Abstract
The objective of this study is developing the multiple emulsions of water-in-oil-in-water (W/O/W) used in pharmaceutical field and investigating the influence of additives and insulin on physicochemical and rheological stability of emulsions. Multiple emulsions based on corn oil, with insulin or without insulin, were formulated with a lipophilic surfactant polymeric in nature (Abil® EM 90) and hydrophilic surfactants of different nature: monomeric (Tween® 80) or polymeric (Lutrol® F127). The preparation was carried out by the two-step process of emulsification at 15 ± 1 °C. The developed multiple emulsions were studied and followed in stability by microscopic observations, granulometric analysis, and conductometric and rheological analyses. The multiple emulsions have been developed with the couple of surfactants: Abil® EM 90-Tween® 80 which exhibited a remarkable stability over 30 months. The mechanism of release of encapsulated substances occurs by swelling-rupture oily membrane. The study also shows that the incorporation of insulin has a significant influence on the physicochemical and the rheological stabilities of multiple emulsions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common multiple emulsions are of W/O/W type, consisting of both water-in-oil (W/O) and oil-in-water (O/W) emulsions. According to Chen et al. [1], the emulsions are heterogeneous systems consisting of droplets of a liquid dispersed in another non-miscible or partly miscible liquid. The role of emulsions is to encapsulate a hydrophilic or lipophilic active molecule inside the dispersed phase, thus ensuring its protection against environmental stress and degradation [2].
Today, emulsions are used in different branches of industry, such as in drilling fluids, in mechanical industry, and in the food industry for the control of microstructure, texture, cosmetic product, and the pharmaceutical field. In pharmaceutical field, the multiple emulsions are widely used as drug delivery systems for parenteral, oral, and topical routes [3, 4]. Despite their potential usefulness, applications of emulsions have been limited because of thermodynamic instability and strong tendency for coalescence over the time which is due to their complex structure. Stability and release characteristics of multiple W/O/W emulsions are affected by several factors, such as the nature of the oil phase, surfactant type, surfactant ratio, the nature of entrapped materials, and some physical properties of the system (globule size, viscosity, conductivity, phase volume ratio…) [5].
The stability of multiple W/O/W emulsions has been widely studied, and it was the subject of many previous works. The release kinetics of a water-soluble of two different multiple W/O/W emulsions prepared with two lipophilic surfactants at different concentrations was studied by Jager-Lezer et al. [6]. It was observed that the water-soluble drug release occurs by a mechanism of swelling followed by a breakdown of the oil globules, in which the lipophilic surfactant is a decisive factor and the increasing of lipophilic surfactant concentration causes amelioration in stability of the multiple emulsions.
Perez-Moral et al. [7] has shown that the addition of calcium during the homogenization stage leads to improvement of the stability against salt concentration in multiple emulsions. According to Kita et al. [8], it is possible to estimate the stability of the W/O/W emulsions by measuring the time dependence of the viscosity in a range of the relatively low disperse phase concentrations, although the presence of ingredients, such as protein, electrolytes, and saccharides, in both aqueous phases affects the rheological properties of the W/O/W emulsions. Cole and Whateley [9] investigated the effects of Pluronic F127/polyacrylic acid (PAA) and 125I-insulin on stability of multiple W/O/W emulsions. The authors have shown that the release pattern of 125I-insulin from the same emulsion after 3 days of storage was not significantly different from the newly prepared emulsion and insulin in multiple W/O/W emulsions where they found them to be stable and could thus be formulated for rapid onset of action or as sustained drug delivery system, depending on the choice of Pluronic/PAA complex in the internal aqueous phase and the type of lipophilic surfactant in the oil phase.
In this study, the effect of several modifications and conditions on the physicochemical properties and rheological stability of multiple emulsions was investigated. The originality of this work lies in the use of corn oil as lipophilic phase which is a vegetable oil rich in essential fatty acids and the increase of the concentration of insulin in the internal aqueous phase of the multiple emulsions.
Materials and methods
Material and composition
The oil phase is consisted of corn oil (Fluka Biochemika, Swiss). The lipophilic surfactant used was cetyl dimethicone copolyol (Abil® EM 90) that was kindly provided by Goldschmidt, France. The hydrophilic surfactants were polyoxyethylene sorbitan monooleate (Tween® 80, Panreac, Spain) or poloxamer 407 (Lutrol® F127, ICI, France). The other substance was NaCl used in the inner water phase as a conductometric tracer; NaCl was supplied in pH. Eur. grade by Prolab (France), recombinant human insulin (28.4 UI/mg) (Sanofi Aventis, Germany). Distilled water (Saidal, Algeria) was used in all experiments.
Preparation of the multiple emulsions
Four different multiple emulsions were prepared either without insulin (ME2 and ME4) or containing insulin in the internal phase (ME1 and ME3), at room temperature.
The internal aqueous phase of the different multiple emulsions were prepared as follows: in order to facilitate dissolution of insulin (3 mg/mL), the estimated quantity was dissolved in dilute hydrochloric acid (3.2 mL of distilled water and 1000 μL of HCl 0.2 N) and then addition of 180 mg NaCl and adjusting the volume of the solution to 100 mL by using the buffered saline solution [KH2PO4 M/15 = 9.08 g/L (39 mL) + Na2HPO4 M/15 = 9.47 g/L (161 mL)]. pH measurements were carried out with a pH meter (InoLab pH 730, WTW GmbH, Weilheim), conductivity measurements were performed with (InoLab cond 720, WTW GmbH, Weilheim), and the osmolar concentration was measured with a cryoscopic osmometer (OSMOMAT 030, Gonotec).
The composition and properties of the internal aqueous phase of the different multiple emulsions are shown in Tables 1 and 2.
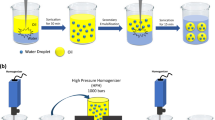
The multiple emulsions were prepared using a two-step process; in the first step, a primary W/O emulsion is prepared by adding during 15 min the aqueous phase containing the electrolyte (and eventually insulin) to the oil phase containing the hydrophobic surfactant and the stirring speed was fixed at 3000 rpm for 30 min with a Rayneri agitator® (Turbotest 33/300P, Bioblock, France). In the second step, 80% of the primary emulsion is dispersed slowly (during 50 min) in an aqueous solution of the hydrophilic surfactant, while the system was stirred at low speed (800 rpm) until the formation of the W/O/W multiple emulsions, which was checked by microscopy observation. The compositions of the multiple emulsions are shown in Table 3, and Table 4 summarizes the characteristics of fabrication process of the multiple emulsions. The two stages of emulsification were carried out at 15 °C ± 1 °C (to preserve insulin from degradation).
The multiple emulsions were stored at +4 °C, and the characteristic properties of the systems were performed at various time intervals during the storage.
Conductivity measurements
The measurements were made at 20 °C on diluted 1/20 multiple emulsions in distilled water or iso-osmotic glucose solution (mean ± S.D, n = 2). The standard deviation was negligible, and therefore, it was not plotted in the graphs.
Conductivity measurements were carried in order to study the conductometric behavior of the freshly prepared multiple emulsions when diluted in different backgrounds and also to evaluate the stability at equal time intervals of the multiple emulsions during storage at 4 ± 2 °C; it should be noted that any increase in conductivity over time indicates an electrolyte release from the internal aqueous phase to the external aqueous phase [10].
Macroscopic and microscopic observations
Microscopic analysis was performed to determine the oily globule size of the W/O/W multiple emulsions, as well as the verification of the multiple structures. It was carried out using an optical immersion microscope equipped with an ocular having a micrometer scale (Leitz Wetzlar, Germany) camera at ×1000 magnifying power (oil immersion).
Observations were made after diluting the samples of multiple emulsions in iso-osmotic NaCl solution with regard to the internal aqueous phase [11].
The mean oily globule diameters and standard deviation (S.D) were calculated for each sample. Also, photomicrographs of the multiple emulsions were taken at various times during the storage.
General characteristics of the multiple emulsions
In order to investigate long-time stability of multiple W/O/W emulsions, macroscopic observation, globule size, conductivity, and measurement rheological analysis of all formulations were analyzed over time.
All the multiple emulsions obtained were macroscopically homogeneous white, fine, and creamy for ME1 and ME2 and fluid in the case of ME3 and ME4. Table 5 shows the organoleptic parameters and properties of the multiple emulsions immediately after the preparation.
The aspect of the multiple emulsions studied through the optical microscopy has shown the presence of the multiple characters of the sample. Microscopic pictures for freshly prepared multiple emulsions are given in Fig. 1.
Rheological measurements
All rheological measurements were performed by using a torque controlled rheometer (RS600 from Thermo-Fischer), equipped with cone-plate geometry (diameter 60 mm; angle 2°; gap 105 μm). It has a Peltier temperature control system that allows having a very quick response to any change in temperature. In order to prevent changes in composition during measurements due to water evaporation, a solvent trap was placed around the measuring device.
Results and discussion
Microscopic and globule size analysis
The comparison and the analysis study of the evolution of the multiple globule dimensions of the multiple W/O/W emulsions show that the characteristics of the four systems developed (ME1–ME4) vary from one emulsion to another. Indeed, in the case of ME1 and ME2 prepared with Abil ® EM 90 as a lipophilic surfactant and Tween® 80 as hydrophilic surfactant, it was found that the introduction of insulin in internal aqueous phase of the multiple emulsions induces the formation of smaller oil globule diameters, respectively, 11 ± 5.6 μm against 18 ± 7.9 μm without insulin.
Indeed, insulin is a protein, and its presence in the formula of a multiple emulsion contributes significantly in the emulsification process; effectively, Table 4 shows that ME1 was formed after 16 min, while it took more 4 min to obtain ME2.
Furthermore, it has been reported in many works [12, 13] that proteins can not only adsorb in the oil/water interface but also interact with the surfactant which increases the elasticity and strength of the globules, thereby avoiding their rupture. It was observed in Fig. 2 that a slow and gradual increase of the oily globule diameters over the time for ME1 and ME2, however, was followed by a decrease of the diameter during the second year of storage. According to Benichou et al. [12], Omotosho et al. [13], and Ursica et al. [14], the increase of the globule sizes would be the consequence of a coalescence phenomenon of oily globules, itself induced by an aqueous stream from the outer aqueous phase to the inner aqueous phase. The increase of globule sizes could be followed by a decrease in its size during storage, which would occur, in a first step, due to the aqueous droplets which contraction is induced by a transfer of water from the inner aqueous phase to the external aqueous phase. In a second step, the possibility of expulsion of the aqueous droplets of the primary emulsion to the external aqueous compartment would happen.
Moreover, when the multiple emulsions are prepared with Lutrol® F127 as a hydrophilic surfactant, the mean size of the multiple globules is much larger with 40 ± 28.8 and 42 ± 24.3 μm, respectively, for ME3 and ME4, which result in a decrease in stability of these systems. Indeed, during the storage, a coalescence phenomenon appeared after 4 months for ME3 and 8 months for ME4 (Fig. 3), which is obvious given the fact that it is well known that the large-diameter particles have a strong tendency to coalesce.
In the case of ME3 and ME4 systems, the surfactant properties of insulin, and the use of a hydrophilic surfactant polymeric nature known for its high performance, have not been demonstrated in this experiment.
Electrical conductivity tests for multiple emulsions
Figure 4 shows the conductivity evolution over the time of the freshly prepared W/O/W emulsion diluted 1/20 in a hypotonic medium. An aqueous flow which causes a progressive swelling of the oily globules until they rupture was observed, which is obvious considering the release of the tracer (NaCl) initially incorporated in the aqueous droplets of the internal phase of each emulsion. In contrast, when the multiple emulsions are diluted 1/20 in iso-osmotic solutions (glucose) to the internal aqueous phase of each emulsion, the absence of evolution of the conductivity indicates good stability of size of the oily globules and hence the absence of release of NaCl in the external aqueous phases.
Figure 5 shows the optical microscopy images of the multiple emulsions ME1 in hypotonic medium after dilution. In these images, two phenomena are observed; globule swelling is observed after 15 h (image A) followed by their rupture in 48 h (image B) later, after maintaining ME1 under hypo-osmotic conditions.
In order to investigate long-time stability of multiple W/O/W emulsions, the conductivity of all formulations were analyzed over a period of 920 days. The samples were stored at temperature of 4 °C. Figure 6 shows conductometric analysis of four systems studied (ME1, ME2, ME3, and ME4) as a function of storage time. It is clear that the emulsions made with Tween® 80 have different characteristics and better stability than those made with Lutrol® F127; this finding is in perfect agreement with the particle size analysis results which are previously described. Although the multiple emulsion ME1 is characterized by multiple smaller globules compared with ME2, unexpectedly, an acceleration of the release of NaCl is observed beyond 300 days of storage particularly with ME1. This latter presents a conductivity of 25.2 S/cm against 16.9 S/cm for ME2; after 800 days later, the conductivity continues its ascent which is 46.6 S/cm for ME1 instead of 28.1 S/cm for the emulsion without insulin; at 920 days of storage, the conductivity of ME1 (59.4 S/cm) is twice higher than that of ME2 (28.7 S/cm).
Furthermore, the monitoring of the stability of the multiple emulsions by microscopic observation at regular times allowed observing, particularly for ME1, after 920 days of storage, the phenomenon illustrated in Fig. 7, which shows in one hand, intact oil globules, and on the other hand, globules having lost their integrity that eventually coalesce. The existence of such aggregates seems to justify the increase of the conductivity of ME1 during storage.
It appears that the decrease in long-term stability of ME1 compared with ME2 is due to the presence of insulin. This situational context was previously highlighted by many authors [13, 15, 16]. According to Benichou et al. [12] and Schmidts et al. [17], the stabilizing power of proteins depends not only on the experimental conditions but also on the concentrations of the protein and the nature of the surfactant used. The substitution of Tween® 80 by Lutrol® F127 for the preparation of ME3 and ME4 has certainly improved the fluidity of these preparations; however, this qualitative change in the starting formula had an impact not only on the properties of obtained systems but also on their long-term stability. This lesser stability can be explained by:
-
The importance of the size of the oily globules ME3 and ME4 emulsions and, consequently, their low rupture strength.
-
A high conductivity immediately after emulsification and its fairly rapid evolution over time, which has resulted in the destabilization of these two systems.
These results suggest that the Lutrol® F127 amount used to formulate ME3 and ME4 is not appropriate to the formation of a strong oil/water interface to prevent the transfer of water through the oil membrane.
Rheological properties of multiple W/O/W emulsions
Effect of swelling–breakdown globules on the apparent viscosity of multiple emulsions
The material transfers between the continuous phase and the inner phase of the multiple emulsions can be evaluated by measuring viscosity over time of the emulsion. After a rest time of 180 s under geometry of measurement, the samples were sheared during 7200 s at constant shear rates (100 s−1) and at a constant temperature of 20 °C. In order to prevent any irreversible evolution of the multiple emulsions diluted, a new fresh sample was used for each emulsion. This study was performed in order to obtain information about the evolution of the volume fraction of the dispersed globules over time.
Figure 8a, b shows the evolution of apparent viscosity as a function of sheeting time of the multiple emulsions ME1 freshly prepared and diluted to one third in distilled water (hypo-osmotic medium) and in a glucose solution (iso-osmotic medium). At a constant shear rate in hypo-osmotic medium, the viscosity of the multiple emulsions significantly increases shearing time to reach a maximum value (Fig. 8a). The observed increase in viscosity for emulsions that were prepared in absence of glucose solution can most likely be explained by the transfer of water between both water phases. Transfer of water from the outer into the inner water phase or vice versa can be excluded since swelling or shrinking of droplets would result and would have been detected as changes of droplet sizes over the time. Thus, sheering of the emulsions caused an increase of viscosity caused by swelling or shrinking droplets. This phase is followed by a decrease of the viscosity up to equilibrium; this corresponds to a rupture of the oily globules; in our case, this rupture occurred on after about 20 min and reaches a constant value corresponding to an equilibrium state after approximately 4000 s. The stability of apparent viscosity of multiple emulsions in hypo-osmotic medium after 4000 s of shearing can be explained by total transfer of water from outer into the inner and, therefore, a stable size of the oily globule.
However, in iso-osmotic conditions, the profile obtained in Fig. 8b shows no change in viscosity for the entire duration of the experiment, causing the absence of aqueous streams between the two internal and external aqueous phases and, therefore, a stable size of the oily globule.
Oscillatory measurements
The aim of the test is to study the oscillatory viscoelastic analysis of multiple emulsions developed ME1 and ME2, which allowed to interpret the structure of the system and evaluate its elasticity; we proceeded to the determination of the transition between the elastic and viscous behavior by observing the elastic modulus G′ and viscous modulus G″ during 7 weeks. The measurements were performed at 25 °C in order to determine the linear viscoelastic region of the samples (amplitude sweep). After the linear viscoelastic region had been determined, the stress was increased from 0.1 to 100 Pa at a fixed frequency of 1 Hz in stress sweep experiments. Figures 9 and 10 show the elastic modulus G′ and viscous modulus G″ of multiple emulsions ME1 (with insulin) and ME2 (without insulin) developed at a frequency of 1 Hz as a function of the amplitude of the oscillatory shear stress for different storage times [18]. The shear stress range in which G′ and G″ are constants determines the viscoelastic linear region of the systems: it is clear that the maximum stress that the ME1 and ME2 can withstand before acquiring a non-linear viscoelasticity is the zero week for all emulsions. The elastic modulus G′ of all multiple emulsions is higher than the viscous modulus G″ at low shear, attesting for their predominant elastic behavior. However, the elastic modulus G′ of ME1 is higher than that of ME2 (Fig. 9).
Consequently, the rheological behavior of ME1 was considered as more elastic than that of ME2. The incorporation of insulin in the formulation of multiple emulsions improves the elasticity of the system by reducing the oil/water interfacial tension. However, the observed decrease in viscoelastic during storage for the multiple emulsions prepared (Figs. 10 and 11) can most likely be explained by the transfer of water and/or insulin between both water phases.
The crossover stress, in which the storage and loss modulus were equal, has been used to characterize the relative importance of elastic and viscous effects in emulsions in which crossover-existed stress corresponds to the stress in which the phase angle δ between the imposed stress and the resulting strain is 45°, i.e., \( tg\left(\delta \right)=\frac{G^{\prime \prime }}{G^{\prime }}=1 \).
Figure 12 shows the variation of characteristic stress for ME1 and ME2 as a function of storage time. The figure shows that characteristic stress decreased with increasing storage time for two multiple emulsions (ME1 and ME2). After 4 weeks, the characteristic stress of multiple emulsions with insulin stabilized, while the characteristic of multiple emulsions without insulin stay in decreasing with storage time. All these facts can be qualitatively explained by the presence of insulin in multiple emulsions which improve their rheological stability.
Conclusion
The aim of this work is developing the multiple W/O/W emulsions used in pharmaceutical field and investigating the influence of additives and insulin on physicochemical and rheological stability of emulsions. The study shows that multiple emulsions developed with the couple of surfactants “Abil®EM90-Tween® 80” exhibited a remarkable stability over 30 months. The hydrophilic surfactant has a significant influence on the stability of multiple emulsions. However, incorporation of insulin has a significant influence on the physicochemical and rheological behaviors of ME, allowing to obtain oily globules with lower diameter while conferring good elasticity at the interface oil/water system formulated with Tween® 80 (ME1) as it was demonstrated in rheological tests; conversely, this protein appears to have an influence on the rate of release of the encapsulated substances and, therefore, the long-term stability. Corn oil proved to be very interesting for formulating the multiple emulsions by reason of its high viscosity; the latter allowed in the conditions of the experiment, obtaining a sufficiently dense oily membrane for slowing transfer of substances between the two internal and external aqueous phases. About the encapsulated substances release mechanism in multiple emulsions studied, the latter is carried out markedly by swelling process, followed by rupture of the oily membrane. Understanding this mechanism was supported by conductivity measurements, viscometric analyses (under hypo-osmotic and iso-osmotic conditions), and microscopic observations.
References
Chen J, Vogel R, Werner S, Heinrich G, Clausse D, Dutschk V (2011) Influence of the particle type on the rheological behavior of Pickering emulsions. Colloids Surf A Physicochem Eng Asp 382:238–245
Bouyer E, Mekhloufi G, Rosilio V, Grossiord JL, Agnely F (2012) Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: alternatives to synthetic surfactants in the pharmaceutical field? Int J Pharm 436:359–378
Rainer HM, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery ± a review of the state of the art. Eur J Pharm Biopharm 50:161–177
Cournarie F, Rosilio V, Vauthier C, Lacour B, Grossiord JL, Seiller M (2004) Improved formulation of W/O/W multiple emulsions for insulin encapsulation. Influence of the chemical structure of insulin. Colloid Polym Sci 282:562–568
Schmidts T, Dobler D, Nissing C, Runkel F (2009) Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J Colloid Interface Sci 338:184–192
Jager-Lezer N, Terrisse I, Bruneau F, Tokgoz S, Ferreira L, Clausse D, Seiller M, Grossiord JL (1997) Influence of lipophilic surfactant on the release kinetics of water-soluble molecules entrapped in a W/O/W multiple emulsion. J Control Release 45:1–13
Perez-Moral N, Watt S, Wilde P (2014) Comparative study of the stability of multiple emulsions containing a gelled or aqueous internal phase. Food Hydrocoll 42:215–222
Kita Y, Matsumoto S, Yonezawa D (1997) Viscometric method for estimating the stability of W/O/W-type multiple-phase emulsion. J Colloid Interface Sci 62:87–94
Cole ML, Whateley TL (1997) Release rate profiles of theophylline and insulin from stable multiple w/o/w emulsions. J Control Release 49:51–58
Cournarie F, Savelli MP, Rosilio V, Bretez F, Vauthier C, Grossiord JL, Seiller M (2004) Insulin-loaded W/O/W multiple emulsions: comparison of the performances of systems prepared with medium-chain-triglycerides and fish oil. Eur J Pharm Sci 58:477–482
Magdassi S, Frenkel M, Garti N (1984) On the factors affecting the yield of preparation and stability of multiple emulsions. J Dispers Sci Technol 5:49–59
Benichou A, Aserin A, Garti N (2004) Oil-in-water-in-oil double emulsions stabilized with WPI-polysaccharide conjugates. Colloids and Surfaces A: Physicochimical and Engineering Aspects 297:211–220
Omotosho JA, Law TK, Wetheley TL, Florence AT (1986) The stabilization of W/O/W emulsions by interfacial interaction between albumin and non ionic surfactants. Colloids and Surfaces 20:133–144
Ursica L, Tita D, Palici I, Tita B, Vlaia V (2005) Particle size analysis of some water/oil/water multiple emulsions. J Pharm Biomed Anal 37:931–936
Florence AT, Whitehill D (1981) Some features of breakdown in water-in-oil-in-water multiple emulsions. J Coll Interf Sci 79:243–256
Garti N, Aserin A (1996) Double emulsions stabilized by macromolecular surfactants. Advenced in Colloid and Interface Science 65:37–69
Schmidts T, Dobler D, Schlupp P, Nissing C, Garn H, Runkel F (2010) Development of multiple W/O/W emulsions as dermal carrier system for oligonucleotides: effect of additives on emulsion stability. Int J Pharm 398:107–113
Benchabane A, Bekkour K (2008) Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym Sci 286:1173–1180
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Moussaoui, N., Hammadi, L., Boudjenane, NE. et al. Development of multiple W/O/W emulsions used in pharmaceutical field: effect of additives and insulin on physicochemical and rheological stability of emulsions. Colloid Polym Sci 295, 125–133 (2017). https://doi.org/10.1007/s00396-016-3989-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3989-1