Abstract
W/O/W multiple emulsions are systems of potential interest in the oral administration of insulin. Although it has been shown that a single oral administration of insulin-loaded W/O/W multiple emulsion to diabetic rats led to the significant decrease of blood glucose levels (Silva Cunha et al., 1998, Int J Pharm 169:33), repeated administrations displayed unpleasant side effects such as diarrhoea and steatosis. These unwanted effects were attributed to the high oil concentration used for their preparation. In the present study, attention was focused on the reduction of oil concentration in the formulation of these systems and on the encapsulation of two different insulins. The physical properties and stability of the multiple emulsions over long periods of time were assessed by conductivity measurements, and granulometric and microscopic analyses. The encapsulation in the inner aqueous phase of two insulins, Umulin and Humalog, differing only by the transposition of one amino acid, had non-negligible effects on the formation and stability of W/O/W multiple emulsions. Both insulins were shown to improve the formation of the multiple emulsions. Circular dichroism studies and surface tension measurements evidenced the contribution of insulin conformation and surface properties in multiple emulsion formation and stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
W/O/W multiple emulsion systems (ME) for the oral administration of insulin were first developed by Engel et al. [1]. A significant reduction in blood glucose was observed when those systems were administered by intraduodenal injection to rats. Since then, various investigations have been performed on insulin-loaded ME [2, 3, 4, 5]. Although ME were considered as interesting vehicles for oral administration of insulin, the heterogeneity of the experimental results led to the conclusion that their formulation should be optimized. De Luca et al. [6] developed for the first time ME with improved stability, which were later used by Silva Cunha et al. [7, 8] for insulin encapsulation. These ME were composed of soybean oil or medium-chain triglycerides easily metabolized by mammals, and stabilized both by a silicone-based copolymer and a polyoxyethylene sorbitan ester. Silva Cunha et al. [7] reported that the stability of those emulsions lasted over a period of six months at 4 °C, and that insulin encapsulation efficiency was higher than 95%. In vitro, the encapsulated insulin was protected against the enzymatic degradation caused by pepsin, trypsin and α-chymotrypsin. In vivo, the decrease of blood glucose levels after a single oral administration to diabetic rats reached 35% [9]. However, diarrhoea and steatosis appeared in rats that received repeated administrations of ME and were attributed to the high oil content of the ME (35%). Introduction of insulin in the inner aqueous phase may alter the formation and stability of ME. Indeed, it is well known that proteins have surface properties and adsorb at the oil/water interface, forming a protein-rich interfacial layer [10]. It is believed that the polypeptide chain at the water/oil interface forms loops floating either in water or in oil, according to the affinity of their amino-acid sequences. Moreover, the surface tension of insulin may vary with its primary structure [11]. Thus, the aim of the present study was double: (i) to form stable ME with a low oil content and (ii) to study the influence of insulin, particularly its chemical structure, on the formation and stability of ME.
Materials and methods
Materials
Biosynthetic human insulins (Umulin and Humalog) were donated by Eli Lilly Company (Indianapolis, USA). Miglyol 810 N constituted the oily phase (Condea Chemie Company, Germany). Abil EM-90, used as the oil-soluble surfactant (low HLB surfactant), was supplied from Goldschmidt (France). The water-soluble surfactants (high HLB surfactants) were either Tween 80 or Arlatone F127G (ICI Surfactants, Belgium). Magnesium sulphate and sodium chloride from Prolabo (France) were used as conductimetric tracers. Glucose (Prolabo, France) was used to prepare iso-osmotic solutions to ME inner aqueous phases. Chloroform and methanol were analytical grade (Prolabo, France). Deionized water (MilliQ, Millipore, France) was used throughout.
Methods
Preparation of the multiple emulsions
A two-step process was used for the preparation of the ME [7]. First, a W/O primary emulsion was formed by adding an aqueous phase to an oily phase containing the low HLB surfactant and stirred for 30 min at 3,000 rpm. Second, 80% of this emulsion was dispersed in an aqueous solution of the high HLB surfactant. The stirring rate and time of ME were adapted to the nature of the constituents. The inner aqueous phases were constituted of 0.6 wt% of MgSO4 for formulation optimization, buffered saline insulin solution (2.8 mg/mL) at pH 7 for insulin-loaded ME, and buffered saline solution for control ME. The insulin solutions were prepared by dissolution of 200 mg of insulin in acidic water (water: 3.2 mL; 0.2N HCl: 800 µL) and then addition of a buffered saline solution (KH2PO4: 0.12 wt%; Na2HPO4: 0.43 wt%; NaCl: 0.18 wt%). The whole emulsification process was performed using a Rayneri centripetal stirrer (3 cm in diameter) at 25±1 and 15±1 °C, for formulation optimization and in the presence of insulin, respectively.
Characterization of the multiple emulsions
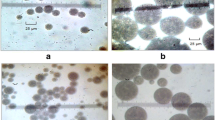
The conductivity of ME diluted 1:20 with iso-osmotic solutions was measured at room temperature by means of a CDM 230 Tassel Conductimeter (Radiometer Copenhagen, France). It allowed determination of the weight fraction of electrolyte released into the outer aqueous phase and consequently the encapsulation efficiency [12]. Microscopic analysis of the W/O/W emulsion was performed to check the multiplicity character of the emulsion (Optical Immersion Laboval 4 microscope, Bioblock, France). The mean size and size distribution of the multiple globules were measured using a laser diffraction granulometer (Coulter LS 230, Coultronics, France) and analysed with the Fraunhofer model. The parameter taken into account was the d43 diameter moment/volume. Stability studies were performed on ME stored at 4±1 and 40±1 °C at different time intervals.
Surface tension measurements
The surface tension of insulin solutions was measured at 22±1 °C using a Krüss K10 tensiometer (Germany) [13]. The accuracy of the measurements was estimated to be 0.2 mN/m. The surface pressure π was deduced from the relationship:
in which γ0 is the surface tension of the buffer solution and γ the surface tension observed in the presence of an adsorbed insulin or spread surfactant monolayer. In experiments where insulin (0.035 mg/mL) was injected beneath a spread Abil EM-90 monolayer, the surface pressure changes, Δπ, were deduced from:
where πT is the total surface pressure measured and πi the initial surface pressure due to the presence of the surfactant monolayer at the air/solution interface.
Dynamic interfacial tension measurements
Dynamic interfacial tension was measured at 20±1 °C by the dynamic drop tensiometer using the Tracker (IT Concept, France) as described by Benjamins et al. [14]. Briefly, a pending drop of Miglyol 810 N was formed at the tip of a syringe into a thermostated optical glass container filled with the aqueous insulin solutions. The interfacial tension γ was determined by analysing the profile of the droplet according to the Laplace equation. The area of the droplet was submitted to sinusoidal fluctuations at a frequency of 0.2 Hz to measure the elastic modulus E given by the equation:
where A is the surface area of the droplet and Δγ the variation of the interfacial tension resulting from the interfacial area change ΔA.
Circular dichroism studies
Circular dichroism spectra of insulin solutions were recorded using a Jasco J-810 dichrograph (Tokyo, Japan) equipped with a Peltier-type temperature control system. Insulin solutions were scanned from 300 to 250 nm and from 250 to 200 nm in 0.1-cm and 0.01-cm path-length cells, respectively, at a scanning rate of 10 nm/min and a temperature of 25±1 °C. The generated ellipticity was measured and subsequently converted to molar circular-dichroic absorption (Δε) using Eq. 4:
where θ is the observed ellipticity, C the insulin concentration (mol/L) and l the path length of the cell. Spectra are expressed in Δε as a function of the wavelength; Δε (M−1 cm−1) is the differential molar dichroic absorption coefficient.
Results
Optimization of W/O/W multiple emulsions without insulin
Effect of oil and surfactants on the formation and stability of ME
In the ME described by Silva Cunha et al. [7, 8], the primary W/O emulsion consisted of 45% Miglyol 810 N, 5% Abil EM-90 and 50% of an insulin solution. The W/O/W ME was then formed from 75% primary emulsion and 25% aqueous solution containing 4% Tween 80. When the oil concentration was reduced from 45 to 25%, a rapidly instable ME was formed (data not shown). Therefore, it appeared necessary to modify the formulation of the ME. The nine new ME, prepared with different concentrations of low and high HLB surfactants, or by changing the nature of the high HLB surfactant, appeared as white creams and microscopic observations confirmed the presence of multiple oily globules. It is worth noting that, for all the prepared ME, the encapsulation efficiency was higher than 96%.
Influence of the concentration of the low HLB surfactant
Concentrations of Abil EM-90 higher than 3% were necessary to obtain a ME still stable after 1 month storage at 4 and 40 °C (Table 1). ME 1 and 2, prepared with high Abil EM-90 concentrations, were stable for 6 months without change of the globules' size. After 2 years of storage at 4 °C, the size increased considerably, while the encapsulation efficiency remained unchanged. Since a lower Abil EM-90 concentration favoured the formation of a 6 months stable ME with smaller globules, and from the perspective of oral administration, the concentration of 4% was selected for further experiments.
Influence of the nature of the high HLB surfactant
The replacement of the monomeric high HLB surfactant (Tween 80) by a polymeric surfactant (Arlatone F127G) led to a significant reduction in both the duration of the second emulsification and the mean globule size (Table 2). Furthermore, the release of electrolyte with time decreased in the ME prepared with Arlatone F127G compared to that prepared with Tween 80 and its stability at 4 °C increased considerably.
Influence of the concentration of the high HLB surfactant
The effects of various Arlatone F127G concentrations (0.5, 1, 1.5 and 2%) on the formation and behaviour of ME were compared. Obviously, Arlatone F127G at the concentration of 1%, allowed formation of ME with the highest entrapment properties and the best long-term stability. Indeed, in the ME prepared with 0.5, 1.5 and 2% of Arlatone F127G, an increase in conductivity, indicating a decrease in encapsulation efficiency, was observed after 1 month of storage at 4 and 40 °C, suggesting multiple globules breaking. The ME prepared with 0.5% of Arlatone F127G also showed larger globules (15±10 µm).
From these data, an appropriate ME formula was elaborated, in which the primary emulsion is formed of 25% Miglyol 810 N, 4% Abil EM-90 and 71% of an aqueous phase. Eighty per cent of this emulsion is dispersed into the second aqueous phase constituted of 1% Arlatone F127G and 19% water. This ME showed an electrolyte entrapment of about 99%, which remained almost constant for more than 6 months.
Effect of insulin encapsulation on the formation and stability of ME
Preparation of insulin ME was performed at a lower temperature to avoid destruction of the protein during the emulsification process. With this in view, we formulated a control ME without insulin at 15 °C, using the previously optimized formula. This ME showed bigger globules compared to that prepared at 25 °C (ME 6). It was obtained after 1 h of emulsification and was only stable for 3 months. Obviously, the lowering of the temperature had an unfavourable effect on ME formation. Insulin encapsulation into the inner aqueous phase improved ME formation (Table 3). Surprisingly, the nature of the insulin introduced in the primary emulsions also had an effect on their consistencies: whereas Umulin led to the formation of a compact primary emulsion, that containing Humalog appeared to be fluid. Moreover, the stirring time for ME formation, the globule size and the final consistency of the obtained emulsions differed significantly. A highly viscous and homogeneous ME was obtained with Humalog after a short stirring time, which exhibited small multiple globules. Conversely, the formation of a fluid ME with Umulin required longer stirring and resulted in much larger sized globules with heterogeneous distribution profiles. Conductivity measurements showed a higher entrapment efficiency for Humalog ME compared to Umulin ME. Moreover, the Umulin ME showed (i) a progressive decrease in encapsulation efficiency, (ii) an increase in emulsion fluidity and, after 3 months of storage, (iii) a transformation into a simple O/W emulsion demonstrating the lower stability of this ME compared to the Humalog-containing ME.
Physicochemical properties of Umulin and Humalog
Interfacial properties of Umulin and Humalog
Insulin adsorption at the air/solution interface
The surface tension of both insulins was measured at the air/solution interface (Fig. 1). A significant drop in surface tension was immediately observed from 72.5 mN/m for the buffer solution, to 56.5 and 55.7 mN/m for Umulin and Humalog, respectively. Equilibrium values were reached in less than 60 min.
Insulin adsorption at the oil/solution interface
Dynamic interfacial tensions between Miglyol and the aqueous phase containing Umulin or Humalog were measured. Figure 2 shows the dynamic surface relaxation after insulin adsorption at the interface. Humalog tends to decrease the interfacial tension to a higher extent than Umulin and its adsorbed film exhibits a higher elasticity than that of Umulin.
Insulin adsorption into an Abil EM-90 monolayer
Injection of insulin beneath an Abil EM-90 monolayer (πi≈20 mN/m) led to significant surface pressure changes Δπ, which account for the adsorption and penetration of both insulins into the surfactant monolayer. From the results plotted in Fig. 3, it appears that Humalog adsorbed more slowly but to a higher extent than Umulin into the spread surfactant monolayer. As shown in the inset to Fig. 3, the maximum surface pressure increments (Δπmax) were reached after 30 min and 10 h following insulin injection, for Umulin and Humalog, respectively.
Adsorption of insulin and Arlatone F127G at the air/water interface
The possible interaction of insulin with Arlatone F127G was also considered. Co-adsorption at the air/solution interface of insulin and the surfactant dissolved in the sub-phase was studied (Fig. 4). For both insulins, the decrease in surface tension appeared to be exclusively controlled by the high HLB surfactant.
Characterization of insulin conformation in solution by circular dichroism
A significant difference between the CD spectra of Umulin and Humalog in aqueous solution was observed. Although a negative maximum was observed for both insulins at 275 nm (Fig. 5), the band was more negative for Umulin than for Humalog. The Umulin CD spectrum also showed an additional negative band at 225 nm (data not shown). These results account for a higher aggregation state of Umulin compared to Humalog.
Discussion
The decrease in oil concentration destabilized the ME. First, it led to the increase of the consistency of the primary emulsion due to the comparatively larger dispersed phase volume with, consequently, a higher risk of multiple globule breakdown during the incorporation of the primary emulsion into the external aqueous phase. Second, the final ME exhibited a thinner oily membrane, unable to protect the multiple globules from breaking. Therefore, it appeared crucial to improve the interfacial films by selecting appropriate surfactants.
The low HLB surfactant is a component of critical importance in the preparation of a primary emulsion and on the stability of the resulting ME. In this regard, Abil EM-90 has proved its efficiency in stabilizing W/O emulsions [7, 8, 9, 15, 16, 17, 18]. ME prepared with this surfactant showed significantly high entrapment efficiencies and slow release of electrolytes [15]. As a polymeric surfactant, Abil EM-90 is able to form a mechanical barrier which opposes micellar transport via reverse micelles. Moreover, as suggested by the results obtained from ME 5 and 6 which are both stable for 6 months, the covering of the inner interface of a ME by Abil EM-90 favours its stability, independently of the nature of the high HLB surfactant used to form the second interface. The stability of ME was maintained even at high Abil EM-90 concentrations (see ME 1 and ME 2 in Table 1), since an excess of low HLB surfactant was shown to have no destabilizing effect [16].
Although it is widely accepted that the nature of the high HLB surfactant is of minor importance when a lipophilic polymeric surfactant is used, our results clearly highlighted the influence of the high HLB surfactant on long-term stability. Whereas ME 5 (Tween 80) and ME 6 (Arlatone F127G) were both still multiple after 6 months, ME 6 remained stable for 2 years with minimal change of its characteristics. Arlatone F127G is a triblock surfactant which exhibits considerable surface activity [19]. On the one hand, its hydrophobic polypropylene oxide chain adsorbs at the oil/water interface, either due to its affinity to the oil molecule or as a result of water rejection. On the other hand, its two polyethylene oxide chains, in the aqueous phase, become strongly solvated by the water molecules as a result of hydrogen bonding. These chains provide strong repulsion upon close approach of two emulsion droplets, preventing flocculation and coalescence. Very stable ME have been obtained with this surfactant [6, 18, 20]. Contrary to oil-soluble surfactants, excess of a water-soluble surfactant has a destabilizing effect. Indeed, high HLB surfactants are able to solubilize some of the low HLB surfactant molecules into mixed micelles [16]. In our experiments, concentrations of Arlatone F127G higher than 1% led to increased electrolyte release rates during storage. Our results also showed that, below a 1% concentration, the high HLB surfactant was insufficient to cover the whole initial globule surface. This leads to the formation of large globules, and consequently, to the breakdown of the ME into a simple O/W emulsion.
The control ME prepared at 15 °C (Table 3) exhibited multiple globules with larger sizes and therefore a lower stability compared to control ME prepared at 25 °C (ME 6). This was not surprising since the processes of emulsification reported in the literature are generally performed at high temperature, in order to weaken the interfacial tension. The introduction of insulin into the inner aqueous phase, especially Humalog, improved the formation of the ME prepared at 15 °C. This was probably due to insulin's ability to adsorb at the W/O interface as inferred from the surface and interfacial tension measurements. Indeed, proteins are able to form interfacial complexes with surfactant at the W/O interface that imparts elasticity and resistance of the interfacial film around the droplets [10, 21, 22, 23, 24]. According to Kanouni et al. [17], a high degree of elasticity of the film formed at the first interface prevents breaking of the globules.
Whereas both insulins did not seem to interact with Arlatone F127G (Fig. 4), their adsorption–penetration into Abil EM-90 monolayers appeared clearly different (Fig. 3). Umulin adsorbed more rapidly into the surfactant monolayer than Humalog but led to a lower maximum surface tension. Such a behaviour could correspond to the adsorption of large and incompressible molecules (i.e. dimers or hexamers). In aqueous solution, due to non-covalent bonding, insulin exists as an equilibrium mixture of monomers, dimers and hexamers depending particularly on insulin species, zinc content, pH, concentration and solvent [25]. It is interesting to note that the primary structure of Humalog is identical to that of Umulin except for the transposition of two amino acids (proline and lysine) at positions 28 and 29 in the C-terminus region of the B chain. As a result, Humalog exhibits a lower ability to self-associate into dimers and hexamers and it dissociates much faster in monomers than Umulin [26].
The bands at 275 and 225 nm of the CD spectra provide an indication of insulin self-association [27, 28, 29]. The 275 nm band is assigned to tyrosine and phenylalanine aromatic residues in the B23–28 region of the anti-parallel structure formed between the monomers of insulin in the hexamers. Attenuation of this band is attributed to hexamer dissociation [30]. As for the 225 nm band, it is assigned in large part to the anti-parallel β structure, which is a predominant feature of the dimer [27]. Since both bands were more negative in the Umulin CD spectrum compared to that of Humalog, the Umulin solution would consist mostly of dimers and hexamers while monomers would predominate in the Humalog solution. The monomeric conformation of Humalog would account for its greater interaction with Abil EM-90 at the interface. Indeed, the surface of insulin monomers bears hydrophobic residues, which could be favourable for hydrophobic interactions with the low HLB surfactant.
Non-polar residues are also involved in the association of monomers into dimers and hexamers, in such a way that surface of the hexamer is therefore almost entirely polar [31, 32]. This could explain why Umulin would not interact as much as Humalog with the low HLB surfactant. It is reasonable to think that Humalog adsorption induced changes in the organization of surfactant molecules at the interface, producing an additional surface pressure increment. It is not clear whether this increment reflects the penetration of more insulin molecules, the result of a change in the orientation of surfactant molecules at the interface, or competitive adsorption between the two molecules at the interface. It is possible that monomers of Humalog compete with Abil EM-90 at the first interface, and enhance the displacement of surfactant molecules towards the second interface covered with Arlatone F127G. The resulting Abil EM-90–Arlatone F127G mixed film would facilitate the formation of small multiple globules since it lowers the interfacial tension (data not shown). Conversely, Umulin and the control ME form large droplets with limited stability with time and a strong tendency to coalesce. This can be explained by the low interaction ability of Umulin with Abil EM-90 due to its complex conformation in solution.
Conclusion
In this study we achieved the formation of a ME with reduced oil concentration (20%), using two polymeric surfactants. The obtained ME was stable for 2 years with minimal changes in its characteristics. Whereas insulin surface properties favoured drug-loaded ME formation, both the characteristics and stability of the ME were influenced by the conformation of the protein in solution, which controlled its interaction with the low HLB surfactant. The efficacy of the obtained insulin-loaded ME will be tested in diabetic rats.
References
Engel RG, Riggi SJ, Fahrenbach MJ (1968) Nature 219:856
Shichiri M, Shimizu Y, Yoshida Y, Kawamori R, Fukuchi M, Shigeta Y, Abe H (1974) Diabetologia 10:317
Shichiri M, Kawamori R, Yoshida Y, Etani N, Hoshi M, Izumy K, Shigeta Y, Abe H (1975) Diabetes 24:971
Shichiri M, Kawamori R, Goriya Y, Oji N, Shigeta Y, Abe H (1976) Endocrinol Jpn 23:493
Matsuzawa A, Morishita M, Takayama K, Nagai T (1995) Biol Pharm Bull 18:1718
De Luca (1991) Les émulsions multiples H/L/H: obtention, validation et libération. PhD thesis, Université Paris XI, Châtenay-Malabry
Silva Cunha A, Grossiord JL, Puisieux F, Seiller M (1997) J Microencapsul 14:311
Silva Cunha A, Grossiord JL, Puisieux F, Seiller M (1998) Int J Pharm 158:79
Silva Cunha A, Chéron M, Grossiord JL, Puisieux F, Seiller M (1998) Int J Pharm 169:33
Douillard R (1994) Colloids Surf A 91:113
Li FYH, Kwok DY, Policova Z, Zingg W, Neumann AW (1995) Colloids Surf B 3:282
Florence AT, Whitehill D (1982) Int J Pharm 11:277
Rosilio V, Albrecht G, Baszkin A, Merle L (2000) Colloids Surf B 19:163
Benjamins J, Cagna A, Lucassen-Reynders EH (1996) Colloids Surf A 114:245
Sela Y, Magdassi S, Garti N (1995) J Control Release 33:1
Jager-Lezer N, Terrisse I, Bruneau F, Tokgoz S, Ferreira L, Clausse D, Seiller M, Grossiord J (1997) J Control Release 45:1
Kanouni M, Rosano HL, Naouli N (2002) Adv Coll Interface Sci 99:229
Tedajo GM, Seiller M, Prognon, P, Grossiord, JL (2001) J Control Release 75:45
Tadros TF, Taelman MC, Dederen JC (1998) Multiple emulsions with polymeric surfactants. In: Grossiord J, Seiller M (eds) Multiple emulsions: structure, properties and applications. Paris, pp 117–137
De Luca M, Grossiord J, Seiller M (2000) Int J Cosmetic Sci 22:157
Law TK, Whateley TL, Florence AT (1986) J Control Release 3:279
Omotosho JA, Law TK, Whateley TL, Florence AT (1986) Colloids Surf 20:133
Garti N, Aserin A, Cohen Y (1994) J Control Release 29:41
Dickinson E, Evison J, Owusu RK, Williams A (1994) Protein-stabilized water-in-oil-in-water emulsions. In: Phillips GO, Wedlock DJ, Williams PA (eds) Gums and stabilizers for the food industry. Oxford University Press, pp 91–101
Brange J (1987) Galenics of insulin. Springer, Berlin Heidelberg New York
Wilde MI, McTavish D (1997) Drugs 54:597
Quadrifoglio F, Urry PW (1968) J Am Chem Soc 90:2760
Goldman J, Carpenter FH (1974) Biochemistry 13:4566
Sato S, Ebert C, Kim SW (1983) J Pharm Sci 72:228
Fredericq E (1953) Nature 171:570
Blundell T, Dodson G, Hodgkin D, Mercola D (1972) Adv Protein Chem 26:279
Renneboog-Squilbin C, Delhaise P, Wodak S (1981) Arch Int Physiol Biochim 89:B192
Acknowledgements
We are indebted to the Eli Lilly Company (Indianapolis, USA) for a generous supply of insulin and to Goldschmit (France) for the supply of Abil EM-90. Fabienne Cournarie is very grateful to the "Chancellerie des Universités de Paris" for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cournarie, F., Rosilio, V., Chéron, M. et al. Improved formulation of W/O/W multiple emulsion for insulin encapsulation. Influence of the chemical structure of insulin. Colloid Polym Sci 282, 562–568 (2004). https://doi.org/10.1007/s00396-003-0960-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-003-0960-8