Abstract
Various drug delivery systems for boron neutron capture therapy (BNCT) have been developed. To selectively destroy cancer cells, the high accumulation and selective delivery of 10B into tumor tissue are required. In this study, a polyborane for BNCT with enhanced hydrophobicity was synthesized from decaborane as a boron carrier, and embedded into bare and PEGylated liposomes. These liposomes having diameters of 40–43 nm were injected into tail vein of tumor-bearing mice to evaluate their biodistribution. Boron concentrations in tumor and tumor/blood ratios of the liposomes were reached over 30 μg/g of tissue and over 5 at 8–24 h, respectively. At 12 h after injection, PEGylated liposomes were found in tumor with high boron level (130.0 μg/g of tissue). This result showed that the PEGylated liposomes with a diameter of 40 nm were able to achieve efficient intratumoral 10B amount without replacing the 11B with 10B.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron neutron capture therapy (BNCT) is a cancer therapy using nuclear reaction between boron and thermal neutron. It is based on the nuclear capture and fission reactions. When boron-10 (10B) is irradiated with low-energy thermal neutrons, it yields high linear energy transfer (LET), α particles (4He), and recoiling lithium-7 (7Li) nuclei. The destructive effects of these high-energy particles are limited to boron containing cells, since the high LET particles have limited path lengths in tissue (5–9 μm). Therefore, BNCT is theoretically possible to kill tumor cells, without affecting adjacent healthy cells, if 10B atoms can be selectively delivered and accumulated in the interstitial space of tumor tissue and/or intracellular space of tumor cell. To improve the efficacy of BNCT, high accumulation and selective delivery of 10B into tumor tissue are the most important requirements. To carry out BNCT efficiently, over 20–30 μg of non-radioactive 10B per gram of tumor tissue and tumor/blood boron concentration ratio of over 5 are required [1,2,3].
A liposome is a closing vesicle formed with phospholipids which are basic components of the biomembrane and can incorporate hydrophilic and/or lipophilic boron compounds [4]. PEGylated liposomes have been prepared to prolong the retention time of liposomes in circulation by avoiding the uptake into the cells of the mononuclear phagocyte system (reticuloendothelial system), and it promotes the enhanced permeability and retention (EPR) effect in solid tumors [5,6,7]. Also, detailed biodistribution studies of liposomes for BNCT were reported. Biodistribution of liposomes encapsulating water-soluble ionic boron compounds having average diameters of 43–115 nm was observed in the blood, tumor, liver, and spleen of tumor-bearing mice [4, 8, 9] and that of 61 and 83 nm were observed in the blood, tumor, liver spleen, kidney, precancerous tissue, and normal pouch tissue in recent years [10]. The biodistribution of liposomes incorporated lipophilic boron compounds having average diameters of 42–114 nm that were observed in the blood, tumor, liver, and spleen of tumor-bearing mice [11]. In the previous study, liposomal drug delivery system for BNCT was reported. Biodistribution of PEGylated liposomes was evaluated by measuring boron concentration at 24 h after injections. Bare liposome with a diameter of 50 nm and PEGylated liposome with a diameter of 100 nm were found in tumor with high boron levels. Moreover, the 50 nm bare liposomes showed high tumor/blood ratios of boron concentration, and their usability for BNCT was suggested [12]. In this study, detailed biodistribution studies of lipophilic boron compound-embedded liposomes were carried out; however, they were insufficient due to that the boron concentration of tissue was measured at only 24 h after sample administration. PEGylation increased the intratumoral boron amount at 24 h after administration in the liposomes with diameters of 100 and 200 nm. In contrast, unexpectedly, the liposomes with a particle size of 50 nm decreased the intratumoral boron amount by PEGylation. Therefore, it was necessary to investigate more detailed biodistribution of PEGylated liposomes with a small particle size. Moreover, the maximum intratumoral boron accumulation amount was 80 μg/g of tumor tissue, and it was necessary to replace a part of 11B in the compound with 10B to satisfy the target value (20 μg of 10B per gram of tumor tissue) of the intratumoral boron amount.
The main aim of the present study was to observe biodistribution of polyborane embedded bare and PEGylated liposomes in tumor-bearing mice at 4–24 h and evaluate their tumor accumulation property by measuring boron amount. Also, smaller liposomes were prepared to achieve intratumoral boron amount of 100 μg/g of tumor tissue. Decaborane, hydrophobic boron compound, was used as a boron carrier because it has ten boron atoms in its structure, and is stable in room temperature. To trap the boron in lipid bilayers of liposomes, we have synthesized a polyborane from decaborane.
Materials and methods
Materials
Decaborane(14) (B10H14, purity ≥98%) was purchased from Santa Cruz Biotechnology Inc. (Dallas, USA). 1,2,-Diiodoethane (C2H4I2, purity ≥98.0%), hexane (CH3(CH2)4CH3, purity ≥95.0%), diethyl ether (C4H10O, purity ≥99.5%), ethyl acetate (CH3COOC2H5, purity ≥99.5%), tetrahydrofuran (THF, C4H8O, purity ≥99.5%, water ≤0.001%), toluene, dichloromethane (CH2Cl2, purity ≥99.5%), chloroform-d (CDCl3, purity ≥99.8%, containing 0.05 vol% TMS), chloroform (CHCl3, purity ≥99.0%), ammonium chloride (NH4Cl, purity ≥99.0%), sodium sulfate (Na2SO4, purity ≥99.0%), sodium thiosulfate (Na2S2O3, purity ≥99.0%), sodium hydrogen carbonate (NaHCO3, purity ≥99.0%), sodium chloride (NaCl, purity ≥99.5%), sodium hydroxide (NaOH, purity ≥97.0%), disodium hydrogenphosphate (Na2HPO4, purity ≥99.0%), potassium hydroxide (KOH, purity ≥85.0%), potassium dihydrogenphosphate (KH2PO4, purity ≥99.0%), and boron standard solution (boron concentration 1000 mg/L) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Silica gel 60 (spherical, particle size 63–210 μm) was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). n-Butyllithium (in hexane, ca. 1.5–1.7 mol/L), 1,2-diphenylacetylene (C14H10, purity ≥98%), tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4, purity >97.0%), and 1-butyl-3-methylimidazolium chloride (bmimCl, C8H15ClN2, purity >98.0%) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Ethynylmagnesium bromide solution (0.5 M, in tetrahydrofuran) was purchased from Sigma-Aldrich Co. LLC (St. Louis, USA). Egg phosphatidylcholine (egg PC) was purchased from Asahi Kasei Kogyo Co., Ltd. (Tokyo, Japan). Sunbright DSPE-020CN (DSPE-PEG, N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt, Mw 2000) was purchased from NOF Corporation (Tokyo, Japan). 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) was purchased from Dojindo Molecular Technologies Inc. (Mashiki, Japan). Isoflurane for the animal was purchased from Mylan Inc. (Pittsburgh, Pennsylvania). Fetal bovine serum (FBS) was purchased from Thermo Fisher Scientific, Inc. (Waltham, USA). All other chemicals were of the highest grade commercially available.
Synthesis of polyborane for BNCT
1,2-Diphenylacetylene (6.0 g, 34 mmol) in THF (14.0 mL) was dropwise added into a n-butyllithium in hexane (1.53 M, 11.0 mL,16.8 mmol) at −10 °C. After stirring for 2 h, the sample solution was cooling down to −78 °C. 1,2,-Diiodoethane (12.3 g, 43.7 mmol) was dropwise added, and then continuously stirred for 1 h. Na2S2O3-saturated aqueous solution and diethyl ether were also added into the reaction vessel, and the reaction mixture was stirred for 10 min at 0 °C. The product was extracted with diethyl ether, and washed with a NaHCO3 saturated aqueous solution, distilled water, and brine. Then, the organic layer was dried with Na2SO4, and concentrated under reduced pressure. The crude solid residue was purified by silica gel column chromatography (hexane/dichloromethane = 300:1), and as shown in Scheme 1, (E)-1,2-diphenyl-1-iodohex-1-ene was obtained as a faint yellow solid (2.5 g, 41.6%) [13]. 1H-NMR (δ, 300 MHz, CDCl3): 0.71 ppm (3H, triplet, J = 7.1 Hz), 1.06–1.26 ppm (4H, multiplet), 2.32 ppm (2H, triplet, J = 15.0 Hz), 7.22–7.40 ppm (10H, multiplet).
(E)-1,2-Diphenyl-1-iodohex-1-ene (2.5 g, 6.9 mmol) and Pd(PPh3)4 (798 mg, 0.69 mmol) were dissolved in THF. Ethynylmagnesium bromide (27.6 mL, 13.8 mmol) was dropwise added into the THF solution, and reacted at 50 °C for overnight. After the reaction mixture was cooling down to 0 °C, NH4Cl saturated aqueous solution was added. The product was extracted with ethyl acetate, and washed with distilled water and brine. Then, the organic layer was dried over Na2SO4, and concentrated under reduced pressure. The crude solid residue was purified by silica gel column chromatography (hexane/ethyl acetate = 150:1), and as shown in Scheme 2, (E)-3,4-diphenyloct-3-ene-1-yne was obtained as a white solid (1.1 g, 60.0%) [14]. 1H-NMR (δ, 300 MHz, CDCl3): 0.73 ppm (3H, triplet, J = 7.0 Hz), 1.10–1.27 ppm (4H, multiplet), 2.44 ppm (2H, triplet, J = 7.5 Hz), 2.86 ppm (1H, singlet), 7.26–7.48 ppm (10H, multiplet).
BmimCl (1.34 g, 7.68 mmol) and decaborane (1.13 g, 9.22 mmol) were dissolved in toluene (30.7 mL) at room temperature. (E)-3,4-Diphenyloct-3-ene-1-yne (2.0 g, 7.7 mmol) in toluene (7.7 mL) was dropwise added into the solution, and heated to reflux for 12 h. After cooling down to room temperature, the desired compound was extracted with ethyl acetate, and washed with distilled water and brine. Then, the organic layer was dried over Na2SO4, and concentrated under reduced pressure. The crude solid residue was purified by silica gel column chromatography (hexane only), and as shown in Scheme 3, (E)-1-(1,2-dicarba-closo-dodecaborane-1-yl)-1,2-diphenylhex-1-ene was obtained as a greenish white solid (0.93 g, 31.8%) [15]. This boron compound included dicarba-closo-dodecaborane (carborane) skeleton, and molecular weight (378.56) was similar to that of cholesterol (386.65). 1H-NMR (δ, 300 MHz, CDCl3): 0.60 ppm (3H, triplet, J = 7.2 Hz), 0.90–1.13 ppm (4H, multiplet), 1.81 ppm (2H, triplet, J = 7.8 Hz), 3.11 ppm (1H, singlet),7.18–7.42 ppm (10H, multiplet).
Preparation of (E)-1-(1,2-dicarba-closo-dodecaborane-1-yl)-1,2-diphenylhex-1-ene embedded liposomes
(E)-1-(1,2-Dicarba-closo-dodecaborane-1-yl)-1,2-diphenylhex-1-ene embedded liposomes were prepared using Bangham method. Compositions of them are shown in Table 1. Lipid mixtures of (E)-1-(1,2-dicarba-closo-dodecaborane-1-yl)-1,2-diphenylhex-1-ene, egg PC and DSPE-PEG were dissolved in chloroform (10 mL) in eggplant flask, and chloroform was evaporated under reduced pressure. After forming a thin film in eggplant flask, it was resuspended with HEPES buffered saline (HBS, pH 7.4, 10 mM). Liposomes were prepared using Nanomizer Mark II (Yoshida Kikai Co., Ltd., Nagoya, Japan) which is one of the mechanochemical systems to prepare particles (flow volume 2.0 mL, pressure 50 MPa, number of passes: ten times). Mean volume diameter, size distribution, and zeta-potential of the prepared liposomes were determined using a dynamic light scattering system, ELSZ-1000ZS (Otsuka Electronics Co., Ltd., Hirakata, Japan). Liposomal suspensions were diluted with HBS. Then, measurement of the liposome size was carried out at 25 °C and electrophoretic mobility was measured at 37 °C. Boron amounts of the liposomes were determined using the inductively coupled plasma atomic emission spectroscopy, ICPE-9000 (Shimadzu Co., Kyoto, Japan). Liposomal suspensions (0.1 mL) were diluted with 6.9 mL of distilled water and 3.0 mL of nitric acid and observed at 249.773 nm. A calibration curve was prepared using boron standard solution. To investigate the stability of bare liposomes, 0.1 mL of liposome suspension was added to 1 mL of FBS and incubated at 37 °C. Then, particle size was measured after 12, 24, and 48 h.
Biodistribution study of liposomes
Mice (ddY, 7–8 weeks old, male) were housed in stainless steel cages and housed under standard environmental conditions (23 ± 1 °C, 55 ± 5% humidity, and a 12/12 h light/dark cycle) and maintained with free access to water and a standard laboratory diet (carbohydrates 30%; proteins 22%; lipids 12%; vitamins 3%) ad libitum (Nihon Nosan Kogyo Co., Yokohama, Japan). They were used in accordance with the Guidelines for Animal Experimentation of Tokyo University of Science, which are based on the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science.
B16 melanoma cells (7.0 × 105 cells in 50 μL PBS) were subcutaneously injected into a footpad of the right hind limb of mice, and tumor-bearing mice were given after 3 weeks. Liposomes (4.4 mg boron/kg, in PBS) were injected via the tail vein into tumor-bearing mice (ddY, 7 weeks old, male) with anesthesia, and animals were maintained in metabolic cages. The injections were well tolerated and no adverse effects were observed. After 4, 8, 12, 16, 20, and 24 h of sample administration, mice were euthanized with isoflurane, and bled at inferior vena cava. The brain, heart, lungs, liver, stomach, pancreas, spleen, and kidneys were immediately taken from the same individual. The blood, urine, feces, and all tissues were weighed and melted using wet ashing method with nitric acid [16]. All boron amounts of samples were determined using ICPE-9000.
Results and discussion
Preparation of (E)-1-(1,2-dicarba-closo-dodecaborane-1-yl)-1,2-diphenylhex-1-ene embedded liposomes
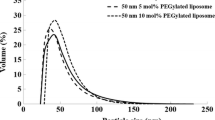
Particle size distributions of prepared liposomes are shown in Fig. 1a. Mean volume diameters of bare and PEGylated liposomes were 40.6 ± 18.2 and 42.4 ± 20.4 nm, respectively (means ± S.D.). The mechanochemical system was useful to prepare liposomes having an average diameter of less than 50 nm, since it could treat the relatively high volume of samples easily and rapidly [4]. Zeta-potential of bare and PEGylated liposomes in PBS (pH 7.4, I = 0.154 M) were −10.1 ± 0.72 and −5.29 ± 0.72 mV, respectively. The absolute value of the zeta-potential of PEGylate liposome decreased as compared to that of bare liposome due to the effect of PEG chain length to the absolute value of the zeta-potential of liposomes [17]. Also, boron amounts of bare and PEGylated liposomes suspension were 1157 and 1328 mg/L, respectively. Figure 1b shows stabilities of bare and PEGylated liposomes in FBS. PEGylated liposomes were stable even after 24 h; however, the particle size of bare liposomes tended to increase. Since the surface of the liposome was negatively charged, we considered that blood components such as proteins and lipids were adsorbed on the surface of the unmodified liposome by electrostatic interaction.
Biodistribution of liposomes
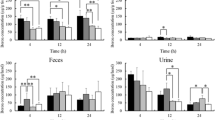
Figure 2 shows boron amounts of the blood, tumor, urine, feces, and all organs (brain, heart, lung, liver, stomach, pancreas, spleen, and kidney). Average boron amount in the tumor of bare and PEGylated liposome reached over 30 μg/g of tissue at 8–24 h after injections. This result indicated that liposomes had leaked out from blood vessels and accumulated in tumor tissues. In particular, intratumoral boron amount of the PEGylated liposomes exceeded 100 μg/g of tissue at 12 h after administration (130.0 μg/g of tissue). Naturally occurring boron contains 19.8% of 10B and 80.2% of 11B [18]. Therefore, this result showed that the PEGylated liposomes with a diameter of 40 nm were able to achieve an intratumoral 10B amount of 20 μg/g without replacing the 11B with 10B. However, boron amount in the tumor of the PEGylated liposome decreased after 12 h and further accumulation was not observed. As shown in Fig. 1b, the stability of PEGylated liposomes was high until 24 h after administration. Therefore, it is assumed that this decrease was caused by liposome leakage from the tumor, not an aggregation of PEGylated liposomes [19]. When this liposome is applied to BNCT, it is recommended to irradiate thermal neutron radiation at 12 h after administration. The bare liposomes reached the maximum intratumoral boron amount at 16 h after administration (95.7 μg/g of tissue), and in other organs, rapid accumulation increases were also observed. We considered that this result was affected by the decrease in stability of bare liposomes after 12 h (Fig. 1b). Interestingly, boron amounts of 3.9–10.9 μg/g of tissue were detected in the brain after 4 h with bare and PEGylated liposomes, and maintained for at least 20 h, which indicated permeation of liposomes through blood–brain barrier. A remarkable difference between two types of liposomes was not observed. In the previous studies using 50-nm PEGylated liposomes, the amount of boron in the brain of 9.5 was observed at 24 h after administration [12]; therefore, PEGylated liposomes with small particle diameter were useful for boron delivery to the brain. However, to deliver 10 B intravenously using these liposomes, it is necessary to replace 11 B with 10 B and further increase the dose. Further detailed study on miniaturization and PEGylation of the liposome is required. From the result of a boron amount of feces, it is suggested that liposomes with an average diameter of less than 50 nm metabolized in the liver due to its size and retention property [4, 19]. As shown in Fig. 3, tumor/blood ratio of bare and PEGylated liposomes reached over 5 at 4–24 h. Though the significant difference was not observed, tumor/blood ratio of the PEGylated liposome had a decreasing trend after 12 h. In this study, liposomes were delivered to the tumor using passive targeting. Active targeting technique using transferrin, antibodies, ligands, and folate will enhance the tumor/blood ratio [19,20,21,22,23].
Conclusions
A polyborane for BNCT with enhanced hydrophobicity was synthesized and successfully embedded into bare and PEGylated liposomes. The detailed biodistribution of them was confirmed by in vivo experiments. Their boron amounts in tumor and tumor/blood ratio were reached over 30 μg/g of tissue and over 5 at 8–24 h after injections, respectively. Intratumoral boron amount of the PEGylated liposomes reached over 100 μg/g of tissue after 12 h post-administration, and leakage from subsequent tumor tissues was suggested. Moreover, considerable boron amounts were detected in the brain at 4–24 h. As a result, detailed biodistributions of bare and PEGylated liposomes with a diameter of 40 nm were revealed, and efficacy of polyborane embedded PEGylated liposomes for BNCT was suggested.
References
Barth RF, Coderrea JA, Vicente MGH (2005) Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 11:3987–4002. doi:10.1158/1078-0432.CCR-05-0035
Maruyama K, Ishida O, Kasaoka S, Takizawa T, Utoguchi N, Shinohara A, Chiba M, Kobayashi H, Eriguchi M, Yanagie H (2004) Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J Control Release 98:195–207. doi:10.1016/j.jconrel.2004.04.018
Vicente MGH, Wickramasinghe A, Nurco DJ, Wang HJH, Nawrocky MM, Makar M, Miura M (2003) Synthesis, toxicity and biodistribution of two 5,15-di[3,5-(nido-carboranylmethyl)phenyl]porphyrins in EMT-6 tumor bearing mice. Bioorganic Med Chem 11:3101–3108. doi:10.1016/S0968-0896(03)00240-2
Hawthorne MF, Shelly K (1997) Liposomes as drug delivery vehicles for boron agents. J Neuro-Oncol 33:53–58. doi:10.1023/A:1005713113990
Allen TM, Hansen C (1991) Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta 1068:133–141. doi:10.1016/0005-2736(91)90201-I
Lasic DD (1996) Doxorubicin in sterically stabilized liposomes. Nature 380:561–562. doi:10.1038/380561a0
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Cont Rel 65:271–284. doi:10.1016/S0168-3659(99)00248-5
Shelly K, Feakes DA, Hawthorne MR, Schmidt PG, Krisch TA, Bauer WF (1992) Model studies directed toward the boron neutron-capture therapy of cancer: boron delivery to murine tumors with liposomes. Proc Natl Acad Sci 89:9039–9043. doi:10.1073/pnas.89.19.9039
Feakes DA, Shelly K, Knobler CB, Hawthorne MF (1994) Na3[B20H17NH3]: synthesis and liposomal delivery to murine tumors. Proc Natl Acad Sci 91:3029–3033. doi:10.1073/pnas.91.8.3029
Heber EM, Kueffer PJ, Lee Jr MW, Hawthorne MF, Garabalino MA, Molinari AJ, Nigg DW, Bauer W, Hughes AM, Pozzi ECC, Trivillin VA, Schwint AE (2012) Boron delivery with liposomes for boron neutron capture therapy (BNCT): biodistribution studies in an experimental model of oral cancer demonstrating therapeutic potential. Radiat Environ Biophys 51:195–204. doi:10.1007/s00411-011-0399-0
Feakes DA, Shelly K, Hawthorne MF (1995) Selective boron delivery to murine tumors by lipophilic species incorporated in the membranes of unilamellar liposomes. Proc Natl Acad Sci 92:1367–1370. doi:10.1073/pnas.92.5.1367
Takeuchi I, Tomoda K, Matsumoto K, Uchiro H, Makino K (2016) PEGylated liposomes prepared with polyborane instead of cholesterol for BNCT: characteristics and biodistribution evaluation. Colloid Polymer Sci 294:1679–1685. doi:10.1007/s00396-016-3925-4
McKinley NF, O’Shea (2006) Carbolithiation of diphenylacetylene as a stereoselective route to (Z)-tamoxifen and related tetrasubstituted olefins. J Org Chem 71:9552–9555. doi:10.1021/jo061949s
Beer ML, Lemon J, Valliant JF (2010) Preparation and evaluation of carborane analogues of tamoxifen. J Med Chem 53:8012–8020. doi:10.1021/jm100758j
Li Y, Carroll PJ, Sneddon LG (2008) Ionic-liquid-promoted decaborane dehydrogenative alkyne-insertion reactions: a new route to o-carboranes. Inorg Chem 47:9193–9202. doi:10.1021/ic800999y
Ueno M, Ban HS, Nakai K, Inomata R, Kaneda Y, Matsumura A, Nakamura H (2010) Dodecaborate lipid liposomes as new vehicles for boron delivery system of neutron capture therapy. Bioorganic Med Chem 18:3059–3065. doi:10.1016/j.bmc.2010.03.050
Hashizaki K, Itoh C, Sakai H, Yokoyama S, Taguchi H, Saito Y, Ogawa N, Abe M (1999) Effects of PEG chain length of phospholipid with covalently attached poly(ethylene glycol) (PEG) on the macroscopic state of liposomes. J Jpn Oil Chem Soc 48:871–876
Kakihana H, Kotaka M, Satoh S, Nomura M, Okamoto M (1977) Fundamental studies on the ion-exchange separation of boron isotopes. Bull Chem Soc Jpn 50:158–163. doi:10.1246/bcsj.50.158
Doi A, Kawabata S, Iida K, Yokoyama K, Kajimoto Y, Kuroiwa T, Shirakawa T, Kirihara M, Kasaoka S, Maruyama K, Kumada H, Sakurai Y, Masunaga SI, Ono K, Miyatake SI (2008) Tumor-specific targeting of sodium borocaptate (BSH) to malignant glioma by transferrin-PEG liposomes: a modality for boron neutron capture therapy. J Neuro-Oncol 87:287–294. doi:10.1007/s11060-008-9522-8
Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP (2004) Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Cont Rel 100:135–144. doi:10.1016/j.jconrel.2004.08.007
Carlsson J, Kullberg EB, Capala J, Sjöberg S, Edwards K, Gedda L (2003) Ligand liposomes and boron neutron capture therapy. J Neuro-Oncol 62:47–59. doi:10.1023/A:1023282818409
Shmeeda H, Tzemach D, Mak L, Gabizon A (2009) Her2-targeted pegylated liposomal doxorubicin: retention of target-specific binding and cytotoxicity after in vivo passage. J Cont Rel 136:155–160. doi:10.1016/j.jconrel.2009.02.002
Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S (2004) Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid–PEG conjugates. Adv Drug Deliv Rev 56:1177–1192. doi:10.1016/j.addr.2004.01.011
Acknowledgements
This work was supported by Program for Development of Strategic Research Center in Private Universities supported by MEXT (2010-2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Mice were used in accordance with the Guidelines for Animal Experimentation of Tokyo University of Science, which are based on the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takeuchi, I., Ishizuka, Y., Uchiro, H. et al. Detailed biodistribution of liposomes prepared with polyborane instead of cholesterol for BNCT: effects of PEGylation. Colloid Polym Sci 295, 1455–1461 (2017). https://doi.org/10.1007/s00396-017-4113-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4113-x