Abstract
A number of recent studies have described cases with tau-positive globular oligodendroglial inclusions (GOIs) and such cases have overlapping pathological features with progressive supranuclear palsy (PSP), but present with clinical features of motor neuron disease (MND) and/or frontotemporal dementia (FTD). These two clinical phenotypes have been published independently and as a result, have come to be considered as distinct disease entities. We describe the clinicopathological and biochemical features of two cases with GOIs: one with clinical symptoms suggestive of MND and the other with FTD. Histological changes in our two cases were consistent with their clinical symptoms; the MND case had severe neurodegeneration in the primary motor cortex and corticospinal tract, whereas the FTD case had severe involvement of the frontotemporal cortices and associated white matter. Immunohistochemistry in both cases revealed significant 4-repeat (4R) tau pathology primarily in the form of GOIs, but also in astrocytes and neurons. Astrocytic tau pathology was morphologically similar to that seen in PSP, but in contrast was consistently negative for Gallyas silver staining. Tau-specific western blotting revealed 68, 64 and 35 kDa bands, showing further overlap with PSP. The underlying neuropathological features of these two cases were similar, with the major difference relating to the regional distribution of pathology and resulting clinical symptoms and signs. The globular nature of glial inclusions and the non-fibrillar properties of tau in astrocytes are characteristic features that allow them to be distinguished from PSP and other tauopathies. We, therefore, propose the term globular glial tauopathy as an encompassing term to classify this emerging class of 4R tauopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From a historical perspective, neurodegenerative diseases have often been classified according to their clinical features. Advances in neuropathology have not only made the diagnosis of neurodegenerative diseases more precise, but have also further emphasised the frequency of clinical heterogeneity.
Progressive supranuclear palsy (PSP) is a neurodegenerative disease that is characterised neuropathologically by the abnormal accumulation of a microtubule-associated protein called tau [16]. The MAPT gene that encodes tau is alternatively spliced, giving rise to six protein isoforms that can have either three or four repeat regions in their microtubule-binding domains, termed 3-repeat (3R) or 4-repeat (4R) tau isoforms [8]. In PSP, 4R isoforms of tau are preferentially deposited and can, therefore, be distinguished from tauopathies that accumulate mainly 3R tau, such as Pick’s disease (PiD) [2]. In PSP, the abnormal 4R tau accumulates in neurons, oligodendrocytes and astrocytes in the form of neurofibrillary tangles (NFT) [33], coiled bodies [17] and tufted astrocytes [15]. These characteristic morphological signatures, in particular, tufted astrocytes allow PSP to be distinguished from another sporadic 4R tauopathy called corticobasal degeneration (CBD) [6]. The same cell types are affected in CBD, but the astrocytic tau lesions have a different distinct morphology termed astrocytic plaque [9]. Both tufted astrocytes and astrocytic plaques are readily positive for Gallyas silver staining indicating the presence of tau filaments [12, 35]. In addition to the contrasting clinical picture in the classical presentations of PSP and CBD, differences are also present in the regional distribution of pathology [5] and the biochemical properties of tau cleavage products when analysed by western blotting [38]. Even within the diagnostic boundaries of PSP (and CBD), there is considerable heterogeneity that has been unravelled, leading to the concept that within the PSP disease spectrum distinct clinicopathological variants are recognisable [7, 37].
A number of recent reports have described another collection of 4R tauopathies characterised by “globular” oligodendroglial inclusions (GOIs) [4, 11, 18, 20] that present with the clinical features of motor neurone disease (MND) and/or frontotemporal dementia (FTD). These cases share some neuropathological features with PSP, such as 4R tau inclusions in neurons, astrocytes and oligodendrocytes. In this study, we compare and contrast the clinical, pathological and biochemical features of two cases with GOIs: case 1 with clinical features of MND and case 2 with FTD.
Materials and methods
Case material
Brain and spinal cord tissue from case 1 was donated to NeuroResource located at UCL Institute of Neurology, London and brain tissue (only) from case 2 was donated to the Queen Square Brain Bank for Neurological Disorders (also located at UCL Institute of Neurology) according to protocols approved by a London Ethics Committee and stored under a licence issued by the Human Tissue Authority (No. 12198). The right half brains of cases 1 and 2 were fixed in 10% buffered formalin and weighed 513 and 580 g, respectively; the other half brains were dissected and flash frozen. Formalin-fixed and/or frozen brain tissues from neuropathologically confirmed PSP and CBD cases stored at QSBB were utilised as disease controls. Clinical information was extracted from all available medical records by KD and LSM; note that the results of brain imaging were not available for examination.
Neuropathological assessment
For neuropathological assessment, formalin-fixed paraffin-embedded tissue sections (8 μm) from cortical, subcortical, brainstem and cerebellar areas were stained using routine histological (H&E, Luxol-fast blue), silver staining (Gallyas) and immunohistochemical (IHC) techniques. The latter was performed using the antibodies summarised in Table 1. For antigen retrieval, deparaffinised and rehydrated sections were heated in a pressure cooker for 10 min in boiling citrate buffer (pH 6.0). Aβ and α-synuclein antibodies required pre-treatment with formic acid for 10 min prior to antigen retrieval. Following endogenous peroxidase blocking (0.3% H202 in methanol for 10 min) sections were incubated in dried milk solution (10% w/v in PBS, 30 min) to prevent non-specific binding. Primary antibodies diluted in PBS were applied for 60 min at room temperature, with the exception of FUS, which required overnight incubation at 4°C. Staining was visualised by the streptavidin–biotin-peroxidase method, using 3,3’-diaminobenzidine as the chromogen. Sections were then counterstained with haematoxylin before cover slipping. Gallyas silver staining was performed as previously described, using PSP and CBD tissues as positive controls [12].
Biochemistry
Tau protein was analysed by western blotting, as previously described [38]. In brief, frozen human brain tissue from the anterior frontal cortex and putamen of cases 1 and 2 was thawed and homogenised in 10 mM Tris–HCl, pH 7.4, 0.8 mM NaCl, 1 mM EDTA, 10% sucrose with complete protease inhibitor (Roche, UK) (0.1 g in 500 μl of buffer). The homogenates were centrifuged at 27,000×g for 30 min. The resulting pellet (27 kP) was dissolved in Laemmli loading buffer and spun at 5,000 rpm for 5 min at 4°C. Proteins were separated on 10% (w/v) SDS-PAGE, electroblotted onto Hybond-P membranes (GE Healthcare). The blots were then blocked with 10% non-fat milk (Marvel) and probed with primary antibodies PHF-1 and TP-70 (Table 1) overnight at 4°C. The membranes were washed thoroughly before being probed with HRP-conjugated secondary antibodies, visualised by enhanced chemiluminescence (Pierce, UK) and captured onto Kodak, X-Omat (Sigma, UK) films. Frozen brain tissue from the anterior frontal cortex of a CBD and PSP case were used as controls and analysed in parallel. Note that prior to using the frozen brain tissue, a 10-μm-thick frozen section was cut for each case using a cryostat and tau-specific IHC performed, to confirm that the relevant tau pathology was present in these cases.
Genetics
MAPT exons 1, 9–13 were PCR amplified from genomic DNA (50 ng) extracted from frozen brain tissue, using primer pairs (0.4 μM each) positioned in the flanking intronic regions of each exon [29]. PCR amplification was carried using a 60–50°C touch-down cycling protocol. PCR amplimers were checked on 2% agarose gel, purified using the QIAquick PCR purification kit (Qiagen) and sequenced using the amplification primers.
Clinical summaries
Patient 1
A 77-year-old woman presented with urinary urgency, mild leg weakness and a 3-year history of stumbling over words. Examination revealed a spastic dysarthria, asymmetrical distal weakness of the right hand, brisk knee jerks and a left Babinski sign. An MRI of brain and cervical cord ruled out any structural cause of her neurological signs. Symptoms progressed and a clinical diagnosis of probable MND was made, although there was no electrophysiological evidence for anterior horn cell disease. After 3 years, the patient had developed swallowing difficulties, a slow shuffling gait, jerky pursuit eye movements and limited upgaze. Multiple system atrophy (MSA) was considered as an alternative diagnosis, and a therapeutic trial of 300 mg levodopa/day for 2 months was unhelpful. After 6 years, she was aphonic, had a PEG tube sited and was wheelchair bound due to frequent falls. She died aged 82 years and donated to her brain to an MND tissue bank, consistent with her clinical diagnosis.
Patient 2
A 67-year-old right-handed man was noted by his wife to be quiet and apathetic for a year following a car accident. In the following months, she noted that he was leaving taps running, needed to be prompted to dress and had urinary frequency and occasional incontinence. On one occasion, his wife found him disorientated after getting lost and she noticed he was dragging his left leg and had a left facial droop, which resolved spontaneously within a few hours. Examination by a geriatrician revealed an abbreviated mental test score of 9/10, brisk left sided reflexes, left ankle clonus and an equivocal left plantar response. A CT scan of his brain revealed atrophy of the frontal lobes and he received a diagnosis of FTD (PiD). 3 years later, aged 70 years, the patient had frequent falls, disinhibited behaviour, minimal speech and difficulty swallowing. He died aged 74 of chest infection with sepsis, while residing in a nursing home, 8 years from symptom onset.
Neuropathology
Gross pathology
Gross pathological assessment of case 1 revealed significant dilatation of the frontal horn of the lateral ventricle and reduction in bulk of the deep frontal white matter; cortical atrophy was difficult to assess due to fixation artefacts. Subcortical structures, including the hippocampus, amygdala, subthalamic nucleus, substantia nigra, dentate nucleus and superior cerebellar peduncle were of normal size and appearance. Gross assessment of case 2 showed significant frontal and temporal atrophy, which was consistent with severe thinning of the cortical ribbon and blurring of the margin between grey and white matter (most severe anteriorly). The anterior horn of the lateral ventricle was severely dilated and the head of the caudate was reduced in bulk with moderate flattening and blurring between the margins of the internal capsule and striatum. The amygdala and the anterior hippocampus were reduced in size and there was severe pallor of the substantia nigra (throughout) and locus coeruleus. Other subcortical regions appeared unremarkable.
Histopathology
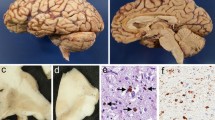
Histopathological assessment of cortical areas in case 1 revealed severe atrophy, superficial microvacuolation of the neuropil, neuronal loss and gliosis restricted largely to the primary motor cortex (Fig. 1a); also confirmed by the presence of a significant reduction in number of Betz cells. The underlying white matter was noticeably vacuolated and had severe myelin pallor and gliosis, with axonal loss; such features were absent in adjacent cortical gyri. In case 2, the anterior frontal cortex (Fig. 1d), the cerebral cortex of the tip of the temporal lobe, insular cortex and anterior cingulate cortex, revealed very severe atrophy, vacuolation, neuronal loss and gliosis. Similar to case 1 (but more severe), the underlying white matter was rarefied, with severe myelin pallor, axonal loss and dense gliosis; these features were more pronounced in deeper white matter areas. Regions of the hippocampus (subiculum, entorhinal cortex and hippocampal white matter) and the amygdala were similarly affected, with the parietal cortex being less affected. Although cortical pathology was generally more severe anteriorly, the primary motor cortex was preserved.
Case 1. a Upper layers of the motor cortex showing significant neuronal loss, gliosis and vacuolation in layer II of the cortex (asterisk H&E), b Significant tau pathology was present in the motor cortex (asterisk), concentrated at the grey–white junction (arrowhead) and lower in the underlying white matter (similar symbol AT8), c Higher magnification of the motor cortex (AT8) shows tau pathology in the form of tufted astrocytes (upper left), more globular astrocytes (upper right), globular oligodendroglial inclusions (middle), coiled bodies (bottom left) and globular neurofibrillary tangles (Betz cell, bottom right). Case 2. d Lower layers of the frontal cortex (asterisk), grey–white junction (arrowhead) and underlying white matter (similar symbol) showing marked neuronal loss, gliosis and vacuolation (H&E), e Tau pathology was present in the frontal cortex (asterisk), enriched at the grey–white junction (arrowhead) and wide spread in the underlying white matter (similar symbol AT8). f Higher magnification of the frontal cortex (AT8) shows tau pathology in the form of tufted astrocytes (upper left), more globular astrocytes (upper right), globular oligodendroglial inclusions (middle), coiled bodies (arrow bottom left) and globular neurofibrillary tangles (Betz cell in motor cortex bottom right). Scale bars 200 μm (a, b, d, e), 20 μm (c, f)
Assessment of white matter structures in case 1 showed severe degeneration of the corticospinal tract (CST). Myelin pallor, axonal loss and macrophages were readily detected in the mid-third of the cerebral peduncle in the midbrain and in the lateral column in the cervical spinal cord (Fig. 2c, d), where one side of the CST was more severely affected than the other. In thoracic and sacral levels of the spinal cord, myelin pallor was less pronounced, but the presence of increased numbers of microglia in the lateral column indicated involvement of the CST. The anterior horn cells were preserved throughout the spinal cord (Fig. 2a). There was mild to moderate loss of melanin-containing neurons and gliosis in the substantia nigra and locus coeruleus. The basal ganglia (including the subthalamic nucleus), the cerebellum and limbic lobe were histologically unremarkable. Subcortical white matter tracts in case 2 also showed severe myelin pallor, axonal loss and gliosis. These included the anterior limb of the internal capsule, the external and extreme capsules, lenticular fasciculus and amygdalostriate transit area. In contrast to case 1, the most medial third and the lateral third of the cerebral peduncle containing fibres of the frontopontine and temporopontine tracts, respectively, were severely atrophic and gliotic, with marked myelin pallor and axonal loss; while the middle third, containing the CST was largely spared. Consistent with gross examination, there was severe loss of melanin-containing neurons and gliosis in the substantia nigra and locus coeruleus. Other nuclei of the basal ganglia and cerebellum were within normal limits; however, in this case, medulla and spinal cord sections were not available for examination.
The spinal cord (cervical level) in case 1 showed preservation of anterior horn cells (a H&E) although some of these cells contained neurofibrillary tangles, pre-tangles and neuropil threads (b AT8). Severe corticospinal tract degeneration was also evident as demonstrated by significant myelin pallor (c Luxol-fast blue) and numerous macrophages (d Iba-1). In case 2, examination of the frontal white matter using an antibody specific to 3R tau isoform (RD3) revealed some diffuse (e) and/or intense punctate (f) immunoreactivity in cells that contained globular oligodendroglial inclusions; insets show higher magnification. Scale bars 100 μm (a), 50 μm (b, d), 20 μm (e, f)
Tau immunohistochemistry
Immunohistochemistry using anti-tau antibodies (AT8, RD3 and RD4) showed significant tau pathology in histologically affected regions of both cases. Using the primary motor cortex as an example in case 1 (Fig. 1c) and the anterior frontal cortex in case 2 (Fig. 1f) the following tau-related inclusions were observed.
Neuronal
In both cases, tau was observed in neurons in the form of pre-tangles, mature neurofibrillary tangles (NFT) and unusual globular inclusions some of which had a Pick body-like morphology. The latter consisted of either a single, large or multiple small, globular cytoplasmic inclusions, which were intensely immunoreactive with 4R tau antibody (RD4) and 3R (RD3) negative.
Astrocytic
Tau-positive astrocytes were detected in grey matter of both cases. Some were morphologically indistinguishable from PSP-type tufted astrocytes, but others were characterised by multiple, small globular tau deposits radiating from the cell body into proximal processes, often in conjunction with more diffuse tau immunoreactivity, termed globular astrocytes. Both the tufted- and the globular astrocytes were 4R positive and present in both the cases.
Oligodendroglial
Tau accumulation in oligodendrocytes was the most prominent feature of both cases. In case 1, the oligodendroglial inclusions were 4R positive and morphologically diverse, including small delicate and larger swollen PSP-type coiled bodies and a smaller number of intensely stained GOIs. Collectively, these inclusions were common in the cerebral cortical grey matter, condensed at the grey–white junction and were less frequent in the underlying white matter. Coiled-body-like inclusions were rare in case 2, with the majority being much larger, intensely 4R-positive GOIs. Interestingly, a smaller subset of GOIs in case 2 were also 3R positive, which consisted of low-level diffuse staining of the actual GOIs and/or numerous small dot-like structures in the cytoplasm of a oligodendrocyte containing a GOI (Fig. 2e, f). In contrast to case 1, the inclusions in case 2 were very frequent in the white matter, enriched at the grey–white junction and less frequent in the cerebral cortical grey matter.
Cell processes
Tau accumulated in cell processes in the form of short, delicate neuropil threads and small tau-positive dots. Similar to the density of oligodendroglial inclusions, these threads were denser in the grey matter of case 1, enriched at the grey white junction of both cases and denser in the white matter of case 2. Case 1 also had a number of longer PSP-type neuropil threads in the grey matter.
Distribution of oligodendroglial inclusions
As a general rule in both cases, the severity of tau-positive oligodendroglial inclusions in white matter structures correlated with the severity of degeneration identified histologically. In case 1, GOIs were common throughout most of the CST, decreasing in severity in craniocaudal direction. For example, the density of GOIs was highest in the primary motor cortex (moderate in the underlying white matter), high in the putamen (pencil fibres rather than grey matter), thalamic fascicle, zona incerta and longitudinal fibres of the comb, moderate in the mid-third of the midbrain cerebral peduncle, mild in the CST fibres of the pontine base and altogether absent in the spinal cord. The brainstem tegmentum (moderate) and red nucleus (mild) had mainly coiled-body inclusions. In contrast to the primary motor cortex, the anterior frontal cortex was minimally affected. In case 2, the underlying white matter of the anterior frontal cortex, inferior temporal gyrus, anterior cingulate cortex and anterior hippocampus had the highest burden of GOIs; white matter of the parietal cortex had a moderate density and the motor cortex had a mild density. Not only was the density lower in the latter two regions, but interestingly, affected oligodendrocytes in these regions often had multiple, smaller GOIs per cell and were similar to the GOIs identified in case 1. The density of GOIs was high in the internal capsule, putamen (pencil fibres more than grey matter), external capsule, extreme capsule, lenticular fascicle and amygdalostriate transit area. In the midbrain cerebral peduncle, the density of GOIs was highest in the medial third (frontopontine tract), mild in the CST and high in the lateral third (temporopontine tract). Only a mild density of GOIs was observed in the longitudinal fibres of the pontine base, with some occasional GOIs in the transverse fibres. Moderate numbers of GOIs were also found in subcortical grey matter structures including the striatum (with the majority in pencil fibres), pallidum and brainstem tegmentum. The subthalamic nucleus (mild) and substantia nigra (moderate) also contained GOIs; the locus coeruleus and cerebellum also had mild densities of GOIs.
Distribution of neuronal and astrocytic inclusions
The regional distribution of neuronal and astrocytic inclusions was consistent with that reported in larger series [18, 20]. In our cases, density of neuronal tau inclusions was marginally, but consistently, higher in the subcortical nuclei of case 2 compared with case 1; these included the subthalamic nucleus, substantia nigra and dentate nucleus of the cerebellum. Analysis of the spinal cord in case 1 revealed anterior horn cells containing NFTs and pre-tangles, and were associated with thicker PSP-like neuropil threads (Fig. 2b). NFTs were present throughout the spinal cord, but had moderate numbers in the cervical cord and sparse in sacral cord, and were consistently more numerous on the side with more severe CST degeneration.
Characterisation of glial inclusions
Detailed characterisation of the glial inclusions in both cases revealed further similarities. It is of note that tau-positive tufted- and globular astrocytes in both cases were mostly Gallyas negative (Fig. 3). Extensive searching in both cases found a very small number of astrocytic cells with short and delicate Gallyas-positive processes, but nothing as convincing as the intense staining achieved by tau IHC (using AT8 and RD4). Analysis of oligodendroglial inclusions and neuropil threads also suggested discrepancies between the Gallyas and tau or p62 staining (Fig. 3). Qualitative analysis in both cases suggested that Gallyas staining identified only a subset of oligodendroglial inclusions with tau and p62 IHC identifying more inclusions.
Tau immunohistochemistry (AT8) in the motor cortex of case 1 (a) showing numerous tau-positive astrocytes, which are mostly negative when using Gallyas silver staining (b), arrow and inset highlight a rare and faint Gallyas-positive astrocyte. Oligodendroglial inclusions in the motor cortex of case 1 are smaller in size and fewer in number using Gallyas staining (c) when compared with tau immunohistochemical staining (d AT8), with p62 staining (e) showing even larger and more numerous oligodendroglial inclusions. Similar to case 1, the motor cortex of case 2 also shows tau-positive astrocytes (f AT8) that are mostly Gallyas negative (g), arrow and inset show a rare and weakly stained Gallyas-positive astrocytic cell. Globular oligodendroglial inclusions in the frontal cortex of case 2 also appeared smaller and fewer in Gallyas stained sections (h) when compared with those stained with tau (i AT8) and p62 staining showing larger and more numerous oligodendroglial inclusions (j). Scale bars 100 μm (a, b, f, g), 50 μm (c, d, e, h, i, j)
Additional pathologies
Case 1 had mild Alzheimer’s disease (AD) type changes (Braak NFT stage III; no plaques), argyrophilic grain disease (AGD) and incidental Lewy body pathology (Braak Lewy body stage IV); no TDP-43 or FUS pathology was observed. Case 2 had minimal AD-type changes (Braak NFT stage I; CERAD sparse mature plaques). AGD was difficult to assess due to extensive tau deposition, but no Lewy body, TDP-43 or FUS pathology was present. AD-type NFTs were positive for both 3R and 4R tau antibodies (Table 1), allowing them to be distinguishing from 4R-positive neuronal tau inclusions described earlier.
Biochemical and genetic analysis
Tau protein analysis was performed on frozen tissue samples from the anterior frontal cortex and putamen of cases 1 and 2. Prior to protein extraction, frozen brain samples were sectioned on a cryostat and tau-specific IHC performed to confirm the presence of tau pathology. Consistent with that seen in the formalin-fixed tissues (see above), significant tau-related pathology was present in the putamen of both cases and in the anterior frontal cortex of case 2, whereas the latter region in case 1 was minimally affected (data not shown).
Representative immunoblots of the 27 kP fraction are shown in Fig. 4. Using PHF-1 antibody (monoclonal, phospho-Ser396/Ser404) all cases and appropriate disease controls revealed two bands at 68 and 64 kDa, but these were absent in the frontal cortex sample of case 1 consistent with the lack of significant pathology in this area. A higher exposure of the same blot revealed a prominent low-molecular weight band at ~35 kDa in the anterior frontal cortex and putamen of case 2, which was also faintly present in the PSP sample. In contrast, the CBD control showed a faint band at ~40 kDa; this was also present in the PSP and case 2 samples where it was relatively less prominent than the ~35 kDa band. Immunoblotting with TP70, a polyclonal non-phosphorylation-dependent antibody recognising residues 428–441 at the C-terminus of tau, was better at highlighting these low-molecular weight tau species, with the putamen of case 1 also revealing a faint band at ~35 kDa. The presence of 68, 64 and ~35 kDa bands in cases 1, 2 and the PSP control is consistent with that reported previously for PSP [38].
Biochemical analysis of tau in 27 kP fractions extracted from the anterior frontal cortex (F) and/or the putamen (P) of cases 1, 2 and disease controls. a Low exposure of an immunoblot stained with PHF-1 shows two prominent bands (black arrows) at ~68 and ~64 kDa in all samples, apart from the frontal cortex of case 1; note that tau pathology was very mild in this region of case 1 when analysed by immunohistochemistry, prior to any biochemical analysis (see “Methods”). b Higher exposure of the same immunoblot also reveals a prominent band at ~35 kDa (white arrow) in both regions of case 2, which was also faintly visible in the PSP control. In contrast, the CBD control showed a ~40 kDa species (grey arrow), which was less prominent in other samples (when compared with the ~35 kDa species). c Immunoblotting with TP70 was better at highlighting lower molecular weight tau species, with the putamen of case 1 also revealing a faint ~35 kDa band (white arrow)
Sequencing of the MAPT gene (exons 1, 9–13) found no pathogenic mutations, although both cases were homozygous for the extended MAPT H1-haplotype [1].
Discussion
We have described the clinical, pathological and biochemical features of two cases that presented with MND and FTD, but had a similar underlying pathology characterised by GOIs. We compare our cases to the few others previously reported in the literature and report some novel findings. This is the first study to directly compare these two clinical phenotypes enabling us to highlight features that will aid in accurate diagnosis, analyse features that overlap with other disorders and suggest a possible classification strategy based on molecular neuropathology.
The clinical presentation of asymmetric upper motor neurone weakness associated with pseudobulbar dysarthria, in the absence of electrophysiological features of chronic partial denervation with fibrillation suggests that case 1 could be a pure upper motor variant of MND known as primary lateral sclerosis (PLS) [34]. Pathological examination identified severe atrophy, vacuolation, neuronal loss and gliosis of the primary motor cortex, along with rarefaction, myelin pallor and axonal loss of the underlying white matter and descending CST. Neuronal loss of upper motor neurons together with preservation of anterior horn cells in the spinal cord, would support a neuropathological diagnosis of PLS [31]; however, these neurodegenerative changes were associated with significant accumulation of 4R tau pathology, which is a feature inconsistent with PLS. The “tufted” morphology of tau-positive astrocytes, predominance of oligodendroglial inclusions and the tau banding pattern on western blots (68, 64 and 35 kDa bands) are features shared with PSP [16, 38]. Nevertheless, the pathology of case 1 deviates from that seen in PSP in a number of ways. First, subcortical structures (subthalamic nucleus, globus pallidus and cerebellar dentate nucleus) that are consistently and severely affected in PSP [16] were relatively spared. Secondly, the severe involvement of the motor cortex is not only atypical for PSP, but would also argue against this case representing a mild or “minimal change” form of PSP. Thirdly and most importantly, the glial tau inclusions were morphologically distinct and had unusual staining properties when compared with those found in PSP. For example, case 1 contained GOIs that were larger and more globular than PSP-type coiled-bodies, which were also present. Similarly, some of the astrocytic inclusions in case 1 were morphologically indistinguishable from PSP-type tufted astrocytes while others had multiple, small, globular tau deposits in proximal processes.
It is important to note that tufted astrocytes are considered pathognomonic for PSP and are Gallyas positive [16]. In contrast tau-positive astrocytes were consistently Gallyas negative in case 1; PSP and CBD cases stained in parallel contained numerous Gallyas-positive astrocytic inclusions (data not shown). Tau pathology in PiD (a 3R tauopathy) is similarly Gallyas negative [19], but case 1 was confirmed as a 4R tauopathy. At this juncture, the significance of these 4R tau-positive Gallyas-negative astrocytic inclusions is unknown, but suggests that tau in these inclusions is non-fibrillar [12] and that its composition and/or aggregation may be different when compared with astrocytic tau in PSP and CBD. In current PSP neuropathological criteria, the presence of tufted astrocytes is supportive of a PSP diagnosis [14, 23], but no distinction is made between those that are Gallyas positive or negative. These criteria rely heavily on the distribution and density of NFT pathology; with case 1 having a NFT distribution similar to that seen in PSP, but with an atypical density (i.e. subthalamic nucleus was only mildly affected). Using present criteria, case 1 might be classified pathologically as atypical PSP [14].
Consistent with this perception, similar cases reported in the literature have been classified as “atypical PSP with corticospinal tract degeneration” (PSP-CST) [18]. In the series of cases reported by Josephs et al. [18], asymmetrical onset of prominent upper motor neurons signs and parkinsonism were described with the pathological findings of severe CST degeneration, relative sparing of subcortical nuclei, GOIs and tau-positive Gallyas-negative tufted astrocytes; although the latter feature was not emphasised. Spinal cord was not available in their cases, but our case showed significant CST degeneration (asymmetrical and more severe in rostral than caudal segments), with no significant loss of anterior horn cells although occasionally they were affected by pre-tangles, NFTs and neuropil threads. Fu et al. [11] also published three cases with striking similarities to our case and those reported by Josephs et al. [18]. Their cases differ by having upper and lower motor neuron signs consistent with the pathological findings of severe loss of upper (motor cortex) and mild to moderate loss of lower (anterior horn cells) motor neurons [11]. They also described numerous tau-positive oligodendroglial and astrocytic inclusions, both of which had a globular morphology and the latter were Gallyas negative [11]. Similar to our case, biochemical analysis of phosphorylated tau in their cases revealed a doublet at 68 and 64 kDa, along with lower molecular weight species at ~33 kDa [11]. Although Fu et al. [11] acknowledged some of the similarities between their cases and those described by Josephs et al. [18], they proposed that their cases represented a distinct clinicopathological entity and classified them as “sporadic four-repeat tauopathy with frontotemporal lobar degeneration, parkinsonism, and motor neuron disease” (FTD-P-MND). The clinicopathological and biochemical similarities between the case we describe (case 1) and those previously reported [11, 18] suggests that collectively these cases might represent a common disease entity or process and such cases present diagnostic difficulties due to overlapping pathological and biochemical features with PSP, but can be distinguished pathologically by the presence of globular–oligodendroglial and astrocytic inclusions, the latter of which are Gallyas negative. Although case 1 has some clinical features reminiscent of Guamanian parkinsonism–dementia complex (PDC) probably due to the involvement of similar brain regions, the predominance of 4R-tau inclusions in case 1 is different from that found in PDC; also tau-positive “granular hazy inclusions” in astrocytes characteristic of PDC are Gallyas positive [28].
Case 2 had apathy, disinhibition and personality change with a steadily progressive cognitive decline. Consistent with this clinical picture, neuropathological examination of case 2 revealed severe neurodegeneration in frontal and temporal cortices (including medial-temporal lobe structures) characterised by atrophy, vacuolation, neuronal loss and gliosis of grey matter, along with rarefaction, myelin pallor and axonal loss of the associated white matter; such features are consistent with a pathological diagnosis of frontotemporal lobar degeneration (FTLD) [25]. More specifically, these neurodegenerative changes were associated with predominately 4R tau pathology. Based on the current nomenclature for FTLD [25], the absence of TDP-43, FUS or any significant AD-type pathology would suggest that case 2 belongs to the FTLD-tau subtype. The most common disorders in this subtype of FTLD, include PiD, PSP and CBD. Although the areas affected and the tau-positive Pick-like neuronal inclusions are suggestive of PiD, such inclusions were 3R negative. CBD can also be excluded from the diagnostic differential as astrocytic plaques were absent, whereas the presence of tau-positive astrocytic inclusions with a “tufted” morphology brings PSP into the differential. For the same reasons, discussed for case 1, such as the regional distribution of neurodegeneration (cortical more than subcortical), presence of GOIs and differences in morphology and histological properties of astrocytic tau inclusions, we feel that a diagnosis of PSP might not be appropriate for this case either.
The pathological features are most compatible with a rare form of the FTLD-tau group, termed “multiple system tauopathy with dementia” (MSTD) [4]. This disorder was first reported as a single case report by Bigio et al. [4] who described a sporadic FTD patient, who on histological examination showed severe neurodegeneration in frontal and temporal cortices associated with tau-positive globular neuronal and glial inclusions in grey and white matter. Bigio et al. [4] also identified “tuft-like astrocytes” and widespread GOIs, which were most numerous at the grey–white junction and in the underlying white matter; Gallyas staining was not performed in their case. In the past, MSTD had only been reported as single case reports [3, 10, 27, 30, 32, 36], but recently Kovacs et al. [20] performed a detailed and systematic evaluation of seven such cases, which they termed “white matter tauopathy with globular glial inclusions” (WMT-GGI). In common with our case 2, descending frontopontine tracts were severely and consistently affected and “tuft-like” astrocytes were present in the cortical grey matter, the majority of which were “non-argyrophilic” (Gallyas negative). Biochemical analysis was performed in one of their cases, which revealed 68 and 64 kDa bands, but also a number of lower molecular weight species (when using a C-terminal specific tau antibody) [20], consistent with the biochemical analysis of case 2. Comparison of the clinicopathological and biochemical features of case 2 with those previously reported for MSTD (and WMT-GGI) suggests that they belong to the same clinicopathological disease entity.
Interestingly, the GOIs in our case of MSTD (case 2) were not only intensely 4R positive (as previously reported [20]), but a subset were also weakly 3R positive; this is a novel finding that has not been described in previous reports of MSTD. Differences in IHC methodology between studies (tissue fixation, antigen retrieval and or antibody source/dilution) may explain such differences. Both 3R and 4R tau isoforms can be co-deposited in certain forms of FTDP-17 [13], but sequencing of the MAPT gene excluded this possibility in our cases. It should be emphasised that 4R tau was the predominant tau species in case 2 and that the presence of 3R tau is unlikely to represent the defining feature of a distinct disease entity as all the other pathological features are compatible with previous reports of MSTD.
Using our case 1 as an example of PSP-CST [18] (or FTD-P-MND [11]) and case 2 as an example of MSTD [4] (or WMT-GGI [20]), this is the first study to provide a detailed comparative clinical, pathological and biochemical study of these two disorders that have, in the past, been reported independently. Table 2 provides a clinicopathological comparison of cases 1 and 2 with similar cases reported in the literature. In terms of similarities, for both cases: (1) specific cortical areas and their associated descending white matter tracts were severely affected; (2) neurodegeneration in subcortical nuclei was largely restricted to the substantia nigra and locus coeruleus; (3) neuronal tau-positive inclusions were 4R positive and present in cortical (predominantly) and subcortical nuclei; (4) tau-positive oligodendroglial inclusions were prominent and characteristic features; (5) a mixture of large GOIs and coiled-body inclusions were present; (6) tau-positive astrocytes were also a prominent feature, with many having a globular morphology; (7) tau-positive astrocytic inclusions were Gallyas negative; (8) biochemical analysis of tau protein revealed strong bands at 68 and 64 kDa, but also weaker lower molecular weight bands, with a ~35 kDa band being the most prominent.
One of the major differences between cases 1 and 2 was the presenting clinical syndrome. In these cases, we propose this variation in phenotype reflects the underlying regional cortical vulnerability; case 1 exhibiting predominant motor cortex pathology and case 2 anterior frontotemporal (and limbic lobe) structures mostly involved, with the motor cortex being spared. Similarly tau pathology in the cortex of case 1 was more pronounced in the grey matter compared with the white matter, whereas the opposite was true for case 2. Neurodegenerative changes in the cortex, descending white matter tracts and subcortical nuclei (e.g. substantia nigra) were more severe in case 2 and GOIs were the most frequent morphology, whereas the GOIs and coiled body were present in similar densities in case 1. The GOIs in case 2 were also larger and more globular than those found in case 1; in the latter some even had an intermediate type morphology being larger, yet retaining an overall coiled-body-like appearance. The pathological similarities between cases 1 and 2, but their vastly different clinical presentations suggests that they are variants which represent the spectrum of a single distinct disease entity; a notion also suggested by Kovacs et al. [20]. This spectrum could be defined clinically by MND at one end and FTD at the other end of the spectrum. A similar spectrum of disease with MND and/or FTD subtypes explains the heterogeneity of FTLD-TDP [24] and FTLD-FUS [22]-type pathology. This study is limited by describing only two cases with GOIs and therefore it is possible that future studies using larger cohorts will reveal further clinicopathological heterogeneity.
As discussed, the neuropathological hallmarks of the two cases described in this study, which allow them to be separated from other members of the 4R-tauopathy family, are the globular nature of tau-positive glial (oligodendroglial and astrocytic) inclusions and the non-fibrillar (Gallyas negative) properties of the latter. Based on these characteristics and the recent trend to classify neurodegenerative diseases based on molecular neuropathology [25, 26], we propose using the term globular glial tauopathy (GGT) as an encompassing term followed by a sub-stratification strategy based on the regional distribution and severity of neurodegeneration. Following these guidelines, case 1 would be termed GGT-CST and case 2 as GGT-FTLD. Such a classification strategy would allow for phenotypic grouping, account for the heterogeneity and avoid confusion or redundancy in terminology; the latter points were demonstrated by the recent FTLD nosology and classification guidelines, which included MSTD and WMT-GGI as separate FTLD-tau subtypes [25]. This study will hopefully pave the way for a formal consensus agreement on the neuropathological terminology of these rare 4R tauopathies.
Conclusion
In conclusion, we describe an emerging class of 4R tauopathy (termed globular glial tauopathy, GGT) that is characterised neuropathologically by globular tau inclusions, primarily in oligodendrocytes, but also in astrocytes that are consistently Gallyas negative. These characteristic features help GGT to be distinguished from other 4R tauopathies, in particular PSP. Whether the variants of GGT (e.g. CST or FTLD) are distinct disease entities or part of a spectrum of disease is open to a similar debate that has been ongoing for PSP and CBD [5]. Ultimately, future studies should be mindful of the expanding heterogeneity of 4R tauopathies. The severe involvement of oligodendroglia and resulting white matter changes (myelin pallor, axonal loss and gliosis) suggests that GGT may represent a primary oligodendrogliopathy. If so, GGT could be the tau equivalent of MSA; a progressive neurodegenerative disease characterised by “globular” α-synuclein inclusions in oligodendrocytes [21].
Abbreviations
- AD:
-
Alzheimer’s disease
- AGD:
-
Argyrophillic grain disease
- ALS:
-
Amyotrophic lateral sclerosis
- CBD:
-
Corticobasal degeneration
- CBS:
-
Corticobasal syndrome
- CST:
-
Corticospinal tract
- FTD:
-
Frontotemporal dementia
- FTDP-17:
-
Frontotemporal dementia with parkinsonism linked to chromosome 17
- FTD-P-MND:
-
Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, parkinsonism, and motor neuron disease
- FTLD:
-
Frontotemporal lobar degeneration
- FUS:
-
Fused in sarcoma protein
- GGT:
-
Globular glial tauopathy
- GOIs:
-
Globular oligodendroglial inclusions
- IHC:
-
Immunohistochemistry
- MND:
-
Motor neuron disease
- MSA:
-
Multiple system atrophy
- MSTD:
-
Multiple system tauopathy with dementia
- NFT:
-
Neurofibrillary tangles
- PiD:
-
Pick’s disease
- PLS:
-
Primary lateral sclerosis
- PSP:
-
Progressive supranuclear palsy
- PSP-CST:
-
Atypical PSP with corticospinal tract degeneration
- TDP-43:
-
TAR DNA-binding protein 43
- WMT-GGI:
-
White matter tauopathy with globular glial inclusions
- 27 kP:
-
27,000×g pellet
- 3R:
-
3-repeat tau
- 4R:
-
4-repeat tau
References
Baker M, Litvan I, Houlden H et al (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 8:711–715
Bergeron C, Morris HR, Rossor M (2003) Pick’s disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 124–131
Berry RW, Quinn B, Johnson N, Cochran EJ, Ghoshal N, Binder LI (2001) Pathological glial tau accumulations in neurodegenerative disease: review and case report. Neurochem Int 39:469–479
Bigio EH, Lipton AM, Yen SH et al (2001) Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol 60:328–341
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246(Suppl 2):II6–II15
Dickson DW, Litvan I (2003) Corticobasal degeneration. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 115–123
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400
Dickson DW, Rademakers R, Hutton ML (2007) Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17:74–82
Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396
Ferrer I, Hernandez I, Boada M et al (2003) Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual tauopathies. Acta Neuropathol 106:419–435
Fu YJ, Nishihira Y, Kuroda S et al (2010) Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol 120:21–32
Gallyas F (1971) Silver staining of Alzheimer’s neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 19:1–8
Ghetti B, Hutton ML, Wszolek ZK (2003) Frontotemporal dementia and parkinsonism linked to chromosome 17 associated with tau gene mutations (FTDP-17T). In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 86–102
Hauw JJ, Daniel SE, Dickson D et al (1994) Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hauw JJ, Verny M, Delaere P, Cervera P, He Y, Duyckaerts C (1990) Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer’s disease and aging. Neurosci Lett 119:182–186
Hauw J, Agid Y (2003) Progressive supranuclear palsy (PSP) or Steele-Richardson-Olszewski disease. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 103–114
Ikeda K, Akiyama H, Haga C, Kondo H, Arima K, Oda T (1994) Argyrophilic thread-like structure in corticobasal degeneration and supranuclear palsy. Neurosci Lett 174:157–159
Josephs KA, Katsuse O, Beccano-Kelly DA et al (2006) Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol 65:396–405
Komori T (1999) Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol 9:663–679
Kovacs GG, Majtenyi K, Spina S et al (2008) White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:963–975
Lantos PL, Quinn N (2003) Multiple system atrophy. In: Dickson DW (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, 1st edn. ISN Neuropath Press, Basel, pp 203–214
Lashley T, Rohrer J, Bandopadhyay R et al (2011) A comparative clinical, pathological and biochemical study of FUS proteinopathies. Brain (in press)
Litvan I, Hauw JJ, Bartko JJ et al (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105
Mackenzie IR (2007) The neuropathology of FTD associated With ALS. Alzheimer Dis Assoc Disord 21:S44–S49
Mackenzie IR, Neumann M, Bigio EH et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Mackenzie IR, Neumann M, Bigio EH et al (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18
Ohara S, Tsuyuzaki J, Oide T et al (2002) A clinical and neuropathological study of an unusual case of sporadic tauopathy. A variant of corticobasal degeneration? Neurosci Lett 330:84–88
Oyanagi K, Wada M (1999) Neuropathology of parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam: an update. J Neurol 246(Suppl 2):II19–II27
Poorkaj P, Bird TD, Wijsman E et al (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–825
Powers JM, Byrne NP, Ito M et al (2003) A novel leukoencephalopathy associated with tau deposits primarily in white matter glia. Acta Neuropathol 106:181–187
Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC (1992) Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 115(Pt 2):495–520
Sakai K, Piao YS, Kikugawa K et al (2006) Corticobasal degeneration with focal, massive tau accumulation in the subcortical white matter astrocytes. Acta Neuropathol 112:341–348
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10:333–359
Swash M, Desai J, Misra VP (1999) What is primary lateral sclerosis? J Neurol Sci 170:5–10
Takahashi T, Amano N, Hanihara T et al (1996) Corticobasal degeneration: widespread argentophilic threads and glia in addition to neurofibrillary tangles. Similarities of cytoskeletal abnormalities in corticobasal degeneration and progressive supranuclear palsy. J Neurol Sci 138:66–77
Tan CF, Piao YS, Kakita A et al (2005) Frontotemporal dementia with co-occurrence of astrocytic plaques and tufted astrocytes, and severe degeneration of the cerebral white matter: a variant of corticobasal degeneration? Acta Neuropathol 109:329–338
Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 8:270–279
Wray S, Saxton M, Anderton BH, Hanger DP (2008) Direct analysis of tau from PSP brain identifies new phosphorylation sites and a major fragment of N-terminally cleaved tau containing four microtubule-binding repeats. J Neurochem 105:2343–2352
Acknowledgments
Professor Revesz, Dr Holton and Dr Ahmed are supported by the Multiple System Atrophy Trust (formerly known as the Sarah Matheson Trust for Multiple System Atrophy). Dr Silveira-Moriyama, Dr Holton, Dr de Silva and Dr Doherty are supported by the Reta Lila Weston Trust for Medical Research. Dr de Silva is funded by grants by the Medical Research Council (G0501560) and Cure PSP+. Part of this work was undertaken at UCLH/UCL, who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Z. Ahmed and K. Doherty contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Ahmed, Z., Doherty, K.M., Silveira-Moriyama, L. et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol 122, 415–428 (2011). https://doi.org/10.1007/s00401-011-0857-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0857-4