Abstract
The hippocampal involvement in amyotrophic lateral sclerosis (ALS) patients has been known for more than a decade, however, its relationship to clinical manifestations including memory deficits and topographical differentiation from Alzheimer disease (AD) remain unclear. In order to clarify the anatomopathological features in the hippocampus and their relevance to disease-specific memory deficits in ALS patients, topography and cytopathology of the hippocampal lesions along the perforant pathway were quantitatively and semiquantitatively surveyed in 14 ALS patients with extramotor involvement. These pathological findings were compared with clinical characteristics assessed from their clinical records. Cytoplasmic inclusions initially appear in the granular cells of the dentate gyrus (DG) and superficial small neurons of the transentorhinal cortex (TEC) with mild subicular degeneration (stage I: inclusion stage). Subsequent gliosis and neuronal loss of the TEC, concomitant with presynaptic degeneration of the outer molecular layer of the DG, suggests an extension of the degeneration through the perforant pathway (stage II: early perforant stage). In a more advanced stage, the presynaptic degeneration is more evident with moderate to severe neuronal loss in the TEC (stage III: advanced perforant stage). This advanced stage was associated with episodic memory deficits mimicking AD in some ALS patients. This ALS pathology initiated by cytoplasmic inclusions and neuronal loss in layer II–III of the TEC is different from neurofibrillary tangles of AD, dominant in layer II–III of the entorhinal cortex. Because this involvement of the TEC-molecular DG projection and subiculum is specific to ALS, it will provide a basis for clinical characterization of memory deficits of ALS, which could be distinct from those of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Involvement of the hippocampal formation [cornu ammonis (CA), dentate gyrus (DG), subiculum, entorhinal/transentorhinal cortex (EC/TEC)] [17], which is relevant to memory function [7, 32], is observed in many neurodegenerative diseases such as Alzheimer disease (AD) [4, 10, 24], Pick body disease [22, 36], Lewy body disease [9], argyrophilic grain disease[34], and amyotrophic lateral sclerosis (ALS)[25, 30, 39]. Recently, we clarified that the perforant pathway was vulnerable in ALS patients and suggested a possible relation of this perforant pathway lesions to memory impairments as seen in AD patients [33]. This awareness may be useful in evaluating patients with memory deficits but may raise controversies in clinical diagnosis of dementia because little is known about how to identify disease-specific memory deficits on clinical grounds. Moreover, it is unfortunate that the anatomical and pathological basis for these hippocampal lesions and their differences, if any, between ALS and AD remain unclear. Because each degenerative process has its own specific topography and cytopathology, we hypothesized that the hippocampal lesions of ALS exhibit a disease-specific topography and cytopathology, which may be distinguishable from other degenerative processes. As expected, topography and cytopathology of the hippocampal lesions, both assessed quantitatively or semiquantitatively in the present study, demonstrated an ALS-specific pattern of extension (staging) along the perforant pathway, which is clearly distinct from that in AD. The perforant pathway has been recognized as projecting fibers from layer II–III neurons of the lateral and medial entorhinal cortex to the molecular layer of the dentate gyrus, CA and subiculum [14, 40]. Moreover, recent sophisticated tracing studies in mammals have revealed that each layer II, layer III, medial and lateral entorhinal region projects to each corresponding area including molecular layer of the dentate gurus, CA and subiculum [40]. This anatomical characterization will provide a basis for further clinical differentiation of memory deficits, which have not been fully characterized but could be disease-specific as well.
Materials and methods
Out of 104 ALS patients autopsied at Tokyo Metropolitan Neurological Hospital from 1980 to 2006, samples from 41 ALS patients were available for histological assessment with conventional pathological and immunohistological evaluation in the central nervous system. Out of these 41 patients, cases with ubiquitin/TDP-43-positive inclusions in the brain and Bunina bodies in the spinal motor neurons were selected to yield 14 cases surveyed in this study. Cases with any evidence of global circulatory disturbance were excluded. The hippocampal formation at the level of the nucleus ruber was routinely fixed in formalin and 5-μm thick sections of paraffin-embedded tissue were stained with hematoxylin and eosin, Klüver–Barrera and Bodian methods for microscopic investigation. Immunostaining was performed with primary antibodies against ubiquitin (polyclonal, 1:1,000, Dako, Glostrup, Denmark), TDP-43 (polyclonal, 1:3,000, Protein Tech, Chicago, Illinois) [2, 28], SNAP-25 (monoclonal, 1:30,000, Sternberger Monoclonals Inc., Lutherville, Maryland) [31] and AT-8 (monoclonal, 1:10,000, Innogenetics, Zwijndrecht, Belgium). Pretreatment of tissues in 10 mM citrate buffer with microwave oven for 18 min was needed for TDP-43 staining. The sections were incubated for 2 or 3 days at 4°C with these primary antibodies diluted with PBS containing 0.03% polyoxylethyleneglycol-p-isooctylphenyl ether (Triton-X) and the corresponding blocking serum. After the incubation, labeling was performed with appropriate secondary antibodies and detected by using the avidin–biotinylated peroxidase complex (ABC) method coupled with a diaminobenzidine (DAB) reaction intensified by ammonium nickel sulfate to yield a black precipitate. Triple-immunofluorescence analysis was also performed with antibodies against ubiquitin (monoclonal, IgG, 1:2,000, Chemicon, Temecula, California), TDP-43 (polyclonal, IgG, 1:600, Protein Tech, Chicago, Illinois) and Alz-50 (monoclonal, IgM, 1:20, generous gift from Prof. P. Davies) [8]. The antibody against ubiquitin was reacted with peroxidase-conjugated anti-mouse IgG antibody specific to γ-chain followed by biotinylated tyramide amplification [35]. The epitope was visualized with streptavidin-conjugated Alexa Fluor 488® (1:200, Molecular Probes, Eugene, Oregon). Another epitope for TDP-43 was visualized with Alexa Fluor 405® conjugated with anti-rabbit IgG (1:200) and that for Alz-50 was with CyTM-5 conjugated with anti-mouse IgM (1:200, Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania).
The cortical degeneration of the EC and TEC was semiquantitatively rated for their severity into four grades, as follows: normal, mild (gliosis with/without spongiotic changes limited to layer II), moderate (spongiotic changes in layers II and III) and severe (transcortical spongiotic changes). The subicular degeneration was also semiquantitatively rated into four grades: normal, mild (mild gliotic changes without neuronal loss), moderate (mild to moderate thinning of the pyramidal layer with gliosis) and severe (significant thinning of the pyramidal layer with severe gliosis). The presynaptic degeneration of the molecular layer of the DG and hippocampus was examined on SNAP-25-stained sections. In order to quantify this degeneration, sections stained with SNAP-25 were photographed with Zeiss MRc5 connected to a conventional light microscope at the same condition and the images were linearly transformed into 8-bit gray scale TIFF files. Ten regions of interest were arbitrarily chosen from each area and the average density (0–255) was calculated by using Photoshop® (Adobe Systems Inc). The average density of SNAP-25 immunoreactivity of the outer molecular layer or CA3 relative to that of the inner molecular layer was calculated as a ratio. The presynaptic degeneration was semiquantitatively rated into four grades according to this ratio: normal (>0.90), mild (0.89 ~ 0.75), moderate (0.74 ~ 0.60), severe (<0.60). Ubiquitin/TDP-43-positive cytoplasmic inclusions (UTCIs) were counted and plotted on brain maps at 200-fold magnification by using Neurolucida® (MicroBrightField Inc., Williston, Vermont). Local densities of the UTCIs were calculated in the EC, TEC and granular layer of the DG. Neurofibrillary tangles (NFTs) were plotted with Neurolucida® as well and were semiquantitatively rated for their severity and distribution into six stages in conformity with progression of AD-NFTs in the entorhinal–hippocampal area according to Braak and Braak stageing [4].
The lateral border of the entorhinal region adjacent to the transentorhinal region was defined as the point where the layer II (Pre-α) began to sweep downward following an oblique course [3]. The medial border between the entorhinal cortex and presubiculum was defined as the point of the end of layer V of the EC because this layer is truncated before presubiculum layer II cells could be observed between the entorhinal layer II islands [38]. The lateral extension of the TEC, not well marginated, was defined as the transition area to isocortex where pre-α layer terminated and layer IV was typically emerged at the deep portion of the rhinal or collateral sulcus [3, 5, 12, 17].
Clinical diagnosis was classified as either ALS or ALS with dementia (ALSD) based on their clinical records from the same neurology department of Tokyo Metropolitan Neurological Hospital over approximately 25 years. Cognitive mental symptoms and memory impairments were also assessed [21, 27]. Dementia was assessed with clinical criteria for frontotemporal dementia [26] and AD (NINCDS-ADRDA) [23].
Statistical analyses were performed by using Kruskal–Wallis test and chi-square test. P values <0.05 were regarded as significant.
Results
The clinicopathological findings are summarized in Tables 1 and 2. There were nine men and five women (Table 1, mean age at death: 66.9 ± 8.7 years; range 54–83 years; mean duration of the illness: 37.3 ± 15.6 months; range 19–69 months). Review of clinical records evidenced the presence of dementia in 9 out of the 14 patients, which provided a proof for the clinical diagnosis of ALSD. Among these nine patients with apparent dementia, memory impairments were described in six cases in their clinical records. However, presumptive frontal dysfunction such as decreased word fluency and loss of attention was noted in five out of these six patients with memory impairments (Table 1, cases 2, 6, 9, 10, 14), three out of the six patients exhibited episodic memory deficit reminiscent of AD in early phase of their illness (Table 1, cases 6, 12, 14). Case 14 had been diagnosed as having AD by a psychiatrist before motor neuron symptoms were clinically apparent.
Pathological changes in the TEC, subiculum and DG are shown in Fig. 1 and their schematic representations traced with Neurolucida® are shown in Fig. 2. In the parahippocampal gyrus, neuronal loss and gliosis were found in 11 out of the 14 cases and accentuated in layer II of the central part of the TEC (Figs. 1a–e, 2). Degeneration of the subiculum, accentuated in the area close to CA1, was observed in 13 out of 14 cases (Figs. 1f–j, 2). Neuronal loss was minimal in the CA and granular cells of the DG. TEC degeneration was accompanied by neuronal loss and UTCIs however, the granular cells in the DG exhibited UTCIs but no neuronal loss. In the subiculum close to CA1, neuronal loss was uniformly severe but UTCIs were rare.
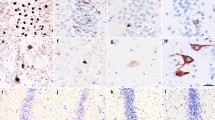
Photomicrographs of the hippocampal lesions in ALS patients (cases 1, 3, 9, 11, 13). a–e TEC on H–E staining. Bars 200 μm. f–j Subiculum on H–E staining. Bars 500 μm. k–o Immunoreactivity for SNAP-25 in the DG, presynaptic degeneration of the DGm was evident in cases with transentorhinal neuronal loss. Bars 100 μm. p–t Immunoreactivity for ubiquitin in the DG. Bars 50 μm. u–y Immunoreactivity for ubiquitin in the TEC. Bars 50 μm. a, f, k, p, u from case 1; UTCIs were observed in the DG and TEC without apparent neuronal loss in these regions and decreased immunoreactivity in the molecular layer of the DG. b, g, l, q, v from case 3; In addition to the UTCIs, degeneration of the subiculum adjacent to CA1 was mild. c, h, m, r, w from case 9; Mild neuronal loss in the transentorhinal region and subiculum was apparent and the presynaptic degeneration of the outer DGm was evident. d, i, n, s, x from case 11; With more and more UTCIs in the DG and TEC, neuronal loss in these areas and presynaptic degeneration of the DGm (star) were more apparent. e, j, o, t, y from case 13; Severe neuronal loss in the TEC and subiculum and presynaptic degeneration of the outer DGm (star) were present. hf Hippocampal fissure, H–E hematoxylin and eosin, il inner layer, ol outer layer, Sp pyramidal layer of the subiculum
Schema representing pathologies of the coronal sections of the hippocampal formation in 14 ALS patients (a–n: cases 1–14). Each red circular dot denotes a UTCI and each green triangular dot denotes a NFT. The severely affected areas are indicated with shading by using different colors (grey: degeneration of the subiculum and entorhinal/transentorhinal regions, purple: presynaptic degeneration of the molecular layer of the dentate gyrus and cornu ammonis)
The presynaptic degeneration of the molecular layer of the DG was noticeable in its outer layer (Fig. 1k–o) and its severity was correlated to both the cortical degeneration (P < 0.001, Table 3) and the inclusion density in the TEC (P = 0.016, Table 3), while its correlation to the inclusion density in granular cells of the DG was not significant (P = 0.104, Table 3). This presynaptic degeneration started from medial region of the dentate fascia and progressed to the lateral area. Inner molecular layer of the DG was well preserved even in the most affected case (Fig. 1k–o).
Distribution and morphology of the ubiquitin-positive inclusions were compared with those of TDP-43-positive inclusions (Fig. 3a, b). UTCIs were observed in all 14 cases in the TEC and granular cells in the DG (Figs. 1p–y, 2). There were a few inclusions in the subiculum and CA1. While these inclusions were more frequent in superficial layer of the TEC, those in the granular cells in the DG distributed uniformly.
a–c Photomicrograph of immunostaining for TDP-43, ubiquitin and AT-8. a Cytoplasmic inclusions immunoreactive for ubiquitin in the TEC (case 11), b TDP-43-positive inclusions in the TEC (case 6), c NFT for AT-8 in the EC case 10. d–o Immunofluorescent signals of the EC-TEC in case 10 are shown. d–g Double-label immunofluorescence with anti-TDP-43 (d) and anti-ubiquitin (e) showed colocalization (g) without tau (Alz-50) (f) in some layer II neurons of the TEC. h–k NFT-like structure which was immunoreacted with ubiquitin (i) and tau (j) was partially immuno-positive for TDP-43 (h) in transient area of the EC-TEC. l–o Typical NFTs showed immuno-negative for TDP-43 (l) but immuno-positive for ubiquitin (m) and Alz-50 (n) in layer II of the EC. Arrowheads: typical motor neuron disease inclusions (ubiquitin/TDP-43-positive cytoplasmic inclusions), arrows: typical NFTs, asterisks: tangle-like structures with immunoreactive for not only tau and ubiquitin but also TDP-43. a, b, d–o Bars 20 μm, c Bar 50 μm. a, d, h, l anti-TDP-43, b, e, i, m anti-ubiquitin, f, j, n: anti-Alz-50, g, k, o merge, c: AT-8
Neurofibrillary tangles were almost independent of UTCIs and were mostly seen in layer III of the TEC and in layer II of the EC (Figs. 2, 3d–g, l–o). Typical NFTs showed no immunoreactivity for TDP-43, however, a small number of Alz-50 positive-pyramidal neurons in layer II–III of the EC were partially immunopositive for TDP-43 (Fig. 3h–k). Amyloid senile plaques were absent on silver-stained sections in our series. The stage of AD pathology was not correlated to that of neuronal loss in the TEC (P = 0.622, Table 3).
Brain weight had a tendency to correlate to the AD-NFT staging without statistical significance (P = 0.079, Table 3) but apparently not to the staging of the ALS-hippocampal pathologies (P = 0.747, Table 3). Duration of the illness was not correlated to the ALS-hippocampal staging (P = 0.400, Table 3).
Discussion
Cytopathology and distribution
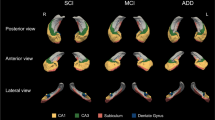
In the hippocampal formation of the ALS patients in the present study, UTCIs commonly appear in superficial layer in the TEC and granular cells in the DG. It is interesting that these inclusions are frequently observed in these two areas that have a close neuronal connection, the so-called perforant pathway [14] (Fig. 4a). Degeneration of the outer molecular layer of the DG was correlated to the neuronal degeneration (P < 0.001) and density of UTCIs (P = 0.017) in the TEC. Its relation to UTCI density in the neighboring granular cells of the DG was not statistically significant (P = 0.104). These suggest that degeneration of the TEC with UTCIs and molecular layer of the DG are possibly linked to each other via the perforant pathway [33]. In contrast, the lack of statistically significant correlation between UTCIs in the granular cells of the DG and presynaptic degeneration is explained either by an enhanced variation of the density of UTCIs, dependent of the area dimension, which is greatly influenced by individual variations of cortical convolution and fixation or by the fact that these two structures belong to different neuronal groups. The non-stereological approach might have enhanced the variability as well. The intrahippocampal projections through the perforant pathway have been clarified by various tracing methods in animals, especially in primates (Fig. 4a) [40]. The molecular layer of the DG is morphologically divided into three layers (Fig. 5). Inner molecular layer of the DG is innervated by CA4 neurons [18], which was less involved in our series. Intermediate layer is the target of projection fibers from layer II of the medial EC where NFTs are often observed (as in cases 4 and 10) (Fig. 3c) [40]. In AD, selective vulnerability of this intermediate layer with preferential formation of senile plaques has been reported (Figs. 4c, 5) [10, 16]. Innervation to the outer one-third layer is from layer II of the lateral entorhinal region in primates [37, 40]. We clarified that this outer layer, less susceptible in AD patients, is involved in ALS patients (Figs. 4b, 5). Although the anatomical correlation between the ‘lateral entorhinal cortex’ in animals (Fig. 4a) [40] and the ‘transentorhinal region’ in human (Fig. 4b, c) [3] remain obscure, early and selective involvement of the superficial TEC with subsequent degeneration in the outer layer of the DG, as we demonstrated in ALS patients, suggests that the so-called transentorhinal region in human might be anatomically a close equivalent to the lateral EC in primates. Conversely, medial EC in primates, known to project to the intermediate layer of the DG [40], may be a counterpart of human EC because appearance of AD-NFTs in this EC is associated with the selective degeneration with senile plaques of the intermediate layer of the DG [10, 16]. It is then possible to consider that two different projections in the so-called perforant pathway are differently involved in AD and ALS; i.e. layer II of the EC (medial EC in primates)-intermediate molecular layer of the DG for AD and layer II of the TEC (lateral EC in primates)-outer molecular layer of the DG for ALS (Fig. 5). Furthermore, these disease-specific involvements were corroborated by site-specific appearance of disease-specific inclusions even in cases with both AD and ALS pathologies (cases 1, 3, 4, 5, 10, 11, 12). Namely, NFTs, preferentially present in the EC, were positive for tau but negative for TDP-43, while motor neuron disease inclusions, preferentially present in the TEC, exhibited the reversed staining profile (negative for tau but positive for TDP-43) [6]. It is interesting that colocalization of tau and TDP-43 epitopes [1] was limited to the boundary between the EC and TEC in case 10 (Fig. 3h–k).
a The schematic representation of intrahippocampal connections in animals. Each colored area represents different neuronal projection. b In ALS patients, layer II of the TEC → outer lamina of the DG and CA3 system is preferentially involved. Subiculum, restricted area close to CA1, is also involved. c The schematic representation of the severity and spreading of AD pathology in the hippocampus described in previous reports [4, 10, 16] was recapitulated in our series with concomitant ALS pathology. f Fornix, LV lateral ventricle
The schema shows terminal laminae of the DGm from the EC, TEC and cornu ammonis. Inner DGm is innervated by projection from CA4. Intermediate layer of the DGm is innervated by the fibers from the EC through the perforant pathway. The perforant pathway from the TEC terminated in the outer one-third of the DGm
Correlation between neuronal loss and intracytoplasmic inclusion (NFTs) as seen in AD brains [11] was observed in the TEC of our ALS patients, where the severity of neuronal loss and the density of UTCIs were correlated (Tables 1, 2). In contrast this correlation was not consistent in the subiculum with predominant neuronal loss but a few UTCIs or in the DG with predominant UTCIs without significant neuronal loss. This regional variation suggests that these two features (UTCIs and neuronal loss) are not necessarily linked or differently regulated in ALS. Indeed, discrepancy between neuronal loss and UTCIs [29] and discrepancy between ubiquitin and TDP-43 immunoreactivity [20] are now being recognized.
Staging
In spite of the awareness of the hippocampal involvement at multiple sites in ALS patients with or without dementia, little has been known about how these lesions evolve during progression of ALS. Once selective involvement of the perforant pathway with concomitant apparition of UTCIs is established as we demonstrated in the previous section, it will provide an axis along which degeneration progresses. Moreover, a wide range of severity of lesions in our series made it possible to classify these cases into three stages, possibly representing the disease-specific progression of hippocampal lesions. UTCIs first appear in the TEC and granular cells in the DG and neurons in the subiculum are simultaneously affected in the early phase. TEC degeneration was invisible in this stage (stage I: inclusion stage) (Tables 1, 2: cases 1–3; Fig. 2). Gliosis accompanied with some degree of spongiotic changes limited to layer II of the TEC is gradually apparent, followed by presynaptic degeneration of the molecular layer of the DG through the perforant pathway (stage II: early perforant stage) (Tables 1, 2: cases 4–10; Fig. 2). In a more advanced stage, involvement of the presynaptic degeneration in the molecular layer in the DG is moderate to severe with approximately equivalent degeneration of the TEC (stage III: advanced perforant stage) (Tables 1, 2: cases 11–14; Fig. 2). Prior to degeneration of the TEC and presynaptic degeneration in the DG, neuronal loss of the subiculum was evident in our series, which suggests that involvement of the subiculum is not completely dependent on that of the perforant pathway.
Correlation between the brain weight and the AD stage exhibited a non-significant trend (P = 0.079; Table 3), which indicates that the AD progression of the whole brain is possibly in parallel with the AD lesions in the hippocampus. The absence of this correlation with ALS staging (P = 0.747; Table 3) suggests that the hippocampal lesions of ALS are, at least partly, independent of the whole brain involvements due to aging or AD lesions, for example.
Our ALS-hippocampal staging failed to exhibit correlation to the duration of their illness (P = 0.400; Table 3) because the illness is often terminated by rapidly progressive motor weakness even when extra-motor involvement remain slight as seen with most ALS patients without cognitive decline.
Clinicopathological correlation
The perforant pathway, which is thought to be related to episodic and contextual memory functions, is commonly involved in AD patients [10, 16, 24]. In our series, three out of the nine ALSD patients (Table 1, cases 6, 12 and 14) were described as having episodic memory deficits in early phase of their illness as seen in AD. Because AD lesions were at most mild in these three patients (Table 1), more evident ALS lesions in the hippocampus were responsible for these episodic and amnesic memory deficits noted in these patients unless otherwise explained. Amnesic memory impairments have been reported in frontotemporal dementia with motor neuron disease inclusions (FTD-MNDI) [13, 15, 19]. It is then possible to speculate that patients with ALSD develop similar amnesic memory impairments because ALSD and FTD-MNDI are sharing a number of cytopathological features. We do not yet know whether memory deficits related to these ALS lesions are clinically distinguishable from those related to AD lesions. Through this study, however, we became aware that the hippocampal lesions involving connections between the TEC and the outer layer of the DG are characteristic of ALS. These are clearly distinguishable from AD-specific hippocampal lesions involving different connections between the EC and the intermediate layer of the DG. It remains to be clarified whether these disease-specific distinctions in the perforant pathway lesions exhibit clinically distinguishable differences in memory deficits between ALS and AD. Even if motor weakness and loss of spontaneity in ALS patients may often hamper detailed neuropsychological evaluation, an enhanced awareness of these disease-specific differences may be useful in deciphering memory deficits and their possible relations to hippocampal lesions. It is reasonable to suppose that the clinical presentation including memory deficits could be disease-specific as well if these are tightly related to hippocampal lesions, which are disease-specific as we demonstrated in this study.
Abbreviations
- AD:
-
Alzheimer disease
- ALS:
-
Amyotrophic lateral sclerosis
- ALSD:
-
Amyotrophic lateral sclerosis with dementia
- CA:
-
Cornu ammonis
- DG:
-
Dentate gyrus
- EC:
-
Entorhinal cortex
- FTD/MND:
-
Frontotemporal dementia with motor neuron disease
- NFTs:
-
Neurofibrillary tangles
- TEC:
-
Transentorhinal cortex
- UTCIs:
-
Ubiquitin/TDP-43-positive cytoplasmic inclusions
References
Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW (2007) Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol (Berl) 113:245–252
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Braak H (1980) Architechtonics of human telencephalic cortex. Springer, Berlin
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82:239–259
Braak H, Braak E (1992) The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res 15:6–31
Cairns NJ, Neumann M, Bigio EH et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Damasio AR (1984) The anatomic basis of memory disorders. Semin Neurol 4:226–228
Davies P (1992) Alz 50 as a reagent to assess animal models of Alzheimer’s disease. Neurobiol Aging 13:613–614
Dickson DW, Ruan D, Crystal H et al (1991) Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer’s disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology 41:1402–1409
Duyckaerts C, Colle MA, Seilhean D, Hauw JJ (1998) Laminar spongiosis of the dentate gyrus: a sign of disconnection, present in cases of severe Alzheimer’s disease. Acta Neuropathol (Berl) 95:413–420
Duyckaerts C, Potier MC, Delatour B (2008) Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol (Berl) 115:5–38
Garey (1999) Common features in cortical architectonics. Brodman’s ‘Localization in the cerebral cortex’. Imperial College Press, London, pp 107–174
Graham A, Davies R, Xuereb J et al (2005) Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain 128:597–605
Hjorth-Simonsen A, Jeune B (1972) Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol 144:215–232
Hodges JR, Davies RR, Xuereb JH et al (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56:399–406
Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR (1986) Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol 20:472–481
Insausti R, Amaral DG (2004) Hippocampal formation. In: Paxinos G, Mai J (eds) The human nervous system. Academic Press, New York, pp 871–914
Ishizuka N, Weber J, Amaral DG (1990) Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol 295:580–623
Jackson M, Lennox G, Lowe J (1996) Motor neurone disease-inclusion dementia. Neurodegeneration 5:339–350
Josephs KA, Lin WL, Ahmed Z, Stroh DA, Graff-Radford NR, Dickson DW (2008) Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol 116:159–167
Lakerveld J, Kotchoubey B, Kubler A (2008) Cognitive function in patients with late stage amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 79:25–29
Lippa CF (2004) Synaptophysin immunoreactivity in Pick’s disease: comparison with Alzheimer’s disease and dementia with Lewy bodies. Am J Alzheimers Dis Other Demen 19:341–344
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Mizutani T, Kasahara M (1995) Degeneration of the intrahippocampal routes of the perforant and alvear pathways in senile dementia of Alzheimer type. Neurosci Lett 184:141–144
Nakano I, Iwatsubo T, Hashizume Y, Mizutani T, Mannen T (1992) Amyotrophic lateral sclerosis with dementia lesions in the apical cortex and some deeper structures of the temporal lobes. Neuropathology 12:69–77
Neary D, Snowden JS, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N (1990) Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 53:23–32
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishihira Y, Tan CF, Onodera O et al (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116:169–182
Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Oyler GA, Higgins GA, Hart RA et al (1989) The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109:3039–3052
Squire LR, Zola-Morgan S (1983) The neurology of memory: the case for correspondance between the findings for human and nonhuman primate. Academic Press, New York
Takeda T, Uchihara T, Mochizuki Y, Mizutani T, Iwata M (2007) Memory deficits in amyotrophic lateral sclerosis patients with dementia and degeneration of the perforant pathway. A clinicopathological study. J Neurol Sci 260:225–230
Tolnay M, Schwietert M, Monsch AU, Staehelin HB, Langui D, Probst A (1997) Argyrophilic grain disease: distribution of grains in patients with and without dementia. Acta Neuropathol 94:353–358
Uchihara T, Nakamura A, Nakayama H et al (2003) Triple immunofluorolabeling with two rabbit polyclonal antibodies and a mouse monoclonal antibody allowing three-dimensional analysis of cotton wool plaques in Alzheimer disease. J Histochem Cytochem 51:1201–1206
Uchihara T, Tsuchiya K (2008) Neuropathology of Pick body disease. In: Duyckaerts C, Litvan I (eds) Handbook of clinical neurology. Elsevier, Amsterdam, pp 395–410
Van Hoesen GW, Pandya DN (1975) Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res 95:39–59
West MJ, Slomianka L (1998) Total number of neurons in the layers of the human entorhinal cortex. Hippocampus 8:69–82
Wightman G, Anderson VE, Martin J et al (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Witter MP, Amaral DG (1991) Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol 307:437–459
Acknowledgments
We thank Dr. Norio Ishizuka, Department of Brain Structure, Tokyo Metropolitan Institute for Neuroscience, Dr. Yoko Mochizuki, Department of Pathology, Tokyo Metropolitan Neurological Hospital, Dr. Naoto Uyama, Department of Neurology, Tokyo Metropolitan Neurological Hospital and Dr. Masako Yamazaki, Department of Neurology, Tokyo Women’s Medical University for their assistance and advice for clinicopathological analyses. We are also grateful to Mr. Yoshitomo Umitsu and Ms. Ayako Nakamura for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeda, T., Uchihara, T., Arai, N. et al. Progression of hippocampal degeneration in amyotrophic lateral sclerosis with or without memory impairment: distinction from Alzheimer disease. Acta Neuropathol 117, 35–44 (2009). https://doi.org/10.1007/s00401-008-0447-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0447-2