Opinion statement
Ventricular tachycardia in patients with structurally normal hearts is most frequently due to adenosine-sensitive, triggered activity. The most common sites of origin are the right and left ventricular outflow tracts. Patients may present with symptoms such as palpitations, or less commonly cardiomyopathy. Treatment options include beta blockers, calcium channel blockers, sodium channel blockers, potassium channel blockers, and catheter ablation. Catheter ablation is highly effective and when performed by a skilled electrophysiologist, can be considered first-line treatment. Knowledge of outflow tract and surrounding anatomy is vital to optimizing results. In this review, we discuss outflow tract anatomy and electrocardiographic morphology, as well as techniques for optimizing ablation outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventricular tachycardia (VT) most often occurs in the setting of structural heart disease, but in 10 % of patients, it occurs in structurally normal hearts and is termed idiopathic [1]. Idiopathic VT most commonly originates from the right and left ventricular outflow tracts (RVOT and LVOT). The anatomic relationships of the RVOT and LVOT to each other and to their surrounding structures are complex. With a detailed understanding of this anatomy, the 12-lead electrocardiogram (ECG) can be used to localize the arrhythmia site of origin and ablation outcomes can be optimized.
Mechanism of outflow tract VT
The mechanism of outflow tract VT is triggered activity resulting from delayed afterdepolarizations, often occurring in high catecholamine states, such as exercise. Burst pacing and isoproterenol infusion are useful techniques for inducing outflow tract VT in the electrophysiology laboratory. The delayed afterdepolarizations result from intracellular calcium release, which is blunted by adenosine [2]. These arrhythmias often terminate with administration of adenosine or calcium channel-blockers.
Clinical presentation
Outflow tract VT classically manifests in younger patients, usually in the third to fifth decade of life [3, 4]. Specific arrhythmia manifestations include premature ventricular contractions (PVCs), salvos of nonsustained VT and sustained VT. Common symptoms include palpitations, chest discomfort, and lightheadedness. Syncope is infrequent and should raise suspicion of structural heart disease or an additional arrhythmia process. Sudden death is exceedingly rare.
High PVC burdens can cause left ventricular dysfunction [5]. Risk for development of cardiomyopathy is generally thought to begin at 20 %, though cardiomyopathy less frequently occurs with lower burdens [6]. Additional risk factors for the development of PVC-induced cardiomyopathy include wider PVC QRS duration, greater coupling interval dispersion, as well as epicardial, RV (as opposed to LV), and non-OT (as opposed to OT) sites of origin [7–10]. The efficacy of catheter ablation is superior to antiarrhythmic medications in the treatment of PVC-induced cardiomyopathy [11•]. Elimination of PVCs typically results in improved LV dimensions, ejection fraction, and mitral regurgitation within several months [12]. However, full recovery has been reported to occur several years after ablation [13]. Although complete elimination of PVCs is optimal, we have found that significant (>80 %) reduction in PVC burden is usually sufficient to lead to recovery [14].
Sudden death risk and distinction from arrhythmogenic right ventricular cardiomyopathy
Sudden death associated with idiopathic outflow tract VT is very rare. However, it is important to differentiate patients with true idiopathic VT from those with scar-based outflow tract VT, such as arrhythmogenic right ventricular cardiomyopathy (ARVC) because the latter patients are at increased risk for lethal arrhythmias and may therefore benefit from implantable cardioverter-defibrillator implantation. In both substrates, VT commonly originates from the RVOT, with a left bundle configuration in lead V1 and inferior axis. A number of ECG signs have been described to distinguish ARVC from idiopathic RVOT VT, including a wider, more notched QRS complex with later precordial transition [15•, 16, 17]. Even when these signs are absent, scar-based VT should be suspected when multiple different morphologies are observed. Cardiac magnetic resonance imaging and signal-averaged ECG are useful for detecting structural and electrical abnormalities respectively [18, 19]. Voltage mapping is usually definitive.
Anatomic and electrocardiographic characteristics of the ventricular outflow tracts
Anatomic orientation of the outflow tracts
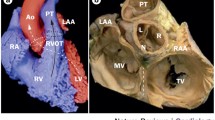
Normally, the RVOT lies anterior to the LVOT as it courses superiorly and leftward to connect to the pulmonary artery. The LVOT is located posteriorly and courses rightward to connect to the aortic root. The pulmonic valve is superior, anterior, and leftward to the aortic valve. The aortic root is centrally located and in direct contact with both atria, the interatrial septum, the mitral valve, and the posterior RVOT (Fig. 1) [20]. It has been well described that myocardial sleeves can extend above the pulmonic and aortic valves into the great vessels, and may be the source of arrhythmias [21]. In nearly three-fourths of patients, sleeves of myocardium extend above the pulmonic valve into the pulmonary artery [22]. Myocardial sleeves similarly extend above the aortic valve into the aortic root in over one-half of patients, most commonly in the right (55 %) and left (24 %) coronary cusps, rarely in the noncoronary cusp (<1 %).
Anatomy of the ventricular outflow tracts and relationship to electrocardiogram morphology. The free wall of the RVOT lies anteriorly within the chest, just beneath the sternum and precordial ECG leads. The electrocardiographic forces generated by ventricular tachycardia from the free wall of the RVOT are negative through much of the precordium, as the activation wavefront moves away from the anterior chest wall. As one progresses posteriorly toward the spine, from the septal RVOT to the right coronary cusp, left coronary cusp, aortomitral continuity, and superior mitral annulus, the activation wavefront moves increasingly toward the precordial leads, generating a greater degree of positivity in lead V1 as well as an earlier precordial transition. We divide the RVOT into sites 1 (most rightward), 2, and 3 (most leftward). Site 1 of the RVOT generates a positive complex in limb lead I, as the wavefront moves toward the left arm. By site 3 of the RVOT, the wavefront moves away from the left arm, generating a negative complex in lead I. RVOT right ventricular outflow tract, RCC right coronary cusp, LCC left coronary cusp, NCC noncoronary cusp, AMC aortomitral continuity.
Electrocardiographic localization of outflow tract VT
As the outflow tracts are superiorly located, positivity in the inferior ECG leads (II, III, and aVF) is the rule. The RVOT free wall is situated most anteriorly within the chest (Fig. 1), just beneath the sternum and precordial ECG leads. As one progresses posteriorly, the septal RVOT is encountered next, followed by the right coronary cusp, left coronary cusp, aortomitral continuity, and superior mitral annulus. The RVOT free wall is therefore the most negative in the precordium, with a left bundle configuration in lead V1 and precordial transition at V4 or later. As one moves posteriorly toward the spine, greater degrees of positivity are observed in lead V1 and the precordium transitions earlier. The precordial transition is typically V3 or V4 for the septal RVOT, V2 or V3 for the right coronary cusp, V2 or right bundle configuration in lead V1 for the left coronary cusp, qR configuration in lead V1 for the aortomitral continuity and positivity throughout the precordium for VT originating from the superior mitral annulus.
These criteria assume accurate positioning of the ECG leads. We have shown that placing leads V1 and V2 too high on the chest results in excess negativity, while placing them too low results in excess positivity [23].
Distinguishing RVOT from LVOT sites of origin
The ability to accurately distinguish RVOT from LVOT sites of origin is desirable to counsel patients about risk of systemic embolism and to decide whether to insert an arterial sheath. VTs with precordial transition at V3 can originate from the septal RVOT or right coronary cusp. We have found that adjusting the VT precordial transition for the sinus rhythm precordial transition is useful for distinguishing these two sites of origin. We do this by calculating the V2 transition ratio, dividing the R/(R + S) during VT by the R/(R + S) during sinus rhythm [24•]. A V2 transition ratio >0.6 is highly specific (91 %) and sensitive (100 %) for an LVOT site origin. Interestingly, the V3 transition ratio was not useful for distinguishing RVOT from LVOT sites of origin for VT transitioning at V3. Others have proposed different algorithms for distinguishing RVOT from LVOT sites of origin, all of which are based on greater degrees of precordial positivity for LVOT sites of origin [25, 26].
In our experience, VT often originates between the septal RVOT and right coronary cusp and can be eliminated with ablation from either side. Alternatively, ablation may be required from both sides to eliminate VT.
RVOT VT
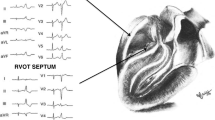
The borders of the RVOT are the pulmonic valve (superiorly), tricuspid valve (inferiorly), interventricular septum (medially), and RV free wall (laterally). The majority (>80 %) of RVOT tachycardias originate along the septum, just beneath the pulmonic valve [27]. We divide the RVOT into free wall and septal aspects, and describe the most rightward extent as site 1 (positive in lead I, Fig. 1 and Table 1), the middle as site 2 (biphasic or isoelectric in lead I), and the most leftward extent as site 3 (negative in lead I). Beneath sites 1, 2, and 3 are sites 4, 5, and 6 [28]. VT originating from the RVOT free wall has notching in the inferior leads and a later precordial transition than VT originating from the septal RVOT.
LVOT VT
LVOT VT can originate from the left ventricular endocardium, the aortic root, and the epicardial LV summit. As one moves along the superior mitral annulus from the septum to lateral wall, the QRS complex widens, with lead V1 becoming more positive and lead I more negative [29].
VT commonly originates from the right and left coronary cusps. The right coronary cusp is in contact with the top of the left ventricular septum. A wide range of ECG morphologies can be observed from the right coronary cusp, though typically the axis is leftward and inferior. As patients age, the aorta may uncoil and contact the septum more inferiorly, leading to less positivity in the inferior leads. The precordial transition is earlier in the left coronary cusp, often with a W or M shaped pattern in lead V1 [30]. VT frequently originates from the commissure between the right and left coronary cusps [31]. The electrocardiographic signature is notching in the downstroke of V1.
Very rarely, VT may originate from the noncoronary cusp. In one series of 90 consecutive patients undergoing ablation of VT in the aortic root, six VTs (7 %) originated from the noncoronary cusp [32]. These patients were younger with narrower VT QRS complexes and smaller III/II ratios than those with VT from the right coronary cusp.
VT can also originate from the LV summit, the superior most portion of the epicardium, near the bifurcation of the left main coronary artery. This location is best accessed through the coronary venous system. Epicardial mapping and ablation are frequently limited by overlying fat. LV summit VT typically has a left bundle configuration in lead V1, with early precordial transition and a pattern break in V2, with more net negativity than lead V1 or V3. Criteria have been proposed to distinguish VT that is accessible for ablation from VT that originates too close to the bifurcation of the left main coronary artery to be safely targeted [33, 34]. As one moves laterally from the anterior interventricular vein to the great cardiac vein, lead V1 develops a right bundle configuration and lead I a qS pattern.
Treatment of outflow tract VT
Medications
Treatment of outflow tract VT is aimed at alleviating symptoms or reversing PVC-induced cardiomyopathy. Because the underlying mechanism of arrhythmogenesis involves triggered activity from delayed afterdepolarizations, which is mediated by cAMP and intracellular calcium, medications that alter the cAMP-mediated calcium influx such as adenosine, calcium channel blockers, and beta-blockers are often effective in acutely terminating VT. For long-term VT suppression, beta-blockers and calcium channel blockers are most commonly prescribed, with modest efficacy in the 25 %–50 % range [35, 36]. Sodium channel blockers are slightly more effective, with 55 % success rate in one comparative study [37]. Potassium channel blockers are used less frequently.
Catheter ablation
Catheter ablation is generally a safe and effective method to permanently cure outflow tract tachycardias and should be considered a reasonable first-line treatment [38•]. When performed by an experienced operator, success rates are in excess of 90 %, with an acceptably low rate of serious complications (approximately 1 %) [39].
To enhance the likelihood of successful ablation, it is important to maximize PVC frequency during the ablation. We typically discontinue beta blockers, calcium channel blockers, and other antiarrhythmic medications several days before ablation. Amiodarone is ideally discontinued several weeks beforehand. As idiopathic VT is sensitive to catecholamine state, we limit the use of propofol and benzodiazepines. Instead, we favor remifentanyl, which is rapidly eliminated following discontinuation and has fewer antiarrhythmic properties [40]. Isoproterenol infusion and burst pacing are used when VT remains infrequent.
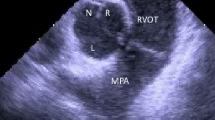
We carefully examine the ECG to anticipate the site of VT origin. A three-dimensional electroanatomic map of that chamber is created, under fluoroscopic and intracardiac echocardiographic (ICE) guidance. We have found ICE to be extremely valuable for understanding anatomic relationships, guiding catheter position, and avoiding/detecting complications. In addition to guiding ablation, voltage mapping identifies myocardial scarring, differentiating idiopathic VT from scar based arrhythmias such as ARVC [41].
When PVCs or VT are frequent enough, we perform activation mapping to identify the site of earliest activation, which should be well before the onset of the QRS complex. The unipolar electrogram should have a qS morphology and simultaneous onset with the bipolar electrogram. When a diffuse area of earliest activation is encountered, one should suspect breakthrough from another chamber, such as the epicardium or contralateral outflow tract [39].
When VT is not frequent enough to activation map, pacemapping is performed at threshold output with the same coupling interval as the spontaneous PVC or cycle length as spontaneous VT. A perfect 12/12 match should be sought. Ablation at sites without perfect 12/12 matches are less likely to be successful [42]. Pacemapping alone results in success rates which are inferior to activation mapping [43]. We have found pacemapping to be highly accurate within the RVOT, but less so within the aortic root, where high pacing outputs are frequently required.
Although either irrigated or nonirrigated ablation is usually sufficient within the RVOT, we exclusively use irrigated ablation within the aortic root and coronary venous system to allow sufficient energy delivery. When ablating within the right or left coronary cusp, care must be taken to define the location of the coronary ostia, either by ICE or angiography, to ensure adequate separation. Coronary injury is uncommon ablating at the bottom of a coronary cusp [44]. Outflow tract VT has rarely been reported to arise from the coronary arteries themselves, and isolation of the coronary ostia has been performed to eliminate these arrhythmia without injuring the artery [45]. The His bundle penetrates the central fibrous body, which is inferior to the noncoronary cusp, so care must be taken when ablating near the junction of the right and noncoronary cusps to avoid damaging the conduction system [46].
Left coronary angiography must be performed prior to targeting LV summit VT from the anterior interventricular vein, to ensure adequate separation. When the VT site of origin is too close, rather than risking injury to the left anterior descending coronary artery, ablation should be attempted from nearby structures including the LV endocardium, left coronary cusp, or septal site 3 of the RVOT [47, 48]. Of note, septal site 3 of the RVOT just beneath the pulmonic valve is close not only to the anterior interventricular vein, but also to the left anterior descending coronary artery, and thus, energy should be delivered judiciously in this location.
If VT is suppressed during ablation but recurs shortly thereafter, the ECG morphology should be reexamined to determine whether the VT exit has changed. For example, after ablating in site 1 of the septal RVOT, the VT exit site may shift to the right coronary cusp. If indeed the ECG morphology has changed, mapping should be repeated, including in adjacent structures. When ablating within a coronary cusp results in suppression and then return of VT, we are often successful ablating the LV endocardium just beneath that cusp. We consider a procedure successful when VT cannot be induced with isoproterenol infusion and burst pacing at least one hour following the last ablation lesion.
Conclusions
Idiopathic outflow tract VT can cause symptoms or cardiomyopathy. Treatment options include medications and catheter ablation. With a detailed understanding of outflow tract and surrounding anatomy, the ECG can be used to predict the site of VT origin. Catheter ablation is a highly successful and generally safe procedure for eliminating outflow tract VT.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Brooks R, Burgess JH. Idiopathic ventricular tachycardia. A review. Medicine. 1988;67(5):271–94.
Lerman BB, Belardinelli L, West GA, et al. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation. 1986;74(2):270–80.
Lerman BB, Stein KM, Markowitz SM. Idiopathic right ventricular outflow tract tachycardia: a clinical approach. Pacing and clinical electrophysiology. PACE. 1996;19(12 Pt 1):2120–37.
Nakagawa M, Takahashi N, Nobe S, et al. Gender differences in various types of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13(7):633–8.
Lee GK, Klarich KW, Grogan M, Cha Y-M. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circulation. 2012;5(1):229–36.
Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7(7):865–9.
Yokokawa M, Kim HM, Good E, et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9(9):1460–4.
Del Carpio MF, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the Burden, Duration, Coupling Interval, Morphology and Site of Origin of PVCs. J Cardiovasc Electrophysiol. 2011;22(7):791–8.
Carballeira Pol L, Deyell MW, Frankel DS, et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014;11(2):299–306.
Kawamura M, Badhwar N, Vedantham V, et al. Coupling interval dispersion and body mass index are independent predictors of idiopathic premature ventricular complex-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25(7):756–62.
Zhong L, Lee YH, Huang XM, et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm. 2014;11(2):187–93. Retrospective study demonstrating superiority of catheter ablation over antiarrhythmic medications for treatment of PVC-induced cardiomyopathy.
Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45(8):1259–65.
Yokokawa M, Good E, Crawford T, et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm. 2013;10(2):172–5.
Mountantonakis SE, Frankel DS, Gerstenfeld EP, et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8(10):1608–14.
Hoffmayer KS, Machado ON, Marcus GM, et al. Electrocardiographic comparison of ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2011;58(8):831–8. Retrospective study proposing ECG criteria for differentiating idiopathic RVOT VT from that occurring in ARVC.
Hoffmayer KS, Bhave PD, Marcus GM, et al. An electrocardiographic scoring system for distinguishing right ventricular outflow tract arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy from idiopathic ventricular tachycardia. Heart Rhythm. 2013;10(4):477–82.
Samol A, Wollmann C, Vahlhaus C, et al. T-wave integral: an electrocardiographic marker discriminating patients with arrhythmogenic right ventricular cardiomyopathy from patients with right ventricular outflow tract tachycardia. Europace. 2013;15(4):582–9.
Kinoshita O, Kamakura S, Ohe T, et al. Frequency analysis of signal-averaged electrocardiogram in patients with right ventricular tachycardia. J Am Coll Cardiol. 1992;20(5):1230–7.
Nagashima K, Tedrow UB, Koplan BA, et al. Reentrant ventricular tachycardia originating from the periaortic region in the absence of overt structural heart disease. Circ Arrhythmia Electrophysiol. 2014;7(1):99–106.
Tabatabaei N, Asirvatham SJ. Supravalvular arrhythmia: identifying and ablating the substrate. Circulation. 2009;2(3):316–26.
Hasdemir CAN, Aktas S, Govsa F, et al. Demonstration of ventricular myocardial extensions into the pulmonary artery and aorta beyond the ventriculo-arterial junction. Pacing Clin Electrophysiol. 2007;30(4):534–9.
Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Intervent Cardiac Electrophysiol. 2011;30(1):5–15.
Anter E, Frankel DS, Marchlinski FE, Dixit S. Effect of electrocardiographic lead placement on localization of outflow tract tachycardias. Heart Rhythm. 2012;9(5):697–703.
Betensky BP, Park RE, Marchlinski FE, et al. The V(2) transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011;57(22):2255–62. Retrospective study describing the V2 transition ratio to distinguish RVOT from LVOT sites of origin for VT transitioning at lead V3.
Cheng Z, Cheng K, Deng H, et al. The R-wave deflection interval in lead V3 combining with R-wave amplitude index in lead V1: a new surface ECG algorithm for distinguishing left from right ventricular outflow tract tachycardia origin in patients with transitional lead at V3. Int J Cardiol. 2013;168(2):1342–8.
Yoshida N, Yamada T, McElderry HT, et al. A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: the V2S/V3R Index. J Cardiovasc Electrophysiol. 2014;25(7):747–53.
Callans DJ, Menz V, Schwartzman D, et al. Repetitive monomorphic tachycardia from the left ventricular outflow tract: electrocardiographic patterns consistent with a left ventricular site of origin. J Am Coll Cardiol. 1997;29(5):1023–7.
Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol. 2003;14(1):1–7.
Dixit S, Gerstenfeld EP, Lin D, et al. Identification of distinct electrocardiographic patterns from the basal left ventricle: distinguishing medial and lateral sites of origin in patients with idiopathic ventricular tachycardia. Heart Rhythm. 2005;2(5):485–91.
Lin D, Ilkhanoff L, Gerstenfeld E, et al. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008;5(5):663–9.
Bala R, Garcia FC, Hutchinson MD, et al. Electrocardiographic and electrophysiologic features of ventricular arrhythmias originating from the right/left coronary cusp commissure. Heart Rhythm. 2010;7(3):312–22.
Yamada T, Lau YR, Litovsky SH, et al. Prevalence and clinical, electrocardiographic, and electrophysiologic characteristics of ventricular arrhythmias originating from the noncoronary sinus of Valsalva. Heart Rhythm. 2013;10(11):1605–12.
Ito S, Tada H, Naito S, et al. Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2003;14(12):1280–6.
Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythmia Electrophysiol. 2010;3(6):616–23.
Gill JS, Blaszyk K, Ward DE, Camm AJ. Verapamil for the suppression of idiopathic ventricular tachycardia of left bundle branch block-like morphology. Am Heart J. 1993;126(5):1126–33.
Issa ZF, Miller JM, Zipes DP. Idiopathic ventricular tachycardia. In: Zipes D, editor. Clinical arrhythmology and electrophysiology. Philadelphia: W.B. Saunders; 2009. p. 440–61.
Stec S, Sikorska A, Zaborska B, et al. Benign symptomatic premature ventricular complexes: short- and long-term efficacy of antiarrhythmic drugs and radiofrequency ablation. Kardiol Pol. 2012;70(4):351–8.
Ling Z, Liu Z, Su L, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythmia Electrophysiol. 2014;7(2):237–43. Prospective, randomized trial demonstrating superiority of catheter ablation over antiarrhythmic medications for treatment of RVOT PVCs.
Dixit S, Lin D, Marchlinski FE. Ablation of ventricular outflow tract tachycardias. In: Huang S, editor. Catheter ablation of cardiac arrhythmias. 2nd ed. Saint Louis: W.B. Saunders; 2011. p. 446–62.
Mandel JE, Hutchinson MD, Marchlinski FE. Remifentanil-midazolam sedation provides hemodynamic stability and comfort during epicardial ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2011;22(4):464–6.
Corrado D, Basso C, Leoni L, et al. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2008;51(7):731–9.
Gerstenfeld EP, Dixit S, Callans DJ, et al. Quantitative comparison of spontaneous and paced 12-lead electrocardiogram during right ventricular outflow tract ventricular tachycardia. J Am Coll Cardiol. 2003;41(11):2046–53.
Chung FP, Chong E, Lin YJ, et al. Different characteristics and electrophysiological properties between early and late recurrences after acute successful catheter ablation of idiopathic right ventricular outflow tract arrhythmias during long-term follow-up. Heart Rhythm. 2014;11(10):1760–9.
Hoffmayer KS, Dewland TA, Hsia HH, et al. Safety of radiofrequency catheter ablation without coronary angiography in aortic cusp ventricular arrhythmias. Heart Rhythm. 2014;11(7):1117–21.
Vaidya V, Syed F, Desimone C, et al. Outflow tract ventricular tachycardia mapped to the coronary arteries: anatomical correlates and management strategies. J Cardiovasc Electrophysiol. 2013;24(12):1416–22.
Yamada T, Litovsky SH, Kay GN. The left ventricular ostium: an anatomic concept relevant to idiopathic ventricular arrhythmias. Circ Arrhythmia Electrophysiol. 2008;1(5):396–404.
Jauregui Abularach ME, Campos B, Park KM, et al. Ablation of ventricular arrhythmias arising near the anterior epicardial veins from the left sinus of Valsalva region: ECG features, anatomic distance, and outcome. Heart Rhythm. 2012;9(6):865–73.
Frankel DS, Mountantonakis SE, Dahu MI, Marchlinski FE. Elimination of ventricular arrhythmias originating from the anterior interventricular vein with ablation in the right ventricular outflow tract. Circ Arrhythmia Electrophysiol. 2014;7(5):984–5.
Compliance with Ethics Guidelines
Conflict of Interest
Jackson J. Liang, Yuchi Y. Han, and David S. Frankel all declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Arrhythmia
Rights and permissions
About this article
Cite this article
Liang, J.J., Han, Y. & Frankel, D.S. Ablation of Outflow Tract Ventricular Tachycardia. Curr Treat Options Cardio Med 17, 4 (2015). https://doi.org/10.1007/s11936-014-0363-9
Published:
DOI: https://doi.org/10.1007/s11936-014-0363-9