant difference between hemispheres ipsilateral or contralateral to the injection site (Supplementary Fig. 4a, online resource). In TgM20+/− mice, DLB injection corresponded with a significantly higher burden of αSyn inclusion pathology, which was measured in the hippocampus ipsilateral to injection (Supplementary Fig. 4b, online resource). Comparison of induced pathology between disease cases revealed a significant difference only in the hippocampus of TgM83+/− mice injected with AD/ALB, where case one resulted in more pathology (Supplementary Fig. 5a, online resource), and TgM20+/− mice injected with DLB, where case one induced more pathology (Supplementary Fig. 5b, online resource).
Elucidation of regional distribution of pathological αSyn spread in TgM83+/− mice after inoculation a Diagram depicting distribution of αSyn pathological deposition (red dots) using an antibody specific for αSyn phosphorylated at pSer129. Column on the right indicates the number of animals that did not exhibit any detectible pathology following inoculation (no pathology in controls) over total number of animals within the corresponding cohort. b Quantitative analysis of percentage of area positive for phosphorylated αSyn deposition, normalized to maximum measured pathology (data represents the mean ± SEM) within indicated brain regions from TgM83+/− mice following inoculation with brain lysates from amygdalae from control, DLB or AD/ALB brains or the cerebellum of control or MSA brains, or human αSyn PFFs. c Representative IHC images of the distinct pSer129 αSyn deposition illustrating the regional differences between cohorts. Scale bar = 25 μm. AD/ALB Alzheimer’s disease with amygdala predominant Lewy bodies, CTX cortex, DLB dementia with Lewy bodies; ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiple system atrophy, MY medulla, PFFs preformed fibrils, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus
Elucidation of regional distribution of pathological αSyn spread in TgM20+/− mice after inoculation a Diagram depicting distribution of αSyn deposition (red dots) using an antibody specific for αSyn phosphorylated at pSer129. Column on the right indicates the number of animals that did not exhibit any detectible pathology following inoculation (no pathology in controls) over total number of animals within the corresponding cohort. *10 mice were harvested at endpoint; however, one sample was lost during tissue processing. b Quantitative analysis of percentage of area positive for phosphorylated αSyn deposition, normalized to maximum measured pathology (data represents the mean ± SEM) within indicated brain regions from TgM20+/− mice following inoculation with brain lysates from amygdalae of control, DLB or AD/ALB brains or the cerebellum of control or MSA brains, or human αSyn PFFs. c Representative IHC images of the distinct pSyn deposition illustrating the regional differences between cohorts. Scale bar = 25 μm. AD/ALB Alzheimer’s disease with amygdala predominant Lewy bodies, CTX cortex, DLB dementia with Lewy bodies, ENTI entorhinal cortex; HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiple system atrophy, MY medulla, PFFs preformed fibrils, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus
The transmission pattern induced by MSA-inoculation varied dramatically between TgM83+/− and TgM20+/− mice. TgM83+/− mice exhibited robust pathology in the hypothalamus, periaqueductal gray, midbrain, medulla, anterior horn and ventral white matter of the spine, with limited pathology in cerebral regions, including those proximal to injection site (Fig. 4a–b; Supplementary Fig. 3a, online resource). Furthermore, pSer129 positive inclusions were generally intraneuronal, occurring both in the neuron body and axon and, very rarely, in the neuropil (Fig. 4c). Comparatively, TgM20+/− mice injected with MSA lysate exhibited pathology predominantly in the hippocampus, entorhinal, piriform and amygdalar cortex; moderate pathology occurred in the motor and somatosensory cortex, as well as the hypothalamus and midbrain, and infrequent pathology was detected in the anterior horn of the spine site (Fig. 5a–b; Supplementary Fig. 3b, online resource). pSer129 αSyn inclusions were typically intraneuronal (Fig. 5c), although some inclusions in astrocytes were observed in the cerebrum (see Supplementary Fig. 6, online resource). Neither cohort of mice injected with MSA lysate demonstrated any significant differences in αSyn inclusion pathology between hemispheres (Supplementary Fig. 4, online resource).
The αSyn pathological lesion profile of PFF injected mice was more similar between both mouse genotypes, with heavy deposition in the hippocampus, entorhinal, piriform, and amygdalar cortex, the hypothalamus and midbrain and moderate pathology in the motor and somatosensory cortex (Figs. 4, 5; Supplementary Fig. 3, online resource), and no significant difference in bilateral distribution of αSyn pathology (Supplementary Fig. 4, online resource). However, analysis of spinal pathology revealed that PFF injected TgM83+/− mice had greater levels of αSyn deposition than similarly inoculated TgM20+/− mice (Figs. 4, 5; Supplementary Fig. 7d, online resource). In addition, in TgM83+/− mice, both PFF and MSA injections resulted in a predilection for the lateral and anterior horn within the spinal cord, with heavy αSyn pathology near the motor nuclei (Fig. 4). Divergently, in TgM20+/− mice, PFF injection resulted in milder pathology in the spinal cord, but this pathology was equally distributed in both the dorsal and ventral horns (Fig. 5; Supplementary Fig. 7, online resource). These findings are consistent with the motor phenotypes, or lack thereof, seen between animals. Taken together, these data not only demonstrate that different αSyn proteoforms exhibit progressive regional tropism, but that this locality profile is extremely host-dependent. PFF and MSA injections may lead to robust αSyn pathology in both cohorts, but intense pathology was more regionally restricted to the cerebrum in TgM20+/− mice, likely resulting in the paucity of severe motor dysfunction, at least within the timeframe of these studies.
Host-dependent variations in astroglial activation to pathological αSyn deposition
Aggregated αSyn can be a potent activator of inflammation [65, 69] and changes in glial activity is hypothesized to be a critical contributor to disease heterogeneity in humans [7, 56, 57, 73]. αSyn PFF injection in mice transgenic for either WT αSyn or A53T human αSyn display significant astrogliosis and αSyn-positive inclusions that can be detected within astrocytes [65, 67]. Therefore, we investigated the levels of astrogliosis in the different experimental animal cohorts using antibodies targeted for glial fibrillary acidic protein (GFAP), a protein expressed by astrocytes that is upregulated upon activation [89]. First, we conducted histological examination on mice injected with control, DLB and AD/ALB lysates, as these cohorts exhibited little to no αSyn pathological deposition. Astrocytic activation was highest in regions immediate to injection site, regardless of inoculum type, and was lower in the cortex of all TgM83+/− mice (Fig. 6a–b). In cohorts inoculated with lysate derived from DLB, GFAP-immunoreactivity was higher in the hippocampus of TgM20+/− mice compared to TgM83+/− mice (Fig. 6; Supplementary Fig. 8, online resource). We then explored astroglial activity in mice laden with αSyn pathology. Here, in addition to our previous question about host-modulation of glial activity, we also wondered whether the location of glial active spots correlated with the location of αSyn pathology. In TgM83+/− and TgM20+/− mice, overall astrocytic activation was highest in PFF injected mice, compared to MSA and control injected mice (Fig. 7). Increased GFAP immunoreactivity was detected in the midbrain and medulla of MSA injected TgM83+/− mice compared to control injected, TgM83+/− mice (Fig. 7a; Supplementary Fig. 9a, online resource). PFF injected TgM83+/− mice exhibited higher GFAP-immunoreactivity in the hippocampus, piriform cortex, hypothalamus, midbrain, entorhinal cortex, and medulla, with moderately heavy positivity in regions of the dorsal cortex (Fig. 7a; Supplementary Fig. 9a, online resource). In TgM20+/− mice inoculated with MSA lysate, GFAP immunoreactivity was robustly detected in the hippocampus and entorhinal cortex, whereas PFF injected TgM20+/− mice demonstrated overwhelmingly robust astrocytic activation in all regions of the cerebrum and brainstem (Fig. 7b; Supplementary Fig. 9b, online resource). Generally, inoculation of TgM83+/− and TgM20+/− mice with either MSA lysates or PFFs, resulted in increased astrocytic activation in regions that corresponded with those high in pathological αSyn burden (Supplementary Fig. 10, online resource). Interestingly, almost all cohorts with αSyn inclusion pathology also exhibited some astrocytes which were positively labeled with pSer129, with MSA-injected TgM83+/− mice being the sole exception (Supplementary Fig. 6, online resource).
Investigation of brain astrocytic activation in Tg mice with low or no αSyn-positive inclusions Representative images showing anti-GFAP immuno-staining counterstained with hematoxylin from a TgM83+/− mice and b TgM20+/− mice inoculated with amygdala lysate from control, DLB or AD/ALB brains. Scatter dot plots compare percent of area with GFAP reactivity within transgenic mice between different inoculums (a–b), (two-way ANOVA followed by Tukey’s multiple comparisons test; data represents individual values and the mean ± SEM), within indicated brain regions. Scale bars = 500 μm. AD/ALB Alzheimer’s disease with amygdala predominant Lewy bodies, CTX cortex, DLB dementia with Lewy bodies, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MY medulla, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus
Characterization of astrocytic activation in Tg mice with extensive αSyn pathology Representative images showing anti-GFAP immuno-staining counterstained with hematoxylin from a TgM83+/− mice and b TgM20+/− mice inoculated with PFFs or brain lysate from the cerebellum of control or MSA brains. Scatter dot plots compare percent of area with GFAP reactivity within transgenic mice between different inoculums (a–b), (two-way ANOVA followed by Tukey’s multiple comparisons test; data represents the individual values and mean ± SEM), within indicated brain regions. Scale bars = 500 μm. CTX cortex, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiple system atrophy, MY medulla, PFFs preformed fibrils, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus
Diverse αSyn inclusion morphology in transgenic mice after inoculation
To further elucidate the consequences of αSyn proteoform and host on pathology, we examined the morphology of αSyn inclusions. We specifically aimed to clarify the extent to which the different hosts and/or seed-types were coupled with distinctive inclusion morphologies. To do this, we compared, between mouse genotypes, the inclusions located in or around the nucleus, in the cell body, or in the axons of neurons and glia to distinguish unique morphological phenotypes. Starting with canonical inclusion morphologies commonly used to histologically describe aggregates in other neurodegenerative disorders, such as Alzheimer’s disease, Huntington’s disease, Creutzfeldt-Jakob, and Amyotrophic Lateral Sclerosis, we categorized neuronal αSyn-positive inclusions into 18 unique, abnormal morphologies (Supplementary Fig. 11, online resource). The most characteristic morphological findings in DLB and AD/ALB-injected cohorts were intraneuronal cell body inclusions, predominantly as globose and LB-like inclusions, and rare ringed inclusions. Axonal pathology, in the form of corkscrew and beaded neurites, were occasionally seen as well (Supplementary Fig. 12, online resource). These cohorts also exhibited αSyn pathology in astrocytes as star-shaped inclusions (Supplementary Fig. 6, online resource). Contrarily, in animals injected with MSA or PFFs, pSer129-positive inclusions exhibited extensive morphological diversity (Figs. 4, 5, 8, 9). Categorization of regional inclusion morphology revealed an emergent, host-dependent, conformational profile (Figs. 8, 9). Specifically, morphological diversity increased in TgM83+/− MSA-injected mice, as distance from the injection site increased (Fig. 8). Comparatively, TgM20+/− mice show the opposite trend, with morphological heterogeneity higher in the cerebrum, specifically in the hippocampus, then following neuroanatomical pathways, with the lowest diversity in the cerebellum/spine (Fig. 8). Interestingly, PFF-injected mice of both cohorts had similar morphological profiles in the cerebrum but diverged caudally with TgM83+/− mice presenting more diversity than TgM20+/− mice in the brainstem region. TgM20+/− mice reduced morphological diversity in the brainstem (Fig. 9) and both cohorts dropped in the number of distinct morphologies in the cerebellum and spine (Fig. 9). This is despite the relative level of pathology remaining moderately high. Taken together, these findings demonstrate that host environment results in distinct protein interactomes, which affect pathological deposition and possibly the severity of disease.
Inoculation with MSA-derived αSyn results in distinct, host-dependent morphological profiles a Representative images of different morphologies from MSA-inoculated TgM83+/− and TgM20+/− mice, shows region-specific morphological profiles of αSyn pSer129 positive inclusions that are discernibly different between hosts. b Heat map showing the percentage of animals within each cohort that were found to have each morphological type, demonstrates that TgM83+/− mice show greater consistency than TgM20+/− mice. c Graph summarizing the fraction of total morphologies found within each cohort and region, shows that morphological variation diminishes in the caudal regions of TgM20+/− mice. Scale bar = 15 μm. BS brainstem, Cb cerebellum, CH cerebrum, Sp spine
Inoculation with PFFs results in manifold morphologies a Representative images of distinct morphologies from PFF-inoculated TgM83+/− and TgM20+/− mice, shows region-specific morphological profiles of pSer129 positive inclusions, that are discernibly different between hosts. b Heat map showing the percentage of animals within each cohort that were found to have each morphological type, demonstrates that TgM83+/− mice show greater consistency than TgM20+/− mice. c Graph summarizing the fraction of total morphologies found within each cohort and region, shows that morphological variation diminishes in the caudal regions of TgM20+/− mice. Scale bar = 15 μm. BS brainstem, Cb cerebellum, CH cerebrum, Sp spine
Discussion
The studies herein demonstrate that αSyn proteoforms derived from DLB, AD/ALB, MSA or PFFs, induce αSyn pathology in two independent transgenic mouse models. We have shown that the resultant neurological and histological features differ in severity and pathological burden, with MSA resulting in the most extreme in inoculated mice, compared to the other synucleinopathies. We have also shown that induction of disease is comparatively mitigated in mice expressing the WT form of human αSyn, as mice expressing the mutated A53T form of human αSyn were the only animals to develop paralysis. This finding is significant as it suggests that induction of neurological dysfunction in propagation studies can be drastically modified by subtle differences between the chosen models. TgM83+/− and TgM20+/− mice were created with a similar transgenic construct; both models use the mouse prion promoter to express human αSyn, but with TgM83+/− mice expressing αSyn with the A53T mutation [24]. The expression of human αSyn in the CNS of both of these mouse lines is comparable, with slightly higher expression in TgM20+/− mice [24]. While previous studies have examined the transmission of neurological disease from MSA brain extracts into TgM83+/− mice [61, 78, 87, 88], these studies either did not analyze comparative host-induced, strain specificity, or were unable to induce αSyn pathology in mouse lines expressing WT human αSyn. Our data suggests that the primary amino acid sequence of αSyn expressed by the host, plays a conspicuous role in the regional distribution, cellular tropism, and, importantly, severity of the neurological disease. The A53T αSyn mutation is more primed to aggregation [11, 26, 53], and therefore, may result in a more rapid spread to the brainstem and spinal cord regions, resulting in the paralysis phenotype. In addition, aggregates comprised of A53T αSyn may be more neurotoxic than those comprised of WT αSyn. These findings are crucial in furthering our ability to appropriately interpret data collected from seeding models, and highlight the importance of the transgenic mouse model utilized when studying synucleinopathies.
Synucleinopathies exhibit a great degree of complexity, differing by age of onset, presenting clinical features, αSyn inclusion morphology, regional and cellular distribution, and co-pathology of amyloidogenic proteins [34, 39, 43]. Distinct conformational strains of fibrillar αSyn and the prion-like, templated recruitment of monomeric αSyn, is an increasingly recognized mechanism to explain the heterogeneity of these diseases [3, 9, 18, 30, 35, 51, 55, 58, 62, 84]. We compared the induced distribution of αSyn pathology in TgM83+/− and TgM20+/− mice injected with DLB-αSyn, AD/ALB-αSyn, MSA-αSyn or PFFs, and found that pathological propagation was dependent on both host and inoculum. Our study demonstrated that αSyn-MSA is the most potent of the selected synucleinopathy-derived proteoforms, inducing widespread αSyn pathology. This finding is consistent with the kinetics of MSA-derived αSyn, illustrated in numerous seeding studies [6, 13, 59, 78, 83, 86,87,88]; αSyn-MSA characteristically exhibits aggressive rates of pathological propagation, a property reminiscent of the more rapid progression of disease in patients with MSA [20, 27]. Analysis of the regional distribution of induced αSyn pathology revealed that MSA-injection resulted in a dramatically diverse pathological distribution in TgM20+/− compared to TgM83+/− mice, with the latter exhibiting significant hindbrain and minimal hippocampal affliction (Figs. 4, 5; Supplementary Fig. 7c, online resource). This is interesting, as MSA seeding studies using TgM83+/− mice have consistently noted this hindbrain preference of MSA-induced pathology, regardless of inoculation route (intraglossal, intraperitoneal, intracerebral or intramuscular) or inoculation site [13, 45, 50, 60, 61, 68, 78, 85, 87], and have consequentially concluded that this regional tropism is determined by the MSA-derived αSyn strain. However, our results argue that regional selectivity is determined by an interaction between strain and substrate properties, as αSyn-MSA injection into TgM20+/- mice resulted in αSyn pathology in the hippocampus and ventral cortex regions, with the hindbrain and spinal cord less affected (Supplementary Fig. 7c, online resource). Conversely, PFF injection resulted in more similar regional distribution patterns in both mouse models, (Supplementary Fig. 7d, online resource), but with more spinal cord pathology in TgM83+/− (Figs. 4, 5). The hindbrain vulnerability of TgM83+/− mice has also been demonstrated in PFF seeding studies [2, 63, 75] and is similar to the strong preferential direction towards hindbrain pathology demonstrated by the TgM83+/− mice in our study.
Another characteristic of disease heterogeneity in synucleinopathies is the variance in cellular tropism. In DLB and AD/ALB, neurons, and to a lesser extent, astrocytes, are targets for αSyn inclusion deposition, while oligodendrocytes bear the brunt of the pathological burden in MSA. Oddly, inoculation of mice with MSA extract reliably results in predominant neuronal pathology, with some rare accumulations detected in oligodendrocytes [13, 45, 46, 50, 61, 68, 78, 85, 87]. The precise reason for this phenomenon is unknown, but recent analysis of MSA tissue, using a novel array of αSyn antibodies targeting carboxy-terminal epitopes, underscored the presence of substantial neuronal inclusions in the pontine nuclei and medullary inferior olivary nucleus [33]. Therefore, we hypothesized that these regions were a potential source of neuron-targeting αSyn seeds, providing a possible explanation for neuronal pathology described in MSA seeding studies. Therefore, in our studies, we used MSA-lysate extracted from the cerebellum, which are predominantly enriched in GCIs, but these homogenates still failed to recapitulate the hallmark glial pathology found in MSA, and predominantly targeted neurons, although rare, αSyn positivity in oligodendrocytes was detected in both models (Supplementary Fig. 13, online resource). Additionally, M20+/− mice exhibited αSyn positivity in astrocytes (Supplementary Fig. 6, online resource). Altogether, these data suggest that cellular tropism is not an obligatory transmissible feature of αSyn-MSA and that strain properties are not the primary predictors of cell type distribution, at least in these experimental studies.
Our studies indicate that the heterogeneity of synucleinopathies cannot be fully determined by disease-specific strains alone. For instance, histological examination of AD/ALB reveals regionally limited αSyn pathology, which we previously hypothesized could possibly be due to a preference for de novo αSyn aggregation in this disease, rather than nucleated polymerization [33]. Biochemical fractions from AD/ALB brains may still contain seeds that can experimentally induce αSyn pathology, but these may not have an intrinsic property to drive widespread αSyn pathology, as was shown in our study. Additionally, while lysates from DLB brains induce more pathology compared to AD/ALB lysates, the spread was not dramatically different. It is possible that the aggregated forms of αSyn that accumulates in the amygdala of both of these diseases are not conducive to transmission, such that even in DLB, amygdala-derived αSyn pathology does not play a major role in progression of pathology.
The operational definition of a prion strain is the resultant pathological profile when strain and host factors remain fixed [5]. Structurally encoded strain characteristics are a key feature of prions, and are categorized by incubation kinetics and subsequent neurological assault, regional distribution and cellular tropism under fixed host and strain parameters [4]. This fluctuation in pathogenic properties can be directly attributed to structural differences, which may be dictated by a single amino acid difference. For example, fibrils assembled from WT αSyn resulted in ‘rod’ polymorphs, with the protofilaments packing densely around the preNAC zipper, and, critically, residue Ala53 deeply incorporated into this zipper motif. However, introduction of a mutation at this residue can rearrange the protofilaments into a ‘twister’ polymorph, with protofilaments packing around the NACore zipper instead [30, 47]. In vitro analysis of self-assembled αSyn filaments demonstrated that PD-related mutations in αSyn, such as the amino acid mutations A53T and A30P, result in different polymorphs, which also differ from fibrils formed by WT αSyn [26]. These types of alterations can explain some of the differences observed here between M20 and M83 αSyn transgenic mice.
Furthermore, our results are consistent with the inoculums used in seeding studies containing an array of diverse prion-like strains, such that, even relatively well-defined inclusion types, like GCIs in MSA, contain an assortment of nucleation abilities, resulting in a diversity of induced αSyn inclusion pathology in the host. Cryo-EM analysis of WT and mutant, in vitro generated or human brain-derived αSyn fibrils, demonstrated morphological distinctions between αSyn proteoforms [30, 47, 71]. MSA-derived inclusions exhibited fibrils made of at least two types of filaments, (termed type I and type II), which themselves were made of two separate protofilaments, and these strains were distinguished from those derived from DLB brains [71]. Additionally, αSyn isolates derived from patients with MSA, have been shown to have lower conformational stability when compared to αSyn derived from the brains of patients with DLB and AD with concomitant deposition of αSyn [45]. These findings are consistent with previous, extensive immunohistological assessments of human brain specimens, suggesting that MSA-derived αSyn aggregates appear inherently unstable and readily mutable [14, 87], resulting in a more stochastic progression process with an array of pathologies observed here in our seeding studies.
Another key feature of prion strains is that their distinctive characteristics are conserved after transmission [4], as it directly templates onto the endogenous protein, conferring its specific conformational properties [4]. However, while biochemical and structural distinctions between different disease-derived αSyn aggregates have been abundantly characterized, it remains enigmatic whether these characteristics are stable enough to persist upon transmission to a new host. Currently, in vivo transmission studies of disease-derived αSyn species suggest that disease-specific αSyn strains are the determining factor, and major cause, of disease heterogeneity, but results from these studies are difficult to compare, due to the diversity of experimental design between research groups. Therefore, our study endeavored to clarify this causal relationship, using parallel models of αSyn transgenic expression. Since TgM20+/− mice do not inevitably develop αSyn pathology, induced inclusions can more confidently be hypothesized to be caused by the inoculating αSyn strain. This is in contrast with TgM83+/− mice that are already primed to develop αSyn pathology. As we have shown quite extensively in our study, the same inoculum may produce wildly different results in mouse models expressing different forms of αSyn, a result reminiscent of prion biology [5].
Our study has shown that both protein-specific nucleating elements, and host cellular environmental factors, likely play interactive roles in the resulting pathology, a phenomenon that is quite relevant in prion biology. Analogous to the evolution of prion strain diversity (reviewed in [5]), misfolded αSyn may exist as clouds of conformational proteoforms, with agnate, but not identical, structural and biophysical features. Theoretically, this variation may initiate different courses of disease. For example, biochemically unstable strains may quickly nucleate in certain new cellular environments, but, due to high reactivity, may be unable to form long stable fibrils. This might result in a rapidly progressing clinical presentation of disease, but biochemically, the misfolded protein may not polymerize in sufficiently long enough polymers that are biochemically insoluble. This hypothesis provides a consistent rationale to explain the curious results that we observed during this study, specifically, the dramatic differences in disease outcome seen between transgenic mice (inoculated with MSA or PFF). Figure 10, illustrates the strain diversity hypothesis as applied to the mice in our study. In theory, the nucleating inoculum contains αSyn folded into a multitude of unique structures, however, not all structures will successfully propagate into the host system. While strain-encoded characteristics are critical to the kinetics of amyloid formation, the strain hypothesis, as well as the findings in our study, suggests that host restricting factors, such as expression level, anatomical connectivity to area of nucleation and activity rate of cells, can contribute to the velocity of amyloid formation. In our study, MSA-lysate proved to be able to potently induce widespread pathology, however, depending on the host, it either resulted in severe, rapid-onset of paralysis, and more predominant hindbrain pathology, compared to more limbic pathology and no overt motor deficit. Host factors in TgM20+/− mice heavily influenced the presenting characteristics of disease, possibly through selective pressures for fibril conformations that happened to be less toxic (as they still exhibited abundant pathology). Alternatively, TgM20+/− host factors may have served to simply reduce the rate of pathological αSyn propagation, thereby only stalling the inevitable neurological disease. It would be interesting for future studies to reproduce this study with a lengthened designated endpoint, in order to observe whether MSA-inoculation of TgM20+/− mice could eventually lead to paralysis. This would help to clarify how the energy landscape for amyloid formation is shifted between these transgenic mice. Furthermore, since most synucleinopathies are sporadic, it would be highly impactful for a mouse model to exhibit neuropathological characteristics of Lewy body diseases without having to harbor disease-associated mutations that are rare in humans. Furthermore, future exploration should examine whether specific morphologies (or clusters of morphologies) correlate to enhanced pathogenicity, and whether certain morphological features garner more reactive immune attention than others.
Theoretical diagram outlining potential interactions between conformation proteoforms and αSyn transgenic mice after inoculation. Nucleating factors in inoculum may contain a limited allotment of conforms, represented by different colors and shapes, of which, only a subset would be able to transmit within the host environment. Intrinsic misfolding properties of the host protein relative to the interactions with the nucleating seeds are important factors in prion-like transmission capability and kinetics, but other factors, such as differences in cellular and regional expression, post-translational modifications, and altered glial activity (e.g. neuroinflammation), may guide the preferential amplification of specific αSyn polymorphs. Once formed, these structures may maintain characteristics that restrict pathogenicity, leading to the formation of strains. In this diagrammed example, ‘clouds’ of αSyn prion-like strains that are preferentially compatible with either the limbic or hindbrain regions in mouse (depicted in the boxes on the left) may undergo further selectivity dictated by mouse host permissivity. Host, region, and cell-specific mechanisms, such as protease expression and type, lysosomal activity, molecular chaperones or homology of amino acid sequence with host protein may be involved in determining the ultimate emergence of pathology. Color in brain indicates generalized level of pathology: red = high, orange. = moderate, yellow = low/none. Gray indicates this region was not quantified. Created with Biorender.com
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Aguzzi A, Rajendran L (2009) The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64:783–790
Ayers JI, Brooks MM, Rutherford NJ, Howard JK, Sorrentino ZA, Riffe CJ et al (2017) Robust central nervous system pathology in transgenic mice following peripheral injection of α-synuclein fibrils. J Virol 91:e02095-e2116. https://doi.org/10.1128/jvi.02095-16
Ayers JI, Lee J, Monteiro O, Woerman AL, Lazar AA, Condello C et al (2022) Different α-synuclein prion strains cause dementia with Lewy bodies and multiple system atrophy. Proc Natl Acad Sci USA 119:e2113489119. https://doi.org/10.1073/pnas.2113489119
Bartz JC (2016) Prion strain diversity. Cold Spring Harb Perspect Med 6:a024349. https://doi.org/10.1101/cshperspect.a024349
Bartz JC (2021) Environmental and host factors that contribute to prion strain evolution. Acta Neuropathol 142:5–16. https://doi.org/10.1007/s00401-021-02310-6
Bernis ME, Babila JT, Breid S, Wüsten KA, Wüllner U, Tamgüney G (2015) Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol Commun 3:75. https://doi.org/10.1186/s40478-015-0254-7
Braak H, Sastre M, Del Tredici K (2007) Development of α-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114:231–241. https://doi.org/10.1007/s00401-007-0244-3
Breid S, Bernis ME, Babila JT, Garza MC, Wille H, Tamgüney G (2016) Neuroinvasion of α-synuclein prionoids after intraperitoneal and intraglossal inoculation. J Virol 90:9182–9193. https://doi.org/10.1128/jvi.01399-16
Chen Y, Peng F, Su T, Yang H, Qiu F (2020) Direct identification of amyloid peptide fragments in human α-synuclein based on consecutive hydrophobic amino acids. ACS Omega 5:11677–11686. https://doi.org/10.1021/acsomega.0c00979
Chiti F, Dobson CM (2017) Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem 86:27–68. https://doi.org/10.1146/annurev-biochem-061516-045115
Conway KA, Harper JD, Lansbury PT (1998) Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med 4:1318–1320. https://doi.org/10.1038/3311
Crystal AS, Giasson BI, Crowe A, Kung MP, Zhuang ZP, Trojanowski JQ et al (2003) A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J Neurochem 86:1359–1368. https://doi.org/10.1046/j.1471-4159.2003.01949.x
Dhillon JKS, Trejo-Lopez JA, Riffe C, Levites Y, Sacino AN, Borchelt DR et al (2019) Comparative analyses of the in vivo induction and transmission of α-synuclein pathology in transgenic mice by MSA brain lysate and recombinant α-synuclein fibrils. Acta Neuropathol Commun 7:80. https://doi.org/10.1186/s40478-019-0733-3
Dhillon JKS, Trejo-Lopez JA, Riffe C, McFarland NR, Hiser WM, Giasson BI et al (2019) Dissecting α-synuclein inclusion pathology diversity in multiple system atrophy: implications for the prion-like transmission hypothesis. Lab Investig 99:982–992. https://doi.org/10.1038/s41374-019-0198-9
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890. https://doi.org/10.1038/nature02261
Duda JE, Giasson BI, Gur TL, Montine TJ, Robertson D, Biaggioni I et al (2000) Immunohistochemical and biochemical studies demonstrate a distinct profile of α-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol 59:830–841. https://doi.org/10.1093/jnen/59.9.830
Dunn SD (1986) Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on western blots by monoclonal antibodies. Anal Biochem 157:144–153. https://doi.org/10.1016/0003-2697(86)90207-1
Eisenberg DS, Sawaya MR (2017) Structural studies of amyloid proteins at the molecular level. Annu Rev Biochem 86:69–95. https://doi.org/10.1146/annurev-biochem-061516-045104
Emmer KL, Waxman EA, Covy JP, Giasson BI (2011) E46K human α-synuclein transgenic mice develop lewy-like and tau pathology associated with age-dependent, detrimental motor impairment. J Biol Chem 286:35104–35118. https://doi.org/10.1074/jbc.M111.247965
Erkkinen MG, Kim M-O, Geschwind MD (2018) Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol 10:a033118. https://doi.org/10.1101/cshperspect.a033118
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS et al (2002) Α-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. https://doi.org/10.1038/ncb748
Geddes AJ, Parker KD, Atkins EDT, Beighton E (1968) “Cross-β” conformation in proteins. J Mol Biol. https://doi.org/10.1016/0022-2836(68)90014-4
George JM, Jin H, Woods WS, Clayton DF (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15:361–372. https://doi.org/10.1016/0896-6273(95)90040-3
Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM-YMY (2002) Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34:521–533. https://doi.org/10.1016/s0896-6273(02)00682-7
Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY (2001) A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem 276:2380–2386. https://doi.org/10.1074/jbc.M008919200
Giasson BI, Uryu K, Trojanowski JQ, Lee VMY (1999) Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 274:7619–7622. https://doi.org/10.1074/jbc.274.12.7619
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676. https://doi.org/10.1212/01.wnl.0000324625.00404.15
Goedert M, Masuda-Suzukake M, Falcon B (2017) Like prions: The propagation of aggregated tau and α-synuclein in neurodegeneration. Brain 140:266–278. https://doi.org/10.1093/brain/aww230
Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW et al (2005) The E46K mutation in α-synuclein increases amyloid fibril formation. J Biol Chem 280:7800–7807. https://doi.org/10.1074/jbc.M411638200
Guerrero-Ferreira R, Taylor NMI, Mona D, Ringler P, Lauer ME, Riek R et al (2018) Cryo-EM structure of alpha-synuclein fibrils. Elife 7:36402. https://doi.org/10.7554/eLife.36402
Guo JL, Lee VMY (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20:130–138
Han H, Weinreb PH, Lansbury PT (1995) The core Alzheimer’s peptide NAC forms amyloid fibrils which seed and are seeded by β-amyloid: is NAC a common trigger or target in neurodegenerative disease? Chem Biol 2:163–169. https://doi.org/10.1016/1074-5521(95)90071-3
Hass EW, Sorrentino ZA, Lloyd GM, McFarland NR, Prokop S, Giasson BI (2021) Robust α-synuclein pathology in select brainstem neuronal populations is a potential instigator of multiple system atrophy. Acta Neuropathol Commun 9:80. https://doi.org/10.1186/s40478-021-01173-y
Hass EW, Sorrentino ZA, Xia Y, Lloyd GM, Trojanowski JQ, Prokop S et al (2021) Disease-, region- and cell type specific diversity of α-synuclein carboxy terminal truncations in synucleinopathies. Acta Neuropathol Commun 9:146. https://doi.org/10.1186/s40478-021-01242-2
Holec SAMM, Woerman AL (2020) Evidence of distinct α-synuclein strains underlying disease heterogeneity. Acta Neuropathol 142:73–86. https://doi.org/10.1007/s00401-020-02163-5
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Rohan Silva HA et al (1995) The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14:467–475. https://doi.org/10.1016/0896-6273(95)90302-X
Ke PC, Zhou R, Serpell LC, Riek R, Knowles TPJ, Lashuel HA et al (2020) Half a century of amyloids: past, present and future. Chem Soc Rev 49:5473–5509. https://doi.org/10.1039/c9cs00199a
Koga S, Sekiya H, Kondru N, Ross OA, Dickson DW (2021) Neuropathology and molecular diagnosis of synucleinopathies. Mol Neurodegener 16:83. https://doi.org/10.1186/s13024-021-00501-z
Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y et al (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12:213–223. https://doi.org/10.1038/ncb2021
Kovacs GG, Wagner U, Dumont B, Pikkarainen M, Osman AA, Streichenberger N et al (2012) An antibody with high reactivity for disease-associated α-synuclein reveals extensive brain pathology. Acta Neuropathol 124:37–50. https://doi.org/10.1007/s00401-012-0964-x
Kuusisto E, Parkkinen L, Alafuzoff I (2003) Morphogenesis of lewy bodies: dissimilar incorporation of α-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 62:1241–1253. https://doi.org/10.1093/jnen/62.12.1241
Laferrière F, Claverol S, Bezard E, De Giorgi F, Ichas F (2022) Similar neuronal imprint and no cross-seeded fibrils in α-synuclein aggregates from MSA and Parkinson’s disease. npj Park Dis 8:10. https://doi.org/10.1038/s41531-021-00264-w
Landreh M, Sawaya MR, Hipp MS, Eisenberg DS, Wüthrich K, Hartl FU (2016) The formation, function and regulation of amyloids: insights from structural biology. J Intern Med 280:164–176. https://doi.org/10.1111/joim.12500
Lau A, So RWL, Lau HHC, Sang JC, Ruiz-Riquelme A, Fleck SC et al (2020) α-synuclein strains target distinct brain regions and cell types. Nat Neurosci 23:21–31. https://doi.org/10.1038/s41593-019-0541-x
Lavenir I, Passarella D, Masuda-Suzukake M, Curry A, Holton JL, Ghetti B et al (2019) Silver staining (campbell-switzer) of neuronal α-synuclein assemblies induced by multiple system atrophy and Parkinson’s disease brain extracts in transgenic mice. Acta Neuropathol Commun 7:148. https://doi.org/10.1186/s40478-019-0804-5
Li Y, Zhao C, Luo F, Liu Z, Gui X, Luo Z et al (2018) Amyloid fibril structure of α-synuclein determined by cryo-electron microscopy. Cell Res 28:897–903. https://doi.org/10.1038/s41422-018-0075-x
Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY (2012) Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med 209:975–988. https://doi.org/10.1084/jem.20112457
Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR et al (2009) Exogenous α-synuclein fibrils seed the formation of lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA 106:20051–20056. https://doi.org/10.1073/pnas.0908005106
Macdonald JA, Chen JL, Masuda-Suzukake M, Schweighauser M, Jaunmuktane Z, Warner T et al (2021) Assembly of α-synuclein and neurodegeneration in the central nervous system of heterozygous M83 mice following the peripheral administration of α-synuclein seeds. Acta Neuropathol Commun 9:189. https://doi.org/10.1186/s40478-021-01291-7
Melki R (2015) Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J Parkinsons Dis 5:217–227. https://doi.org/10.3233/JPD-150543
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2012) National institute on aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. https://doi.org/10.1007/s00401-011-0910-3
Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D et al (1999) Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J Biol Chem 274:9843–9846. https://doi.org/10.1074/jbc.274.14.9843
Nelson PT, Abner EL, Patel E, Anderson S, Wilcock DM, Kryscio RJ et al (2018) The amygdala as a locus of pathologic misfolding in neurodegenerative diseases. J Neuropathol Exp Neurol 77:2–20. https://doi.org/10.1093/jnen/nlx099
Peelaerts W, Bousset L, Baekelandt V, Melki R (2018) α-synuclein strains and seeding in Parkinson’s disease, incidental lewy body disease, dementia with lewy bodies and multiple system atrophy: similarities and differences. Cell Tissue Res 373:195–212. https://doi.org/10.1007/s00441-018-2839-5
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M et al (2015) α-synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–344. https://doi.org/10.1038/nature14547
Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL et al (2018) Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557:558–563. https://doi.org/10.1038/s41586-018-0104-4
Peng C, Gathagan RJ, Lee VMY (2018) Distinct α-synuclein strains and implications for heterogeneity among α-synucleinopathies. Neurobiol Dis(PtB) 109:209–218
Van der Perren A, Gelders G, Fenyi A, Bousset L, Brito F, Peelaerts W et al (2020) The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with lewy bodies. Acta Neuropathol 139:977–1000. https://doi.org/10.1007/s00401-020-02157-3
Polinski NK (2021) A summary of phenotypes observed in the in vivo rodent alpha-synuclein preformed fibril model. J Parkinsons Dis 11:1555–1567. https://doi.org/10.3233/jpd-212847
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB et al (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci USA 112:E5308–E5317. https://doi.org/10.1073/pnas.1514475112
Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E (2014) Alpha-synuclein transfers from neurons to oligodendrocytes. Glia 62:387–398. https://doi.org/10.1002/glia.22611
Rutherford NJ, Dhillon JKS, Riffe CJ, Howard JK, Brooks M, Giasson BI (2017) Comparison of the in vivo induction and transmission of α-synuclein pathology by mutant α-synuclein fibril seeds in transgenic mice. Hum Mol Genet 26:4906–4915. https://doi.org/10.1093/hmg/ddx371
Sacino AN, Brooks M, McGarvey NH, McKinney AB, Thomas MA, Levites Y et al (2014) Induction of CNS α-synuclein pathology by fibrillar and non-amyloidogenic recombinant α-synuclein. Acta Neuropathol Commun 2:38. https://doi.org/10.1186/2051-5960-1-38
Sacino AN, Brooks M, Shaw G, Golde TE, Giasson BI, McKinney AB et al (2014) Brain injection of α-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J Neurosci 34:12368–12378. https://doi.org/10.1523/JNEUROSCI.2102-14.2014
Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW et al (2014) Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci 111:10732–10737. https://doi.org/10.1073/pnas.1321785111
Sacino AN, Thomas MA, Ceballos-Diaz C, Cruz PE, Rosario AM, Lewis J et al (2013) Conformational templating of α-synuclein aggregates in neuronal-glial cultures. Mol Neurodegener 8:17. https://doi.org/10.1186/1750-1326-8-17
Sargent D, Verchère J, Lazizzera C, Gaillard D, Lakhdar L, Streichenberger N et al (2017) ‘Prion-like’ propagation of the synucleinopathy of M83 transgenic mice depends on the mouse genotype and type of inoculum. J Neurochem 143:126–135. https://doi.org/10.1111/jnc.14139
Sarkar S, Dammer EB, Malovic E, Olsen AL, Raza SA, Gao T et al (2020) Molecular signatures of neuroinflammation induced by αsynuclein aggregates in microglial cells. Front Immunol 11:33. https://doi.org/10.3389/fimmu.2020.00033
Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI et al (2007) Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 447:453–457. https://doi.org/10.1038/nature05695
Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B et al (2020) Structures of α-synuclein filaments from multiple system atrophy. Nature 585:464–469. https://doi.org/10.1038/s41586-020-2317-6
Sorrentino ZA, Brooks MMT, Hudson V, Rutherford NJ, Golde TE, Giasson BI et al (2017) Intrastriatal injection of α-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity. Mol Neurodegener 12:40. https://doi.org/10.1186/s13024-017-0182-z
Sorrentino ZA, Giasson BI, Chakrabarty P (2019) α-Synuclein and astrocytes: tracing the pathways from homeostasis to neurodegeneration in Lewy body disease. Acta Neuropathol 138:1–21. https://doi.org/10.1007/s00401-019-01977-2
Sorrentino ZA, Goodwin MS, Riffe CJ, Dhillon J-KS, Xia Y, Gorion K-M et al (2019) Unique α-synuclein pathology within the amygdala in Lewy body dementia: implications for disease initiation and progression. Acta Neuropathol Commun 7:142. https://doi.org/10.1186/s40478-019-0787-2
Sorrentino ZA, Xia Y, Funk C, Riffe CJ, Rutherford NJ, Ceballos Diaz C et al (2018) Motor neuron loss and neuroinflammation in a model of α-synuclein-induced neurodegeneration. Neurobiol Dis 120:98–106. https://doi.org/10.1016/j.nbd.2018.09.005
Spillantini MG, Goedert M (2018) Neurodegeneration and the ordered assembly of α-synuclein. Cell Tissue Res 373:137–148. https://doi.org/10.1007/s00441-017-2706-9
Uchihara T, Giasson BI (2016) Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131:49–73. https://doi.org/10.1007/s00401-015-1485-1
Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM et al (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA 110:19555–19560. https://doi.org/10.1073/pnas.1318268110
Waxman EA, Giasson BI (2008) Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol 67:402–416. https://doi.org/10.1097/NEN.0b013e31816fc995
Waxman EA, Giasson BI (2010) A novel, high-efficiency cellular model of fibrillar α-synuclein inclusions and the examination of mutations that inhibit amyloid formation. J Neurochem 113:374–388. https://doi.org/10.1111/j.1471-4159.2010.06592.x
Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT (1996) NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 35:13709–13715. https://doi.org/10.1021/bi961799n
Wiltzius JJW, Landau M, Nelson R, Sawaya MR, Apostol MI, Goldschmidt L et al (2009) Molecular mechanisms for protein-encoded inheritance. Nat Struct Mol Biol 16:973–978. https://doi.org/10.1038/nsmb.1643
Woerman AL (2017) The importance of developing strain-specific models of neurodegenerative disease. Acta Neuropathol 134:809–812. https://doi.org/10.1007/s00401-017-1761-3
Woerman AL (2021) Strain diversity in neurodegenerative disease: an argument for a personalized medicine approach to diagnosis and treatment. Acta Neuropathol 142:1–3. https://doi.org/10.1007/s00401-021-02311-5
Woerman AL, Kazmi SA, Patel S, Freyman Y, Oehler A, Aoyagi A et al (2018) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol 135:49–63. https://doi.org/10.1007/s00401-017-1762-2
Woerman AL, Oehler A, Kazmi SA, Lee J, Halliday GM, Middleton LT et al (2019) Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg (SNCA*A53T) mouse lines. Acta Neuropathol 137:437–454. https://doi.org/10.1007/s00401-019-01959-4
Woerman AL, Patel S, Kazmi SA, Oehler A, Lee J, Mordes DA et al (2020) Kinetics of α-synuclein prions preceding neuropathological inclusions in multiple system atrophy. PLoS Pathog 16:e1008222. https://doi.org/10.1371/journal.ppat.1008222
Woerman AL, Stöhr J, Aoyagi A, Rampersaud R, Krejciova Z, Watts JC et al (2015) Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci USA 112:E4949–E4958. https://doi.org/10.1073/pnas.1513426112
Xu K, Malouf AT, Messing A, Silver J (1999) Glial fibrillary acidic protein is necessary for mature astrocytes to react to β-amyloid. Glia 25:390–403. https://doi.org/10.1002/(SICI)1098-1136(19990215)25:4%3c390::AID-GLIA8%3e3.0.CO;2-7
Acknowledgements
This work was supported by grants from the National Institute on Aging (P50AG047266; P30AG066506) and the National Institute of Neurological Disorders and Stroke (R01NS089022, R01NS100876) and (T32 NS082168)(GML). We would like to thank the patients and their caregivers for their contributions to our study.
Author information
Authors and Affiliations
Contributions
GML, ZAS, BIG conceived and designed the experiments; GML, ZAS, SQ, BIG, KG, BB, GP, BL performed the experiments; GML, BIG, SQ, SP analyzed the data; GML and BIG wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest in this manuscript.
Ethical approval
All procedures were performed according to the National Institute of Health Guide for the Care and Use of Experimental Animals and were approved by the University of Florida Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
401_2022_2425_MOESM1_ESM.pdf
Supplementary file1 Supplementary fig. 1 Western blot analysis to refine the epitope map and characterize the specificity of the new αSyn antibody, 3H19 Immunoblot analysis using recombinant human γSyn, human βSyn, mouse (m) αSyn, human (h) αSyn and a series of carboxy-truncated human αSyn (1-119, 1-115 and 1-102) proteins. 100 ng of each protein was loaded per lane and membranes were probed with the antibodies labeled above. Antibody 3H11 was also used as a control. The relative mobility of molecular mass markers is indicated on the left of each blot. Supplementary fig. 2 Western blot analysis of αSyn species excluded by the aggregation-specific antibody, 5G4 Immunoblot analysis using recombinant human (h) αSyn and mouse (m) αSyn and a series of αSyn mutants (A30P, E46K, H50Q, G51D, A53E, A53T) proteins. 150 ng of each protein was loaded per lane and membranes were probed with the antibody specific for aggregated αSyn, 5G4, labeled above. Antibody 94-3A10 was also used as a control. The relative mobility of molecular mass markers is indicated on the left of each blot. Supplementary fig. 3 Variability of pSer129 αSyn load and regional spread between a TgM83+/− mice and b TgM20+/− mice after inoculum. Values were normalized within group for comparison; plots deliberately emphasize variability within each cohort. AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, AMY amygdala, CB cerebellum, CTX cortex, DLB dementia with Lewy bodies, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiple system atrophy, MY medulla; PFFs preformed fibrils, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus. Supplementary fig. 4 Comparison between bilateral distribution of αSyn pathology in a TgM83+/− mice and b TgM20+/- mice inoculated with PFFs or lysates from DLB, AD/ALB and MSA brains. (Two-way ANOVA followed by Tukey’s multiple comparisons test; data represents the mean ± SEM). AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, CTX cortex, DLB dementia with Lewy bodies, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiple system atrophy, MY medulla, PFFs preformed fibrils, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus. (c indicates region measured is contralateral to injection site; i indicates region measured is ipsilateral to injection site). Supplementary fig. 5 Comparison of induced pathological load between disease case lysates in a TgM83+/− mice and b TgM20+/− mice. (Two-way ANOVA followed by Šídák's multiple comparisons test; data represents the mean ± SEM). AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, CTX cortex, DLB dementia with Lewy bodies, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiple system atrophy, MY medulla, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus. Supplementary fig. 6 Astroglial aversion in TgM83+/− mice. Representative IHC images of pSer129 positive astrocytic inclusions from the hippocampus and midbrain of TgM83+/− and TgM20+/− mice following inoculation with either brain-derived lysate or PFFs. Scale bar = 15 μm. AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, DLB dementia with Lewy bodies, MSA multiple system atrophy, PFFs preformed fibrils. Supplementary fig. 7 Comparison of pathological αSyn pattern of regional spread between TgM83+/− and TgM20+/− mice after inoculation with a DLB, b AD/ALB, or c MSA lysates and d PFFs. Values were normalized within group for comparison. (Two-way ANOVA followed by Šídák's multiple comparisons test; data represents the mean ± SEM). AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, CTX cortex, DLB dementia with Lewy bodies, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus; MB midbrain, MSA multiple system atrophy, MY medulla, PFFs preformed fibrils, PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus. Supplementary fig. 8 Representative images of astrocytic activation in DLB and AD/ALB injected mice High magnification images comparing levels of astrocytic activation in the hippocampus between a TgM83+/− mice and b TgM20+/− mice injected with amygdala lysate from a control, DLB and AD/ALB brain. analyzed using a modified Color Deconvolution Algorithm to highlight relative levels of astrocytic activation. Red/orange colors indicate GFAP positivity. Blue indicates nuclei. AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, DLB dementia with Lewy bodies. Scale bar = 200 μm Supplementary fig. 9 Representative images of astrocytic activation in MSA and PFF injected mice High magnification images comparing levels of astrocytic activation between regions in a TgM83+/− mice and b TgM20+/− mice injected with PFFs and cerebellum lysate from a control and MSA brain. Images were analyzed using a modified Color Deconvolution Algorithm to highlight relative levels of astrocytic activation. Red/orange colors indicate GFAP positivity. Blue indicates nuclei. CB cerebellum, CTX cortex, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiple system atrophy, PFFs preformed fibrils, PIR piriform cortex. Scale bar = 200 μm. Supplementary fig. 10 Analysis of the correlation between αSyn pathology and astrocytic activation in transgenic mice Scatterplots and Pearson correlation coefficients of percent positivity of 81A versus GFAP for corresponding regions plotted for a TgM83+/− mice and b TgM20+/− mice. Simple linear regressions were performed for each correlation plot. The line of best fit is represented by the black lines, with the shaded region signifying the 95% confidence intervals. R2 values (indicate goodness of fit) and p values (two-sided) are labeled on corresponding plots. AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, AMY amygdala, CTX cortex, DLB dementia with Lewy bodies, ENTI entorhinal cortex, HPF hippocampal formation, HY hypothalamus, MB midbrain, MSA multiplesystem atrophy, MY medulla, PFFs preformed fibrils; PAG periaqueductal gray, PIR piriform cortex, SUBv ventral subiculum, TH thalamus. Supplementary fig. 11 Visual dictionary of αSyn inclusion morphologies Scale bar = 15 μm. Supplementary fig. 12 Limited induction of αSyn pathology corresponds with limited inclusion morphology types Representative images of different morphologies from TgM83+/− and TgM20+/− mice inoculated with a DLB-lysate and b AD/ALB lysate shows restricted morphological distinctions, although DLB-inoculated TgM20+/− mice developed 8 different morphology types affecting the nucleus, cell body and processes. See supplementary fig. 11 for visual descriptions of morphologies. AD/ALB alzheimer’s disease with amygdala predominant Lewy bodies, DLB dementia with Lewy bodies. Scale bar = 15 μm. Supplementary fig. 13 Representative images of pSer129 positive oligodendrocyte inclusions from a TgM83+/− mice and b TgM20+/− mice injected with MSA and PFFs. Scale bar = 50 μm. MSA multiple system atrophy, PFFs preformed fibrils. (PDF 28830 KB)
Rights and permissions
About this article
Cite this article
Lloyd, G.M., Sorrentino, Z.A., Quintin, S. et al. Unique seeding profiles and prion-like propagation of synucleinopathies are highly dependent on the host in human α-synuclein transgenic mice. Acta Neuropathol 143, 663–685 (2022). https://doi.org/10.1007/s00401-022-02425-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02425-4