Abstract
Recently, boron neutron capture therapy (BNCT) has been focused on, which is a cancer therapy using nuclear reaction between boron and thermal neutron. To selectively destroy cancer cells, the high accumulation and selective delivery of boron-10 (10B) into tumor tissue are required. We have developed polyborane from 1,7-dicarba-closo-dodecaborane as a boron carrier. To evaluate tumor accumulation of polyborane, PEGylated liposomes were chosen as carrier. The mean volume diameters of polyborane-embedded liposomes were 50, 100, and 200 nm, respectively. They were injected into the tail vein of tumor-bearing mice. Twenty-four hours later, mice were killed and biodistribution of boron was determined using the inductively coupled plasma atomic emission spectrometry. At 24 h after injection, 50 nm bare liposome and 100 nm PEGylated liposome were found in tumor with high boron levels. Moreover, 50 nm bare liposome showed high tumor/blood ratios of boron concentration, and their usability for BNCT was suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron neutron capture therapy (BNCT) is a cancer therapy using nuclear reaction between boron and thermal neutron. It is based on the nuclear capture and fission reactions. When boron-10 (10B) is irradiated with low-energy thermal neutrons, it yields high linear energy transfer (LET), α particles (4He), and recoiling lithium-7 (7Li) nuclei. The high LET particles have limited path lengths in tissue (5–9 μm). The destructive effects of these high-energy particles are limited to boron containing cells. Therefore, it is possible to selectively destroy cancer cells if 10B is selectively delivered into the cancer cells. The high accumulation and selective delivery of 10B into tumor tissue are the most important requirements. In order to BNCT be successful, a minimum of 20–30 μg of non-radioactive 10B per gram of tumor tissue and tumor/blood boron concentration ratio of over 5 are required [1–3].
Liposomal drug delivery system on tumor-selective boron delivery in BNCT has been reported [2, 4–6]. A liposome is a closing vesicle formed with phospholipids, which are basic components of the biomembrane. It can encapsulate hydrophilic boron compounds within the aqueous core of the liposome, or lipophilic boron compounds within the lipid bilayer, or both types of boron compounds can be incorporated into the same liposome formulation [6]. Liposomes can also be PEGylated and grafted with targeting ligands [7]. PEGylation prolongs the retention time of liposomes in circulation by avoiding the uptake into the cells of the mononuclear phagocyte system (reticuloendothelial system), and it promotes the enhanced permeability and retention (EPR) effect in solid tumors [8–10]. Ligands are used to bind liposomes and tumor cells, and they allow receptor-directed targeting and intracellular delivery of liposomal drugs [11, 12]. Moreover, liposome size affects its biodistribution and tumor uptake [13]. In previous studies for BNCT, sodium or cesium salt of mercaptoundecahydrododecaborate (BSH) and boronophenylalanine (BPA) were used as boron carriers [2, 4, 5, 14, 15]. BSH, having 12 10B atoms, is known as a water-soluble cluster with low toxicity [16]. It is encapsulated into the aqueous compartment of liposomes. BPA, having one 10B atom, is known as hydrophobic boron compound, and it attains a high tumor concentration in brain [14]. Lipid analogs were synthesized using BPA to incorporate into phospholipid bilayers of liposomes [15].
In this study, we have developed polyborane for BNCT using liposomal drug delivery system, and evaluated its tumor accumulation property by measuring biodistribution of boron in mice. 1,7-Dicarba-closo-dodecaborane, a hydrophobic boron compound, was used as a boron carrier because dicarba-closo-dodecaborane has 10 boron atoms in its structure and exists as ortho, meta and para isomers, which differ in the relative positions of the carbon atoms in the cluster [17]. Although this boron compound is stable in the presence of many chemical reagents and it also has significant thermal stability, various synthetic routes were reported [18–21]. Moreover, it has hydrophobic property and easily available. To trap the boron in lipid bilayers of liposomes, we have synthesized a polyborane from 1,7-dicarba-closo-dodecaborane [22]. Moreover, bare and PEGylated liposomes, with diameter of 50, 100, and 200 nm, were prepared to study effects of particle size and PEGylation.
Materials and methods
Materials
1,7-dicarba-closo-dodecaborane (C2H12B10, purity ≥97 %), tetrahydrofuran (THF; C4H8O, purity ≥99.5 %, water ≤0.001 %), boron tribromide (BBr3; 99.85 %), chloroform-d (CDCl3; purity ≥99.8 %, containing 0.05 vol% TMS), boron standard solution (boron concentration 1000 mg/L), and Celite no. 503 (Imerys Minerals California, Inc.) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Egg phosphatidylcholine (egg PC) was purchased from Asahi Kasei Kogyo Co., Ltd. (Tokyo, Japan). Sunbright DSPE-020CN (DSPE-PEG, N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt, Mw 2000) was purchased from NOF Corporation (Tokyo, Japan). 1,2-Dimethoxyethane (DME) was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). n-Butyllithium (in hexane, ca. 1.5–1.7 mol/L) and heptyl p-toluene-sulfonate were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). 2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES) was purchased from Dojindo Molecular Technologies Inc. (Mashiki, Japan). All other chemicals were of the highest grade commercially available.
Synthesis of polyborane for BNCT
1,7-dicarba-closo-dodecaborane (3.5 g, 24.3 mmol) in DME (97.2 mL) was dropwise added into an n-butyllithium in hexane (1.57 M, 16.3 mL, 25.5 mmol) at 0 °C under Ar atmosphere. After stirring for 30 min at room temperature, copper chloride (3.13 g, 31.6 mmol) was added and then continuously stirred for 1 h. Pyridine (14.7 mL, 182 mmol) and 4-iodoanisole (5.97 g, 25.5 mmol) were also dropwise added into the reaction vessel, and the reaction mixture was stirred for 48 h at 100 °C. After cooling down to room temperature, the reaction mixture was diluted with diethyl ether and stirred for 15 h to quench the reaction. To remove insoluble materials, the reaction mixture was filtered through Celite no. 503. The filtrate was washed with hydrochloric acid (2 N), aqueous sodium thiosulfate, distilled water, and brine. Then, the organic layer was dried with anhydrous sodium sulfate and concentrated under reduced pressure. The crude solid residue was purified by silica gel column chromatography (hexane/ethyl acetate = 40:1), and as shown in Scheme 1, 1-(4-methoxyphenyl)-1,7-dicarba-closo-dodecaborane was obtained as a white amorphous solid (2.17 g, 35.7 %) [23]. 1H-NMR (δ, 300 MHz, CDCl3): 1.50–3.70 (10H, broad multiplet), 3.04 (1H, broad singlet), 3.77 (3H, singlet), 6.76 (2H, doublet, J = 9.0 Hz), and 7.33 (2H, doublet, J = 9.0 Hz).
1-(4-Methoxyphenyl)-1,7-dicarba-closo-dodecaborane (2.2 g, 8.67 mmol) in THF (86.7 mL) was dropwise added into a n-butyllithium in hexane (1.57 M, 5.8 mL, 9.11 mmol) over 45 min at 0 °C under Ar atmosphere. After stirring the mixture at room temperature for 1 h, heptyl p-toluene-sulfonate (2.46 g, 9.11 mmol) and n-butyllithium (1.57 M) in hexane were dropwise added, and continuously stirred at room temperature. After 12 h, cooled distilled water was added into the reaction mixture, and the desired compound was extracted with diethyl ether. The organic layer was washed with brine and dried with anhydrous sodium sulfate, and then concentrated under reduced pressure. The crude solid residue was purified by silica gel column chromatography (hexane/ethyl acetate = 40:1), and as shown in Scheme 2, 1-(4-methoxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane was obtained as a white amorphous solid (2.12 g, 70.3 %) [22]. 1H-NMR (δ, 300 MHz, CDCl3): 0.88 (3H, triplet, J = 6.8 Hz), 1.24–1.43 (10H, broad multiplet), 1.93–1.99 (2H, multiplet), 3.78 (3H, singlet), 6.75 (2H, doublet, J = 9.0 Hz), and 7.33 (2H, doublet, J = 9.0 Hz).
1-(4-Methoxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane (2.1 g, 6.10 mmol) in dichloromethane (61.0 mL) was dropwise added into a BBr3 in dichloromethane (1 M, 11.7 mL, 11.7 mmol) at −78 °C under Ar atmosphere. After stirred it at room temperature for 2 h, water was added at 0 °C, and the desired compound was extracted with diethyl ether. The extracted organic layer was washed with distilled water and brine. After drying with anhydrous sodium sulfate and concentrating under reduced pressure, the crude solid residue was purified by silica gel column chromatography (hexane/ethyl acetate = 10:1), and as shown in Scheme 3, 1-(4-hydroxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane was obtained as a white amorphous solid (1.91 g, 96.9 %) [22]. 1H-NMR (δ, 300 MHz, CDCl3): 0.88 (3H, triplet, J = 6.8 Hz), 1.24–1.38 (10H, broad multiplet), 1.93–2.04 (2H, multiplet), 4.97 (1H, singlet), 6.69 (2H, doublet, J = 9.0 Hz), and 7.30 (2H, doublet, J = 9.0 Hz).
Preparation of 1-(4-hydroxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane-embedded liposomes
1-(4-Hydroxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane-embedded liposomes were prepared using Bangham method. Compositions of them are shown in Table 1.
Lipid mixtures of 1-(4-hydroxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane, egg PC, and DSPE-PEG were dissolved in chloroform (10 mL) in eggplant flask, and chloroform was evaporated under reduced pressure. After a thin film was formed in eggplant flask, the film was resuspended with HEPES buffered saline (HBS, pH 7.4, 10 mM), and it was vortexed and sonicated 5 min, respectively. Fifty nanometer liposomes were prepared using Nanomizer Mark II (Yoshida Kikai Co., Ltd., Nagoya, Japan). It is one of the mechanochemical systems to prepare particles, and preparing conditions are shown in Table 2.
Liposomes with the diameters of 100 and 200 nm were prepared using Lipex Extruder (Northern Lipid Inc., Burnaby, Canada) and polycarbonate membrane (Nuclepore Track-Etched Membranes, Whatman Inc., Florham Park, USA). Samples were heated to 75 °C, and to obtain liposomes with the diameters of 100 and 200 nm, they were passed through polycarbonate membrane (pore sizes 100 nm) ten times and passed through polycarbonate membrane (pore sizes 200 nm) five times, respectively. The average diameters of the liposomes were determined using ELSZ-1000ZS (Otsuka Electronics Co., Ltd., Hirakata, Japan) which is one of the dynamic light scattering systems. Liposomal suspensions were diluted with HBS and measured at 25 °C. Boron concentrations of the liposomes were determined using the inductively coupled plasma atomic emission spectroscopy, ICPE-9000 (Shimadzu Corporation, Kyoto, Japan). Liposomal suspensions (0.1 mL) were diluted with 6.9 mL of distilled water and 3.0 mL of nitric acid and observed at 249.773 nm. A calibration curve was prepared using boron standard solution.
Biodistribution of liposomes
Twenty-seven mice (ddY, 7–8 weeks old, male) were housed in stainless steel cages and housed under standard environmental conditions (23 ± 1 °C, 55 ± 5 % humidity and a 12/12 h light/dark cycle) and maintained with free access to water and a standard laboratory diet (carbohydrates 30 %, proteins 22 %, lipids 12 %, vitamins 3 %) ad libitum (Nihon Nosan Kogyo Co., Yokohama, Japan). They were used in accordance with the Guidelines for Animal Experimentation of Tokyo University of Science, which are based on the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science.
B16 melanoma cells (2 × 105 cells in 50 μL PBS) were subcutaneously injected into footpad of right hind limb of mice, and tumor-bearing mice were given after 3–5 weeks (diameter of tumor 8–10 mm). Liposomes (8.7 mg boron/kg in PBS) were injected via the tail vein into tumor-bearing mice (ddY, 7–9 weeks old, male) with anesthesia, and animals were maintained in metabolic cages. The injections were well tolerated and no adverse effects were observed during the 24-h observation period. After 24 h, mice were killed by cervical dislocation with anesthesia, and blood at inferior vena cava and brain, heart, lungs, liver, stomach, pancreas, spleen, and kidneys was taken. Blood, urine, feces, and all tissues were weighed and melted using wet ashing method with nitric acid [24]. All boron concentrations of samples were determined using ICPE-9000. Multiple comparisons between groups were made by Tukey-Kramer test.
Results and discussion
Preparation of 1-(4-hydroxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane-embedded liposomes
Size distributions of prepared 50 nm liposomes are shown in Fig. 1. Mean volume diameters of 50 nm bare liposome, 50 nm 5 mol% PEGylated liposome, and 50 nm 10 mol% PEGylated liposome were 48.4 ± 20.7, 45.5 ± 18.6, and 51.1 ± 16.7 nm, respectively (means ± SD). Mechanochemical system was useful to prepare 50 nm liposomes, since it could treat relatively high volume of samples easily and rapidly. Size distributions of prepared 100 nm liposomes are shown in Fig. 2. Mean volume diameters of 100 nm bare liposome, 100 nm 5 mol% PEGylated liposome, and 100 nm 10 mol% PEGylated liposome were 98.9 ± 34.6, 110.9 ± 41.9, and 103.9 ± 36.6 nm, respectively. Size distributions of prepared 200 nm liposomes are shown in Fig. 3. Mean volume diameters of 200 nm bare liposome, 200 nm 5 mol% PEGylated liposome, and 200 nm 10 mol% PEGylated liposome were 200.4 ± 81.4, 200.8 ± 82.4, and 199.5 ± 88.8 nm, respectively.
Biodistribution of liposomes
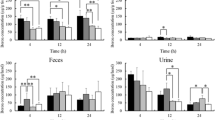
Boron concentrations of blood, tumor, urine, feces, and all organs (brain, heart, lung, liver, stomach, pancreas, spleen, and kidney) are shown in Figs. 4, 5, and 6. Average boron concentrations in tumor of 50 nm bare liposome, 50 nm 5 mol% PEGylated liposome, 100 nm 5 mol% PEGylated liposome, 100 nm 10 mol% PEGylated liposome, and 200 nm 10 mol% PEGylated liposome reached over 30 μg/g tissue. Fifty nanometer bare liposome and 100 nm 10 mol% PEGylated liposome had especially high values, 79.8 ± 33.1 and 71.6 ± 24.9 μg/g tissue, respectively (means ± SD). Table 3 shows tumor/blood ratios of boron concentration which is the ratio of boron concentration of tumor divided by that of blood. Average tumor/blood ratios of 50 nm bare liposome, 50 nm 5 mol% PEGylated liposome, 100 nm 10 mol% PEGylated liposome, 200 nm 5 mol% PEGylated liposome, and 200 nm 10 mol% PEGylated liposome reached over 5. As shown in Figs. 4, 5, and 6, high accumulations of boron in feces were confirmed. 1-(4-Hydroxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane is a lipophilic compound. Therefore, it is assumed that this compound is eliminated by hepatic metabolism.
From the results of Figs. 4, 5, and 6 and Table 3, it is found that 50 nm 1-(4-hydroxyphenyl)-7-heptyl-1,7-dicarba-closo-dodecaborane-embedded bare liposome is most suitable to BNCT because it showed high tumor accumulation and tumor/blood ratios of boron concentration, and it is suggested that PEGylation increases the tumor/blood ratios of boron concentration of prepared 100 and 200 nm polyborane-embedded liposomes. However, at 50 nm, PEGylation did not enhance the tumor/blood ratios. It is already reported that liposomes with a diameter less than 70 nm mainly accumulated in liver [11]. From the result of boron concentration of feces shown in Fig. 4, it is suggested that 50 nm PEGylated liposomes leak out from the tumor tissue and are released into the bloodstream after once accumulated in it, because of its size and retention property, and they are metabolized in liver.
Conclusions
We have synthesized polyborane for BNCT and successfully embedded it into liposomes. Its high tumor accumulation and selectivity were confirmed from the results of biodistribution study, and its usability for BNCT was suggested. Effects of PEGylation were also studied. In polyborane-embedded liposomes having diameter of 100 and 200 nm, tumor/blood ratios of boron concentration were increased by using PEGylation technique.
References
Barth RF, Coderrea JA, Vicente MGH (2005) Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 11:3987–4002. doi:10.1158/1078-0432.CCR-05-0035
Maruyama K, Ishida O, Kasaoka S, Takizawa T, Utoguchi N, Shinohara A, Chiba M, Kobayashi H, Eriguchi M, Yanagie H (2004) Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J Control Rel 98:195–207. doi:10.1016/j.jconrel.2004.04.018
Vicente MGH, Wickramasinghe A, Nurco DJ, Wang HJH, Nawrocky MM, Makar M, Miura M (2003) Synthesis, toxicity and biodistribution of two 5,15-di[3,5-(nidocarboranylmethyl)phenyl]porphyrins in EMT-6 tumor bearing mice. Bioorganic Med Chem 11:3101–3108. doi:10.1016/S0968-0896(03)00240-2
Yanagië H, Tomita T, Kobayashi H, Fujii Y, Takahashi T, Hasumi K, Nariuchi H, Sekiguchi M (1991) Application of boronated anti-CEA immunoliposome to tumour cell growth inhibition in in vitro boron neutron capture therapy model. Br J Cancer 63:522–526
Yanagië H, Tomita T, Kobayashi H, Fujii Y, Nonaka Y, Saefusa Y, Hasumi K, Eriguchi M, Kobayashi T, Ono K (1997) Inhibition of human pancreatic cancer growth in nude mice by boron neutron capture therapy. Br J Cancer 75:660–665
Hawthorne MF, Shelly K (1997) Liposomes as drug delivery vehicles for boron agents. J Neuro Oncol 33:53–58
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Cont Rel 148:135–146. doi:10.1016/j.jconrel.2010.08.027
Allen TM, Hansen C (1991) Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta 1068:133–141. doi:10.1016/0005-2736(91)90201-I
Lasic DD (1996) Doxorubicin in sterically stabilized liposomes. Nature 380:561–562. doi:10.1038/380561a0
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Cont Rel 65:271–284. doi:10.1016/S0168-3659(99)00248-5
Carlsson J, Kullberg EB, Capala J, Sjöberg S, Edwards K, Gedd L (2003) Ligand liposomes and boron neutron capture therapy. J Neuro Oncol 62:47–59. doi:10.1023/A:1023282818409
Shmeeda H, Tzemach D, Mak L, Gabizon A (2009) Her2-targeted pegylated liposomal doxorubicin: retention of target-specific binding and cytotoxicity after in vivo passage. J Cont Rel 136:155–160. doi:10.1016/j.jconrel.2009.02.002
Liu D, Mori A, Huang L (1992) Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta 1104:95–101. doi:10.1016/0005-2736(92)90136-A
Yang W, Barth RF, Rotaru JH, Moeschberger ML, Joel DD, Nawrocky MM, Goodman JH (1997) Enhanced survival of glioma bearing rats following boron neutron capture therapy with blood-brain barrier disruption and intracarotid injection of boronophenylalanine. J Neuro-Oncol 33:59–70. doi:10.1023/A:1005769214899
Shirakawa M, Yamamto T, Nakai K, Aburai K, Kawatobi S, Tsurubuchi T, Yamamoto Y, Yokoyama Y, Okuno H, Matsumura A (2009) Synthesis and evaluation of a novel liposome containing BPA–peptide conjugate for BNCT. Appl Radiat Isot 67:S88–S90. doi:10.1016/j.apradiso.2009.03.101
Lee JD, Ueno M, Miyajima Y, Nakamura H (2007) Synthesis of boron cluster lipids: closo-dodecaborate as an alternative hydrophilic function of boronated liposomes for neutron capture therapy. Org Lett 9:323–326. doi:10.1021/ol062840+
Valliant JF, Guenther KJ, King AS, Morel P, Schaffer P, Sogbein OO, Stephenson KA (2002) The medicinal chemistry of carboranes. Coord Chem Rev 232:173–230. doi:10.1016/S0010-8545(02)00087-5
Bregadze VI (1992) Dicarba-closo-dodecaboranes C2Bl0H12 and their derivatives. Chem Rev 92:209–223. doi:10.1021/cr00010a002
Coult R, Fox MA, Gill WR, Herbertson PL, Macbride JAH, Wade K (1993) C-arylation and C-heteroarylation of icosahedral carboranes via their copper (I) derivatives. J Organomet Chem 462:19–29. doi:10.1016/0022-328x(93)83337-U
Zheng Z, Jiang W, Zinn AA, Knobler CB, Hawthorne MF (1995) Facile electrophilic iodination of icosahedral carboranes. Synthesis of carborane derivatives with boron-carbon bonds via the palladium-catalyzed reaction of diiodocarboranes with Grignard reagents. Inorg Chem 34:2095–2100. doi:10.1021/ic00112a023
Endo Y, Iijima T, Yamakoshi Y, Fukasawa H, Miyaura C, Inada M, Kubo A, Itai A (2001) Receptor-targeted liposomal delivery of boron-containing cholesterol mimics for boron neutron capture therapy (BNCT). Chem Biol 8:341–355. doi:10.1021/bc060075d
Thirumamagal BTS, Zhao XB, Bandyopadhyaya AK, Narayanasamy S, Johnsamuel J, Tiwari R, Golightly DW, Patel V, Jehning BT, Backer MV, Barth RF, Lee RJ, Backer JM, Tjarks W (2006) Potent estrogenic agonists bearing dicarba-closo-dodecaborane as a hydrophobic pharmacophore. Bioconjug Chem 17:1141–1150. doi:10.1021/jm9900725
Endo Y, Iijima T, Yamakoshi Y, Yamaguchi M, Fukasawa H, Shudo K (1999) Potent estrogen agonists based on carborane as a hydrophobic skeletal structure: a new medicinal application of boron clusters. J Med Chem 42:1501–1504. doi:10.1016/S1074-5521(01)00016-3
Ueno M, Ban HS, Nakai K, Inomata R, Kaneda Y, Matsumura A, Nakamura H (2010) Dodecaborate lipid liposomes as new vehicles for boron delivery system of neutron capture therapy. Bioorganic Med Chem 18:3059–3065. doi:10.1016/j.bmc.2010.03.050
Acknowledgments
This work was supported by Program for Development of Strategic Research Center in Private Universities supported by MEXT (2010–2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Takeuchi, I., Tomoda, K., Matsumoto, K. et al. PEGylated liposomes prepared with polyborane instead of cholesterol for BNCT: characteristics and biodistribution evaluation. Colloid Polym Sci 294, 1679–1685 (2016). https://doi.org/10.1007/s00396-016-3925-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3925-4