Abstract
Ventricular arrhythmia arising from the outflow tracts can manifest itself as frequent premature ventricular complexes (PVCs), salvos of ventricular tachycardia (VT), and/or sustained VT. It is amenable to management with medication and catheter ablation without need for an intracardiac defibrillator. The electrocardiogram (ECG) is a crucial tool in the management of these patients as it is can help localize the site of origin, thereby helping guide the electrophysiologist. An appreciation of the unique anatomy of the outflow tracts as well as their relationships with the surrounding structures is essential in interpreting the ECG. In this review, we examine the ECG features of the various outflow tract arrhythmia morphologies with a focus on anatomy and provide an approach to the ablation of these abnormal rhythms.
Zusammenfassung
Die ventrikuläre Arrhythmie aus einem Ausflusstrakt kann sich als häufige vorzeitige ventrikuläre Komplexe (PVC), Salven von ventrikulären Tachykardien (VT) und/oder anhaltende VT manifestieren. Sie ist mittels medikamentöser Therapie und Katheterablation behandelbar, ohne dass ein intrakardialer Defibrillator notwendig ist. Das Elektrokardiogramm (EKG) ist ein wichtiges Instrument bei der Behandlung dieser Patienten, da es bei der Lokalisierung des Ursprungsorts helfen kann und damit dem Elektrophysiologen leitende Hilfestellung bietet. Die Beachtung der einzigartigen Anatomie der Ausflusstrakte sowie ihrer Beziehung zu den umliegenden Strukturen ist für die Interpretation des EKG essenziell. In diesem Review untersuchen wir die EKG-Charakteristika der unterschiedlichen Ausflusstraktarrhythmie-Morphologien mit dem Fokus auf die Anatomie und stellen ein Verfahren für die Ablation dieser Arrhythmien vor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Outflow tract tachycardia (OT-VT) classically presents in young females (30–50 years) without any underlying structural heart disease and is frequently triggered by stress and exercise [1]. It has three distinct phenotypes, namely, monomorphic premature ventricular contractions (PVCs), nonsustained monomorphic ventricular tachycardia, and sustained VT [2]. Although the left and right outflow tracts (LVOT and RVOT, respectively) are the most common sites for VT, with the right more common (60–80%), there are other variants of OT-VT including origins from the pulmonic valve [3], the aortic sinus of Valsalva [4, 5], near the His bundle [6], and the epicardium of the ventricles [7]. Preliminary localization from surface ECG analysis is critical to aid in ablation [8]. In this paper, we will discuss the basic and advanced interpretation of the ECG in OT-VT as well as outline the approach to mapping these arrhythmias.
Anatomy
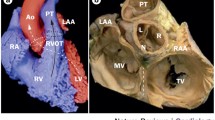
The OT are structures located at the base of the heart [9] (Video 1). The proximal RVOT begins at the superior margin of the tricuspid annulus and lies to the right of the LVOT here. As the RVOT extends superiorly, the mid and distal RVOT wrap around the LVOT and therefore contrary to its name the RVOT is located anteriorly and more leftward than the LVOT. The structure therefore immediately anterior to the aortic valve is the posterior muscular infundibular portion of the RVOT (Fig. 1). The pulmonic valve is located to the left and anterior (5–10 cm) to the aortic valve. The posterior RVOT is immediately anterior to the right coronary sinus of Valsalva cusp (RCSoV) and a portion of the left coronary sinus of Valsalva (LCSoV). Therefore, the posterior RVOT has no true epicardial surface in contrast to the anterior RVOT. The LVOT is not entirely muscular (like the RVOT) and has a fibrous portion. There is no infundibulum below the aortic valve. This difference in anatomy can be attributed to the differential absorption of the subvalvular infudibulae below the aortic (AoV) and pulmonary valves (PV) [10]. Extensions of muscle sleeves are noted above the valves in both the outflow tracts (LCSoV 24%, RCSoV 54% [11], PV 74% [12]).
The aortic valve is the central valve of the heart and has an intimate relation with all the other valves of the heart. The membranous interventricular septum lies just below the junction of the RCSoV and the noncoronary sinus of Valsalva (NCSoV). This corresponds to the junction of the anterior and septal leaflet of the tricuspid valve on the right side. The aortic valve annulus is anatomically not a true annulus. It is a composite junction between the left ventricle and the aorta. The left and posterior part of this annulus is fibrous and is structurally continuous with the anterior leaflet of the mitral valve [13]. Apart from the anterior RVOT, in structurally normal hearts, all the other sites have proximity to the coronary arteries. As the pulmonary valve is superior to the aortic valve, the posterior RVOT is located at the level of the aortic cusps and coronary artery ostia. The left main coronary artery runs posterior to the distal RVOT and can be at risk of injury due to ablation in its posteroseptal aspect [14].
The region on the epicardial aspect bounded by the division of the left main coronary artery (LM) into its main branches and the great cardiac vein (GCV) laterally forms the triangle of Brocq and Mouchet [15]. This surface is close to the anteroseptal RVOT and the LCSoV forming the highest portion of the LV epicardium (LV summit).
Generic ECG
Any VT that originates from the LVOT and RVOT will tend to have an inferior axis (tall positive R waves in leads II, III, and aVF) with negative QS complexes in leads aVR and aVL [8]. If the origin is from the RVOT, the ECG will typically display a left bundle branch block (LBBB) and R wave transition at lead V4 or greater, while LVOT origin will typically have a right bundle branch block (RBBB) with R wave transition leads at V3 or earlier [16].
Importance of lead V1
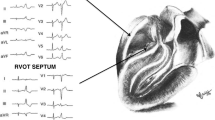
Lead V1 is a right sided and anterior chest lead. It sits directly above and very close to the anterior RVOT. Therefore, the vector of activation from the anterior RVOT (directly behind V1) will be away from this lead resulting in a predominately negative complex in lead V1 (QS wave, left bundle branch morphology). The presence of an R wave in lead V1 should raise suspicion of the possibility of a more posterior origin owing to anteriorly directed forces and myocardial activation towards V1 [17] including the posterior RVOT, RCSoV/LCSoV or aorto-mitral continuity (AMC).A posterior RVOT origin therefore will have a small but noticeable R wave in V1, while origin in either sinus of Valsalva or the AMC will have a larger R wave due to their more posterior location (Fig. 2).
Importance of lead I
Lead I being a left sided lead provides valuable insight in differentiating between the various sites of origin of OT-VT with an R wave in lead V1 [18]. Presence of a large R wave in lead I is suggestive of an origin from the right and posterior RVOT or the anterior and right LVOT (RCSoV). A biphasic QRS in lead I with predominant S wave (R/R + S ratio ≤ 0.7) localizes the origin to the peri PV region. This corresponds to the anatomical location of these sites with the peri PV region being located more leftward than the posterior RVOT or the RCSoV. In keeping with its leftward and anterior location, the anterior RVOT has the smallest R wave (R/R + S ratio < 0.4) in lead I, but the absence of R wave in lead V1 makes the distinction relatively easier (Fig. 3)
Reconstructed computed tomographic image showing the anatomy and interrelationship between the outflow tracts. RVOT right ventricular outflow tract; LVOT left ventricular outflow tract; RV right ventricle; LV left ventricle; PA pulmonary artery; SoV sinuses of Valsalva; CS coronary sinus; MCV middle cardiac vein; GCV great cardiac vein, RAO right anterior oblique; LAO left anterior oblique
Epicardial versus endocardial origin
PVCs arising from the epicardial surface at the LV summit show slurring of the initial portion of the QRS complex which can be quantified by use of the maximum deflection index (MDI: shortest interval to the maximal positive or negative deflection divided by the QRS duration) ≥55 ms. Another useful clue is the presence of a ‘pattern break’ in the precordial lead progression of the R wave consisting of an abrupt loss of the R wave in lead V2 coinciding with a septal epicardial origin of the PVC (opposite lead V2) [19].
In addition, a QS pattern in lead I also suggests an epicardial origin over an endocardial origin. This is attributed to the absence of any vector towards lead I when the origin is epicardial as compared to an endocardial site where the depolarization of the myocardium begins endocardially and is directed to the surface of the heart traveling through the transmural myocardial thickness [20].
No specific ECG features differentiating intramural origin of PVCs from endocardial origin have been described.
Other unique features in the origin of PVCs from various sites are summarized in Table 1.
Approach to mapping
Role of ECG and Holter
PVC mapping is tailored to the mechanism of the underlying PVC. It begins with the recording of the PVC morphology and estimation of the PVC count on surface ECG recordings and 24–48 h Holter records which are extremely useful in the following ways:
-
Estimation of total PVC burden
-
Identification of the predominant PVC morphology
-
Comparative assessment to identify morphological variants of PVC
-
Assessment of the sinus beat–PVC coupling interval (Table 2 )
Role of preoperative imaging
Preoperative imaging with magnetic resonance imaging (MRI) can help in the identification of abnormal substrate in arrhythmogenic right ventricular dysplasia (ARVC), subepicardial scar in the RVOT in athletes having ventricular arrhythmias and periaortic scar in elderly patients due to localized fibrosis. In patients with surgically repaired conotruncal anomalies, it provides detailed information about the variant coronary anatomy and cardiac structure. Functional mapping using positron emission tomography (PET) detects areas of increased glucose uptake in suspected inflammatory cardiomyopathies [30].
Catheterization laboratory
Anesthesia has an unquantified role in PVC suppression and should be used minimally at the outset. If PVC numbers are low, pacing maneuvers from ventricle or the atrium, adrenergic medications, caffeine, and calcium may be used to induce PVCs. Caution may need to be exercised in the patients with coronary artery disease, chronic kidney disease, and the elderly.
After catheters have been placed, an assessment of the site of PVC origin is done by triangulating its location (Fig. 4). A suitable stable reference either off the surface QRS or off an intracardiac catheter is then chosen. The window of interest can be initially broad to include an interval that is split in half before and after the reference and encompassing the entire coupling interval of the PVC. Care to map the same PVC is important with appropriate exclusion of fused or variant PVC morphologies from the map.
Mapping of a premature ventricular complex (PVC) with triangulation of intracardiac signals based on catheter position and relative anatomy. a During the PVC, the timing of the far field (FF) ventricular signals on CS 1.2 corresponds to the FF ventricular signals on the His (H) catheter indicating that neither of these sites are early and a third site along a line connecting these is the site of origin. The ventricular signals on the right ventricular (RV) catheter is late. The FF signals on the ablation catheter in the RV basally is the earliest signal during the PVC indicating that the His location is also late, but a nearby site is the early site. b The right anterior oblique (RAO) and left anterior oblique (LAO) views show the catheter positions at the site of final ablation of the PVC. The distal tip of the ablation catheter lies along a line between the tip of the His catheter and the tip of the coronary sinus (CS) catheter in both views validating the observation made in a
A high-density map at the site of interest is important with analysis of the prepotentials and their timing in sinus rhythm. There may be reversal of signals with the prepotential coming earlier than the PVC (Fig. 5).
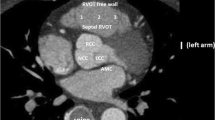
Intraprocedural echocardiography is useful to assess regional anatomy and contact (Fig. 6). If the PVC burden is low, then pace mapping may be a useful technique. Potential pitfalls include capture of tissue at remote sites, inability to capture local tissue, and the phenomenon of preferential conduction [31]. Pace mapping at an output just above the threshold at the local site with adequate contact is important.
Imaging of the left ventricular outflow tract (LVOT). a,b Reconstructed computed tomographic images of the LVOT showing the relation of the LVOT to the mitral valve (M). c Intracardiac echocardiographic image showing the ablation catheter beneath the left coronary sinus of Valsalva: ablation catheter tip (white arrow), left coronary sinus of Valsalva (black arrow). d Biplanar fluoroscopic image of the catheter position in image: ablation catheter tip (white arrow)
It is important to map anatomically contiguous regions since the unique anatomy of the outflow tracts predispose to functionally disparate structures contributing to the production of similar PVCs. Biplanar fluoroscopic imaging may help with the understanding of the regional anatomy during mapping (Fig. 7). Reentry may involve components of all these structures and may need to be mapped separately. Several theories including scar formation [3], loss of electronic interaction with surrounding myocardium in cul-de-sac regions [32] are proposed for reentry. Clues for identification of reentry during mapping include mapping >70% of coupling interval of PVC, reset of PVC with fusion, long stimulus to R wave during pace mapping, and suppression of PVC using subthreshold stimuli. Additional clues may include multiple closely related morphologies, inducibility of PVC using programmed ventricular stimulation and presence of fragmented electrograms in areas of interest [29].
Utility of biplanar views in the mapping of the outflow tract and anatomically contiguous regions. a Relation of the AIC-GCV junction and the aortomitral continuity. b Relation of the of the AIC-GCV junction and the anterior RVOT. RAO right anterior oblique; LAO left anterior oblique; RVOT right ventricular outflow tract, AIC-GCV anterior interventricular vein–great cardiac vein
After mapping the site of origin for focal PVCs or the circuit for reentrant PVCs, it is important to assess the safety of ablation [14, 33]. A routine angiogram to demarcate the course of the vessels, keeping the ablation catheter at the proposed site of energy delivery, is the safest way to gauge the risk. Ablation from multiple sites may be needed especially if there is proximity to coronary vessels (Fig. 8). In case suppression of PVCs is not achievable by this technique, it may be of use to revisit the mapping to identify alternate sites or reevaluate the decision to ablate in consultation with the patient. Ablation within the coronary venous system may be difficult owing to increased impedance and poor delivery of power in this region along with concerns of causing damage to the arterial system. For PVCs arising from the LV summit, success is usually dependent on the proximity to the coronary arteries with greater success achieved at the more lateral regions away from the bifurcation of the LM (Fig. 8).
Proximity of the coronary vessels to the posteroseptal RVOT. a Ablation catheter in the posteroseptal RVOT close to the origin of the left main coronary artery (LM) and the left anterior descending artery. b Reconstructed computed tomographic image showing the proximity of the coronary arteries and the posteroseptal RVOT. RAO right anterior oblique; LAO Left anterior oblique; RVOT right ventricular outflow tract, AIC-GCV anterior interventricular vein–great cardiac vein; LV left ventricle; RV right ventricle; LCX left circumflex artery; LAD left anterior descending artery; GCV great cardiac vein
Intramural origin of PVCs can be recognized by the absence of a clear early site of activation during mapping >10 ms ahead of the PVC, poor pace maps, early far field prepotentials in the venous system or at the AMC, transient suppression of PVC despite ablation from corresponding sites at both sides of the muscle [34]. Potential solutions may include ablating at all sites of relative early activation or use of bipolar ablation techniques or use of venous tributaries for ablation.
Origin from the conus papillary muscle should be suspected when there are spatially displaced sites of early signals within the RVOT with prepotentials suggestive of conduction tissue when mapping. Adequate contact is achieved by use of intracardiac echocardiography during mapping and ablation. Ventricular fibrillation may be induced when ablating at this site [22].
Sites lower in the outflow tracts and in the basal ventricular septum carry the potential to cause atrioventricular blocks while ablation [35]. Cryomapping and ablation may be preferable to use of radiofrequency energy here [36]. At the AMC, prepotentials during mapping are associated with narrower QRS duration of the PVC, poorer pace map scores, and short local electrogram to QRS duration (VEGM-QRS) at sites of successful ablation [37].
Post ablation, it is important to check for inducibility of sustained monomorphic arrhythmia localized to the site of ablation. If present, additional ablation lesions to ensure noninducibility should also be given.
Special situations
ARVC.
Testing for sustained VT and additional substrate mapping of the RV and LV (if indicated) to ablate areas of abnormal substrate may be needed.
Congenital heart disease.
Inaccessible areas owing to recreation of the outflow tract by synthetic material or xenograft tissue may pose special challenges necessitating mapping at contiguous sites from the adjacent cardiac chamber or puncturing baffles or homografts to gain access to the underlying native cardiac tissue.
Conclusion
Arrhythmias arising from the outflow tract may either be independent in their etiology or a part of a larger spectrum of disease. They are usually calcium-dependent for their production; however, alternate mechanisms are currently being recognized. ECG provides a clue to the site of origin and the success rates of ablation are high with low incidence of major complications.
References
Nakagawa M et al (2002) Gender differences in various types of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol 13(7):633–638
Kim RJ et al (2007) Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 49(20):2035–2043
Mulpuru SK et al (2014) Atypical variants of right ventricular outflow arrhythmias. J Cardiovasc Electrophysiol 25(12):1321–1327
Kanagaratnam L et al (2001) Ventricular tachycardias arising from the aortic sinus of valsalva: an under-recognized variant of left outflow tract ventricular tachycardia. J Am Coll Cardiol 37(5):1408–1414
Ouyang F et al (2002) Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol 39(3):500–508
Yamauchi Y et al (2005) Electrocardiographic characteristics of repetitive monomorphic right ventricular tachycardia originating near the His-bundle. J Cardiovasc Electrophysiol 16(10):1041–1048
Chun KR et al (2007) Left ventricular outflow tract tachycardia including ventricular tachycardia from the aortic cusps and epicardial ventricular tachycardia. Herz 32(3):226–232
Asirvatham SJ (2009) Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol 20(8):955–968
Walmsley R (1979) Anatomy of left ventricular outflow tract. Br Heart J 41(3):263–267
Boukens BJ, Coronel R, Christoffels VM (2016) Embryonic development of the right ventricular outflow tract and arrhythmias. Heart Rhythm 13(2):616–622
Gami AS et al (2011) Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 30(1):5–15
Liu CF et al (2014) Ubiquitous myocardial extensions into the pulmonary artery demonstrated by integrated intracardiac echocardiography and electroanatomic mapping: changing the paradigm of idiopathic right ventricular outflow tract arrhythmias. Circ Arrhythm Electrophysiol 7(4):691–700
Sutton JP 3rd, Ho SY, Anderson RH (1995) The forgotten interleaflet triangles: a review of the surgical anatomy of the aortic valve. Ann Thorac Surg 59(2):419–427
Walsh KA, Fahy GJ (2014) Anatomy of the left main coronary artery of particular relevance to ablation of left atrial and outflow tract arrhythmias. Heart Rhythm 11(12):2231–2238
Halm MA (2016) Specific needs, concerns, strategies and advice of caregivers after coronary artery bypass surgery. Heart Lung 45(5):416–422
Asirvatham SJ, Cha Y‑M, Friedman PA (2013) Mayo Clinic electrophysiology manual. Mayo Clinic Scientific Press/Oxford University Press, New York
Cheng Z et al (2013) The R‑wave deflection interval in lead V3 combining with R‑wave amplitude index in lead V1: a new surface ECG algorithm for distinguishing left from right ventricular outflow tract tachycardia origin in patients with transitional lead at V3. Int J Cardiol 168(2):1342–1348
Ebrille E et al (2014) Distinguishing ventricular arrhythmia originating from the right coronary cusp, peripulmonic valve area, and the right ventricular outflow tract: utility of lead I. J Cardiovasc Electrophysiol 25(4):404–410
Enriquez A et al (2017) How to map and ablate left ventricular summit arrhythmias. Heart Rhythm 14(1):141–148
Nagashima K et al (2014) Ventricular arrhythmias near the distal great cardiac vein: challenging arrhythmia for ablation. Circ Arrhythm Electrophysiol 7(5):906–912
Komatsu Y et al (2012) Two distinct electrocardiographic forms of idiopathic ventricular arrhythmia originating in the vicinity of the His bundle. Europace 14(12):1778–1785
Hai JJ et al (2014) Endocavitary structures in the outflow tract: anatomy and electrophysiology of the conus papillary muscles. J Cardiovasc Electrophysiol 25(1):94–98
Callans DJ et al (1997) Repetitive monomorphic tachycardia from the left ventricular outflow tract: electrocardiographic patterns consistent with a left ventricular site of origin. J Am Coll Cardiol 29(5):1023–1027
Bala R et al (2010) Electrocardiographic and electrophysiologic features of ventricular arrhythmias originating from the right/left coronary cusp commissure. Heart Rhythm 7(3):312–322
Lin D et al (2008) Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm 5(5):663–669
Hai JJ et al (2015) Electrophysiologic characteristics of ventricular arrhythmias arising from the aortic mitral continuity-potential role of the conduction system. J Cardiovasc Electrophysiol 26(2):158–163
Ouyang F et al (2014) Ventricular arrhythmias arising from the left ventricular outflow tract below the aortic sinus cusps: mapping and catheter ablation via transseptal approach and electrocardiographic characteristics. Circ Arrhythm Electrophysiol 7(3):445–455
Tada H et al (2005) Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 45(6):877–886
Nagashima K et al (2014) Reentrant ventricular tachycardia originating from the periaortic region in the absence of overt structural heart disease. Circ Arrhythm Electrophysiol 7(1):99–106
Hamzeh N et al (2015) Pathophysiology and clinical management of cardiac sarcoidosis. Nat Rev Cardiol 12(5):278–288
Yamada T, Litovsky SH, Kay GN (2008) The left ventricular ostium: an anatomic concept relevant to idiopathic ventricular arrhythmias. Circ Arrhythm Electrophysiol 1(5):396–404
Bradfield JS et al (2014) Coupling interval variability differentiates ventricular ectopic complexes arising in the aortic sinus of valsalva and great cardiac vein from other sources: mechanistic and arrhythmic risk implications. J Am Coll Cardiol 63(20):2151–2158
Benhayon D et al (2017) Catheter Ablation in the Right Ventricular Outflow Tract Associated With Occlusion of Left Anterior Descending Coronary Artery. Journal of cardiovascular electrophysiology 28(3):347–350
Yamada T et al (2015) Radiofrequency catheter ablation of idiopathic ventricular arrhythmias originating from intramural foci in the left ventricular outflow tract: efficacy of sequential versus simultaneous unipolar catheter ablation. Circ Arrhythm Electrophysiol 8(2):344–352
Yamada T et al (2012) Idiopathic premature ventricular contractions arising from the intraventricular septum adjacent to the his bundle. Pacing Clin Electrophysiol 35(5):e108–11
Di Biase L et al (2011) Safety and outcomes of cryoablation for ventricular tachyarrhythmias: results from a multicenter experience. Heart Rhythm 8(7):968–974
Zera E, Rydlewska-Sadowska W, Kompiel J (1970) Evaluation of the performance of a cardiological intensive care unit on the basis of our own observations. Pol Tyg Lek 25(22):812–814
Acknowledgements
The authors acknowledge the efforts of Margot Potts and Jennifer Mears in formatting and creating this manuscript with its figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Padmanabhan, A. Sugrue, P. Gaba and S.J. Asirvatham declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Caption Electronic Supplementary Material
Video 1 Reconstructed computed tomographic image showing the anatomy of the outflow tracts
Rights and permissions
About this article
Cite this article
Padmanabhan, D., Sugrue, A., Gaba, P. et al. Outflow tract ventricular arrhythmias. Herzschr Elektrophys 28, 177–186 (2017). https://doi.org/10.1007/s00399-017-0507-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00399-017-0507-1