Abstract
A star-shaped copolymer, poly(N-isopropylacrylamide-co-itaconamic acid (poly(NIPAAm-co-IAM)), being pH- and thermo-dual-responsive, was synthesized by atomic transfer radical polymerization (ATRP) and characterized in this work. The lower critical solution temperature (LCST) of the star copolymer increases with the molar fraction of IAM. The particle size decreases as the temperature increases but increases as the pH value increases. Transmission electron microscopy (TEM) reveals that the star-shaped copolymer has a near-spherical core-shell structure that favors drug delivery. The star copolymer can be used in drug encapsulation as well as drug release. The star copolymer has different drug release rates in environments of different pH, and thus it can carry drugs in an acidic (gastric) environment and release the drugs in a neutral or less acidic (intestinal) environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Functional star polymers defined as polymers that consist of several linear polymer chains connected at one point have been increasingly studied because of its special three-dimensional structure to exhibit special chemical and physical properties [9, 12]. Especially, through appropriate molecular design, many specific chemical and physical properties can be integrated into a star copolymer. For example, most star copolymers have lower viscosity and a lower melting point than a linear polymer with the same molecular weight [9, 12]. Hence, star copolymers have been utilized in various fields, such as drug delivery, surface modifiers, hydrogels, coatings, etc. [1, 9, 12]. In drug delivery, the challenge of using star polymers is to utilize properties of polymers to carry and release drugs in response to different environments (e.g., temperature and pH) with a controllable release rate. Various approaches [3, 5, 6, 10, 18, 23] were reported.
Star polymers can be designed to function as smart polymers which respond to different stimuli, especially multiple stimuli-responsive polymers [2, 4, 8, 11, 13–15, 22]. Smart polymers generally are used in fields such as drug delivery systems, biomimetic tissues, functional membranes, chemical valves, etc. For example, a pH- and thermo-responsive copolymer, poly(N-isopropylacrylamide-co-methacrylic acid-co-methyl methacrylate), as a drug delivery system was reported [7] where the polymer was designed to be insoluble at pH = 1.2 but soluble at pH = 7.4 at a body temperature (37 °C). Star polymers are prepared by a method that can simply control their molecular weight and conformation in order to satisfactorily achieve the targeted physical and chemical properties of the star polymers. Atomic transfer radical polymerization (ATRP) has been extensively used to precisely prepare polymers because it provides a linear conversion rate, a linear increase in polymer molecular weight, a narrow distribution of molecular weight, and active polymer chain ends. Usually, synthesis of a star polymer may have two types of methods, either arm first or core first [17]. In this study, core first and ATRP methods are chosen because a core molecule is first modified to have multiple reactive halides to be used as an initiator for subsequent ATRP.
Cyclodextrin, sometimes called “cycloamylose” made up of sugar molecules bound together in a ring, has been used as a core for star polymers because it has not only a hydrophobic cavity and a hydrophilic outer shell but also has many hydroxyl groups on its outer shell. Therefore, cyclodextrin and its derivatives have been designed to exhibit various characteristics including stimuli-responsiveness and drug release [16, 21]. To provide more functionality of cyclodextrin as a drug carrier, this work is focused on star copolymers using cyclodextrin as a central core. A pH- and thermo-responsive polymer or copolymer, star poly(NIPAAm) or star poly(NIPAAm-co-IAM), is prepared herein using NIPAAm, cyclodextrin, or/and IAM [20] as monomers to provide thermal and pH sensitivity and a core-shell structure.
Materials and methods
Materials
N-isopropylacrylamide (NIPAAm; C6H11NO) obtained from Aldrich Chemical Corp. was purified through recrystallization from n-hexane. Itaconamic acid (IAM; also called “4-amino-2-methylene-4-oxobutanoic acid”) was prepared according to the method disclosed in the US patent publication No. 2013/0172490. β-cyclodextrin (β-CD) obtained from TCI was purified through recrystallization and vacuum filtration. Copper(I) bromide (CuBr), obtained from Aldrich Chemical Corp., was purified by the following procedure: placing the raw CuBr in a flask, adding acetic acid to the flask in an environment of nitrogen, stirring for one day, removing acetic acid from the solution mixture, rinsing with ethanol and ether until the powder became light white, and drying the resulting powder. 2-Bromopropinyl bromide was obtained from Alfa; 2,2′-bipyridyl was obtained from Aldrich Chemical Corp.; 1-methyl-2-pyrrolidinone (NMP) was obtained from TEDIA; and tetrahydrofuran (THF) and dimethyl formamide (DMF) were obtained from ECHO. The buffer solution of pH 2, obtained from Fluka, was prepared from citric acid, hydrochlocic acid and chloride; the buffer solution of pH 4, obtained from Aldrich, was prepared from potassium hydrogen phthalate; the buffer solution of pH 7, obtained from Aldrich, was prepared from potassium dihydrogen phosphate and disodium hydrogen phosphate; the buffer solution of pH 10, obtained from Aldrich, was prepared from potassium dihydrogen phosphate and disodium hydrogen; the buffer solution of pH 12, obtained from Aldrich, was prepared from disodium hydrogen phosphate and sodium hydroxide solution; and the buffer solution of pH 7.4, obtained from Sigma, was prepared from phosphate buffered saline.
Methods
Synthesis of initiator
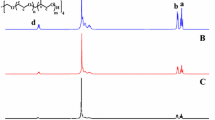
Dried β-CD (3 g, 2.64 mmol) was weighed and added to NMP (40 ml, 414.80 mmol) in a reaction flask. The mixture in the flask was stirred until becoming a non-viscous solid. Then, 2-bromopropinyl bromide (12 g, 55.5 mmol) was added to the reaction flask to react for 2 h at 0 °C. Before the reaction, the flask was repeatedly filled with nitrogen and degassed. The flask was kept for 22 h at room temperature to complete the reaction. After the reaction, the liquid in the flask turned into reddish brown. The liquid was dissolved in dichloromethane (55 ml) and extracted by 0.1 N HCl solution (100 ml) three times, saturated NaHCO3 solution (100 ml) three times, and deionized water. The organic layer of the liquid was separated from the aqueous layer, dried by MgSO4, and added to n-hexane for precipitation. Light-yellow solids (initiator; (Br)18-β-CD) were obtained through filtration and vacuum-drying. The initiator was identified and characterized by 1H-NMR shown in Fig. 1. Eighteen OH groups among 21 OH groups in β-CD were reacted and substituted based on calculation from 1H-NMR of Fig. 1.
Synthesis of star poly(NIPAAm)
Star poly(NIPAAm) was prepared by ATRP (atomic transfer radical polymerization) using the previously prepared (Br)18-β-CD as the initiator.
NIPAAm (2.50 g, 22.1 mmol) and the initiator ((Br)18-β-CD) (0.7883 g, 0.221 mmol) were placed in a 250-ml flask I and dissolved in THF (15 ml). After flask I was repeatedly filled with nitrogen and degassed, it was placed in an oil bath at 60 °C for 10 min. In another flask II, 2,2′-bipyridyl (0.173 g, 1.105 mmol) and purified CuBr (catalyst) (0.063 g, 0.442 mmol) were dissolved in THF (5 ml), and flask II was repeatedly degassed and filled with nitrogen to remove oxygen. The solution in flask II was added to flask I by a syringe and then the mixture in flask I reacted for 24 h at 60 °C. Then, flask I was opened to bring its contents in contact with air to terminate the reaction. After the reaction, THF was added and a mixture of aluminum oxide and celite was added to remove the metal catalyst. After vacuum filtration and drying, white solids (star poly(NIPAAm)) were yielded. Star poly(NIPAAm) was identified and characterized by 1H-NMR shown in Fig. 1 and labeled as star-0.
Synthesis of star poly(NIPAAm-co-IAM)
Star poly(NIPAAm-co-IAM) was prepared by the same method as was used to prepare star poly(NIPAAm) except that NIPAAm and IAM in various molar ratios (10/1, 10/1.25, 10/3.33) were used instead of NIPAAm. They were placed in a 250-ml flask I and dissolved in THF (15 ml). Three star poly(NIPAAm-co-IAM) were obtained and were characterized by 1H-NMR shown in Fig. 1 and labeled as star-1, star-2, and star-3. Scheme 1 shows the reaction of (Br)18-β-CD with NIPAAm and IAM.
Identification and characterization
NMR spectra were obtained using a Bruker Advance 300 MHz spectrometer by weighing 10 mg of each sample and dissolving it in 1 ml of DMSO-d6 placed in a standard 507-HP NMR test tube. FT-IR spectra were measured by a Perkin Elmer Spectrum RXI FTIR in the range of 4000–400 cm−1 having spectral resolution of 4.00 cm−1. The sample to be measured by RXI FTIR was prepared by dissolving a small amount of the tested material in water to form into a testing film.
Star copolymer of 0.1 g was added to deionized water of 100 ml to obtain a diluted solution for dynamic light scattering (DLS) measurement. Transmission electron microscopy (TEM) images of the samples were measured by a high-resolution transmission microscope (JEM-2100 by JEOL). Each sample was prepared as a 1 wt.% solution, of which 10 μl was drawn by a micro pipette, dripped on a metal mesh. The mesh was placed on a filtration paper, and the sample on the mesh was placed in an oven to dry for observation.
The weight average molecular weight (Mw), the number average molecular weight (Mn), PDI (Mw/Mn), and the values in the Mark-Houwink equation, a and K, of star polymers were determined by gel permeation chromatography (GPC) by Viscotek 270max Advanced, Malvern. The test sample was prepared by weighing 25 mg of the test material and dissolving it in 5 ml of DMF. A column 300*810 mm and a flow rate of 1 ml/min were used, the temperature of the column was set to 50 °C, the temperature of the detector was set to 50 °C, and the injection quantity of the test sample and the standard material, separately, was 50 μl.
The LCST was determined from the transmittance of the samples containing star polymers as a function of temperature using a laser transmittance meter (LASOS LGK 7628). The LCST value was the temperature as the transmittance is 50 %. The samples containing star polymers were prepared by adding star polymers (star-0, 1, 2, 3) to water to obtain samples with different concentration (1, 3, 5, 7, or 10 wt.%) or adding 10 ml of the previously prepared solutions which contain 3 wt.% star copolymers to various buffer solutions to obtain the samples with 3 wt.% of star copolymers for comparison in different pH values.
Drug release
Preparation of drug carrier
Aripiprazole was used as a representative drug in this work. Aripiprazole was added to each of star polymers to have concentration of 20 wt.%, and 2 ml of DMF was added to the mixture. The resulting mixture was stirred for 4 h to obtain a test sample for the drug encapsulation test. After stirring, a dialysis bag (MWCO 3500) was filled with the test sample and then placed in 1 L of deionized water for 4 days. The deionized water was replaced with fresh deionized water every 6 h to remove unreacted monomers and solvent. After dialysis, the sample was dried in a freeze dryer to remove water and finally a cotton-like drug carrier (weighted as W encap) was obtained. The drug carrier was dissolved in a buffer solution for 1 day, and the upper layer was used to measure the encapsulation efficiency and calculate the amount of the drug which was encapsulated by the star polymer. The encapsulation efficiency (EE) is defined as follows:
where EE represents encapsulation efficiency, W encap represents weight after encapsulation and W drug represents initial weight of the drug.
Drug release experiment
Aripiprazole has a maximum absorbance peak at 247 nm in the UV-vis spectrum and thus a UV absorbance vs. drug concentration calibration curve for aripiprazole was made. Specifically, the solutions with various concentration of aripiprazole were prepared by weighing aripiprazole to dissolve in water; the absorbance of the solutions was measured using a UV-vis spectrometer at 247 nm; and the calibration curve of aripiprazole in water was obtained by linear regression with an R square value of 0.9977. Similarly, the previously prepared solutions with various concentration of aripiprazole were used and dissolved in the buffer solution pH = 2 or pH = 7.4; the absorbance of the solutions was measured; and the calibration of aripiprazole in the buffer solution pH = 2 or pH = 7.4 was obtained by linear regression with an R square value of 0.9992 or 0.9990, respectively. The aripiprazole encapsulation efficiency using 20 mg of aripiprazole for star-0, 1, 2, and 3 was 93.9, 93.8, 93.7, and 94.0 %, respectively.
Two milligrams of the dried drug carrier was dispersed in 4 ml of buffer solutions pH 2 and pH 7.4 (PBS; phosphate buffered saline) and then the resulting mixture was placed in a dialysis bag (MWCO 3500). The dialysis bag was then placed in 100 ml of the same buffer solution at 25 or 37 °C. The drug was released through diffusion. At fixed intervals, 2 ml of liquid in the dialysis bag was taken to measure and calculate the concentration of aripiprazole by the UV-vis spectrometer based on the previous calibration curves, and 2 ml of the same buffer solution was added into the bag at the same time. A cumulative release (CR) rate can be calculated as follows:
where
- Wrelease,n :
-
Vs × (C1 + … + Cn-1) + V × Cn
- Wrelease,n (mg):
-
weight of aripiprazole taken from the sample for the nth time
- Cn (mg/ml):
-
concentration of aripiprazole taken from the sample for the nth time
- Vs (ml):
-
volume taken from the sample each time
- V (ml):
-
total volume of sample.
Results and discussion
Initiator ((Br)18-β-CD) preparation and star polymer characterization
The initiator is identified and characterized by 1H-NMR shown in Fig. 1. The peak at δ = 1.8–2 ppm is due to CH3 at the position –CH(CH 3 )Br; and the peak at δ = 3.5–6.3 ppm is due to H at –CH(CH3)Br and unreacted H on β-CD. Therefore, based on NMR spectrum, the initiator can be determined to have about 18 (−OH) groups among 21 be replaced by Br.
Star poly(NIPAAm) (star-0)
From 1H-NMR in Fig. 1, the peak at δ = 0.8–1.2 ppm is due to –NH–CH–(CH 3 )2; the peak at δ = 1.2–1.6 ppm is due to –CH 2 –CH–CO–; the peak at δ = 1.6–2.1 ppm is due to –CH2–CH–CO–; the peak at δ = 3.7–4 ppm is due to –NH–CH–(CH3)2; the peak at δ = 6.9–7.6 ppm is due to –NH–CH–(CH3)2. Comparing 1H-NMR of star-0 with that of NIPAAm monomer, an absence of the peaks at δ = 5.5 ppm (CH 2 = CH–CO–) and δ = 6.2 ppm (CH2 = CH–CO–) and the appearance of the peaks at δ = 1.2–2.2 ppm (–CH 2 –CH–CO–) show successful synthesis of star poly(NIPAAm) (star-0).
Star poly(NIPAAm-co-IAM) (star-1, star-2, star-3)
From 1H-NMR in Fig. 1, the spectra of star-1, star-2, and star-3 are similar to that of star-0 except that for characteristic peaks from IAM at δ = 0.8–1.5 ppm (–CH–C–), δ = 1.5–2.4 ppm (–CH 2 –CO–NH2–) and δ = 7–8 ppm (–CH2–CO–NH 2 –). Therefore, star poly(NIPAAm-co-IAM) (star-1, star-2 or star-3) was successfully synthesized.
The ratio of composition units from NIPAAm to composition units from IAM in star-1, star-2, and star-3 was determined by 1H-NMR where the area of the peak at δ = 4.0 ppm (–CH–NH–) is defined as 1 and the peaks at δ = 1.2–1.8 ppm and δ = 1.8–2.32 ppm are used to quantize composition units from NIPAAm and composition units from IAM. Table 1 shows the feed ratios of NIPAAm to IAM monomers compared with the polymerized ratios (NIPAAm/IAM) in copolymers. From Table 1, it is found that the polymerized molar fractions of composition units from NIPAAm in copolymers are consistently lower than the feed molar fractions probably because IAM has two electron-withdrawing groups and thus has better reactivity so as to lower the reaction rate of NIPAAm.
The FT-IR spectra of the cylcodextrin monomer, the initiator, the NIPAAm monomer, and the star polymers are shown in Fig. 2. In Fig. 2, the O–H stretching peak is shown at 3329 cm−1 for β-CD and disappears after esterification while the C=O stretching peak is shown at 1743 cm−1 to thereby determine that OH is esterified. Moreover, the C–H asymmetric bending and symmetric bending peaks of the CH2 group on Br are shown at 1448 and 1375 cm−1, respectively, and the C-Br stretching peak is shown at 667 cm−1. The C–O ether stretching peak for β-CD is shown at 1157 cm−1, and the un-esterified OH peaks are shown at 1409 and 1336 cm−1 for primary and secondary alcohols, respectively.
From Fig. 2, for NIPAAm monomer, the amide C=O stretching peak is shown at 1654 cm−1; the secondary amine N–H stretching peak is shown at 3310 cm−1; and the mono-substituted C=C (vinyl) peak is shown at 990 cm−1. After reacting with the initiator, an absence of the 990 cm−1 peak indicates C=C is formed into C–C in poly(NIPAAm). The C=O stretching peak is shown at 1655 cm−1; the N–H bending and C–N stretching peaks overlap each other at 1550 cm−1; the N–H stretching peak is shown at 3500 ∼ 3300 cm−1. The FT-IR spectra of the star polymers that indicate the synthesized star polymers have the structures that are consistent with NMR spectra.
Comparing the FTIR spectra of poly(NIPAAm-co-IAM) (star-1, star-2, star-3), it is found that the intensity of the 1710 cm−1 peak (due to C=O stretch of carboxylic group of IAM) increases with the molar fraction of IAM. The carboxylic O–H peak (3500 ∼ 3300 cm−1) overlaps with the N–H stretching peak from NIPAAm.
Gel permeation chromatography analysis
The GPC spectra of the star polymers (star 0 ∼ 3) were shown in Fig. 3, and the weight average molecular weight (Mw), the number average molecular weight (Mn), PDI (Mw/Mn), and the values in the Mark-Houwink equation, a and K, for star polymers calculated from GPC are shown in Table 2. Table 2 shows that the star polymers prepared by ATRP have a relatively narrow molecular weight distribution, that is, PDI is within 1.2 ∼ 1.4. According to Mark-Houwink equation, the Mark-Houwink parameters a and K can be determined graphically from a line of best fit to define the relationship of intrinsic viscosity and molecular weight; and the parameters a and K are related to the properties of the polymer and the solvent. DMF is a polar protonic solvent and can form hydrogen bonds with polymers. Accompanying with polymerization processing, more hydrophilic groups are introduced and thus the structure of the polymer becomes more swelling to result in a higher molecular weight and increased solubility in DMF due to hydrogen bond formation so as to have a higher Mark-Houwink K value when the molar fraction of IAM is increased. It is indicated that, for star-1, star-2, and star-3, DMF is a good solvent. For most flexible polymers, 0.5 ≦ a ≦ 0.8; for semi-flexible polymers, a ≧ 0.8; and for polymers with an absolute rigid rod, a becomes as high as 2.0. The results listed in Table 2 indicate that, in the DMF system, molecular chains of star-3 are swelling to extend outwardly so that it has a near-spherical shape where the Mark-Houwink a value is close to 0.5. For star-0, since the Mark-Houwink a value, 0.9, is relatively large, it is implicated that molecular chains of star polymer star-0 are close to or curly attached to surfaces of the conical β-CD, and thus the bulk property of the wrapped particle is semi-flexible.
Lower critical solution temperature analysis
The dependence of LCST on concentration (1, 3, 5, 7, and 10 wt.%) is shown in Fig. 4a. It is found that LCST of copolymer has an increasing trend when the molar fraction of IAM in the copolymer is increased, that is, star-3 has the higher LCST values for different concentration. This trend may result from the hydrophilic carboxylic and amino groups in IAM. After the reaction of NIPAAm with IAM, more functional groups to form hydrogen bonds are introduced in the copolymer to suppress the interaction of the hydrophobic groups of NIPAAm to thereby have an increasing trend for LCST. From the result, it shows introducing a hydrophilic monomer tends to raise the LCST.
The dependence of LCST on pH for star-0 ∼ 3 is shown in Fig. 4b where the concentration of the star polymer is 3 wt.%. Since the pH responsive segment (IAM) is introduced into star-1 ∼ 3, it is expected that the LCST values are influenced by different pH values due to protonation or de-protonation of the carboxylic groups on IAM so as to achieve the purpose of obtaining pH- and thermo-responsive star polymers. From the experimental result, at a low pH value, the carboxylic groups on IAM are not charged and lead to enhance the formation of hydrogen bonds between polymer chain segments; it would make the polymer have lower water solubility so that the LCST of the phase separation is lowered. On the contrary, at a high pH value, the carboxylic groups are ionized (de-protonation) to become charged to be easily dissolved in water so that LCST is increased.
Morphological and dimensional analysis
Schematic structural diagrams of star polymer at different temperatures and pH values are shown in Fig. 5a, b, respectively. The effect of temperature on the effective diameters for star-0 ∼ 3 is shown in Fig. 6a while the effect of pH on the effective diameters thereof in a buffer solution is shown in Fig. 6b. The effective diameters of the star polymer were defined as the diameter of the CD/polymer cluster as shown in Fig. 5 and were determined using dynamic light scattering (DLS).
In Fig. 6a, at a particular temperature, such as 25 °C, a higher molar fraction of IAM is associated with a larger particle diameter. It is implicated that the hydrophilicity of the copolymer increases with the number of hydrophilic segments (IAM) so that the volume of the copolymer is increased by encapsulating more water and thus its particle diameter increases. For a specific polymer, for example star-0, 1, 2, or 3, as the temperature is higher, the particle diameter becomes smaller. It revealed that the interaction between the hydrophobic groups (–CH(CH3)2) becomes stronger than the hydrogen bonds between the amide groups and water so as to deswell its volume by expelling water to lower its particle diameter.
In Fig. 6b, regarding the effect of pH on LCST, it is expected and also observed that star-0 has the least or almost no effect due to the pH changes because it contains no segment from IAM. In addition, the slope of LCST vs. pH is expected and also observed to increase as the molar fraction of IAM increases in star polymers (that is, star-3 > star-2 > star-1). In an environment with a low pH value, the carboxylic groups on IAM are not charged and the hydrogen bonds easily form between the NHCO chain segments in NIPAAm to have chain strangling so as to lower its particle diameter. In contrast, in an environment with a high pH value, the carboxylic groups are ionized (de-protonation) to become charged where the positive charges are dissociated and dissolved in water. This effect is probably responsible for the swelling of the copolymer shown at the upper right hand side of Fig. 5b and a larger particle diameter is shown [19]. Li et al. [13] reported that the diameter decreases from 514 to 293 nm as the temperature increased from 25 to 45 °C with the most drastic decrease occurring at the LCST of 32 °C for poly(NIPAAm-co-glycidyl methacrylate). Liu et al. [15] reported that the particle size of the micelle shows small changes with pH value which is 113 nm at pH = 5 and 85 nm at pH = 9 for poly(NIPAAm-co-DMAEMA) star-shaped copolymer. In this work, the particle size of star-3 shows both pH and thermal sensitive and has higher sensitivity in pH compared to the previously reported poly(NIPAAm-co-DMAEMA) star-shaped copolymer.
In Fig. 7, the TEM image of star-0 shows a circular structure via self-assembly and the TEM images of star-1 ∼ 3 show a core-shell structure where the hydrophilic segments originated from IAM aggregate on the periphery of the outer layer and the hydrophobic segments originated from NIPAAm inwardly aggregate to form a typical micelle structure. Therefore, the shell (hydrophilic IAM) shows the dependence on pH to exhibit either swelling or de-swelling at different pH values.
Drug release application
In this study, 25 and 37 °C are chosen to simulate room temperature and human body temperature, respectively, and the environments at pH 2 and pH 7.4 are chosen to simulate acidic (gastric fluid) or neutral (intestinal fluid) environments, respectively. Figure 8a, b shows drug release profiles of various drug carriers at 25 °C in buffer solutions pH 2 and pH 7.4, respectively. Figure 8c, d shows drug release profiles of various drug carriers at 37 °C in buffer solutions pH 2 and pH 7.4, respectively.
a Drug release profile of various drug carriers at 25 °C in buffer solution pH 2. b Drug release profile of various drug carriers at 25 °C in buffer solution pH 7.4. c Drug release profile of various drug carriers at 37 °C in buffer solution pH 2. d Drug release profile of various drug carriers at 37 °C in buffer solution pH 7.4
In Fig. 8a, b, the cumulative release rate is generally higher at a higher temperature. For star-3, at pH 2, the cumulative release rate at 37 °C is about 24 % higher than that at 25 °C for about 250 min while at pH 7.4, the cumulative release rate at 37 °C is about 1 % higher than at 25 °C. This result is consistent with our previous finding. Firstly, referring to Fig. 4b, the LCSTs of star-1, star-2 and star-3 are 34.2, 34.5, 37 °C at pH 2, respectively, while the LCSTs of star-1, star-2 and star-3 are 36.2 °C, 38.1 °C, 42.3 °C at pH7. Secondly, as discussion in paragraph of morpholigical and dimensional analysis, the particle size at 37 °C is smaller than at 25 °C. Therefore, the cumulative release rate is expected to increase with temperature.
For each copolymer (star-1, star-2, or star-3), at each temperature (either 25 or 37 °C), the release rate at pH 2 is lower than that at pH 7.4 and the drug carrier is more stable in the acidic environment than in the neutral environment. It shows applicability in drug release in the intestinal environment although the time of drug release may be optimized and adjusted by modification of copolymer composition. Therefore, pH- and thermo-dual reponsive star coplymers have potential to be applied to drug release.
Conclusions
pH- and thermo-responsive star polymers star poly(NIPAAm) and star poly(NIPAAm-co-IAM) were prepared as new materials by ATRP using NIPAAm, IAM, and cyclodextrin as monomers to provide thermal and pH sensitive properties as well as a core-shell structure. The results show that the segments from cyclodextrin provide a star skeleton for star polymers, the segments from IAM containing COOH groups provide the pH sensitive property, and the segments from NIPAAm provide the thermal sensitive property. The solubility of star polymers below and above the lower critical solution temperature (LCST), and the particle diameter changes of the star polymers at different pH values at the body temperature show that the prepared star polymers have potential to be utilized in drug release application. Moreover, the spherical shape and the core-shell structure of these star polymers are also advantageous to drug release application. Besides, the LCSTs of the star polymers prepared in this study are close to the body temperature which is not able to be achieve by poly(NIPAAm) alone. In addition, dual-responsive copolymers are able to be achieved by introducing the IAM segments having a free carboxylic group remained after polymerization together with NIPAAm segments where the carboxylic group also provides the hydrophilic property on the shell of the copolymer. The star polymers prepared in this work show significant size changes as either pH value or the temperature changes, indicating higher sensitivity in pH and temperature changes. The cell toxicity of the star polymers should be studied in the future in order to actually apply in drug release.
References
Aloorkar NH, Kulkarmi AS, Patil RA, Ingale DJ (2012) Star polymers: an overview. Int J Pharm Sci Nanotech 5:1675–1684
Bai Y, Wei J, Yang L, He C, Lu X (2012) Temperature and pH dual-responsive behavior of polyhedral oligomeric silsesquioxane-based star-block copolymer with poly(acrylic acid-block-N-isopropylacrylamide) as arms. Colloid Polym Sci 290(6):507–515
Cai J, Guo J, Ji M, Yang W, Wang C, Fu S (2007) Preparation and characterization of multiresponsive polymer composite microspheres with core–shell structure. Colloid Polym Sci 285(14):1607–1615
Carreira A, Gonçalves F, Mendonça P, Gil M, Coelho J (2010) Temperature and pH responsive polymers based on chitosan: applications and new graft copolymerization strategies based on living radical polymerization. Carbohydr Polym 80(3):618–630
Cirillo G, Iemma F, Spizzirri U, Puoci F, Curcio M, Parisi O, Picci N (2011) Synthesis of stimuli-responsive microgels for in vitro release of diclofenac diethyl ammonium. J Biomater Sci 22(4–6):823–844
Fang S, Kawaguchi H (2002) A thermosensitive amphoteric microsphere and its potential application as a biological carrier. Colloid Polym Sci 280(11):984–989
Fundueanu G, Constantin M, Stanciu C, Theodoridis G, Ascenzi P (2009) pH- and temperature-sensitive polymeric microspheres for drug delivery: the dissolution of copolymers modulates drug release. J Mater Sci Mater Med 20(12):2465–2475
Gupta B, Kumari M, Ikram S (2013) Drug release studies of N-isopropyl acrylamide/acrylic acid grafted polypropylene nonwoven fabric. J Polym Res 20(3):95
Hadjichristidis N, Pitsikalis M, Iatrou H, Driva P, Sakellariou G, Chatzichristidi M (2012) 6.03—polymers with star-related structures: synthesis, properties, and applications. In: Matyjaszewski K, Möller M (eds) Polymer science: a comprehensive reference. Elsevier, Amsterdam, pp 29–111. doi:10.1016/B978-0-444-53349-4.00161-8
Ho KM, Li WY, Wong CH, Li P (2010) Amphiphilic polymeric particles with core–shell nanostructures: emulsion-based syntheses and potential applications. Colloid Polym Sci 288(16):1503–1523
Ivan Meléndez-Ortiz H, Bucio E (2009) Stimuli-sensitive behaviour of binary graft Co-polymers (PP-g-DMAEMA)-g-NIPAAm and (PP-g-4VP)-g-NIPAAm in acidic and basic medium. Designed Monomers Polym 12(1):99–108
Lapienis G (2009) Star-shaped polymers having PEO arms. Prog Polym Sci 34(9):852–892. doi:10.1016/j.progpolymsci.2009.04.006
Li P, Xu R, Wang W, Li X, Xu Z, Yeung K, Chu P (2013) Thermosensitive poly(N-isopropylacrylamide-co-glycidyl methacrylate) microgels for controlled drug release. Colloid Surf B: Biointerfaces 101:251–255
Ling Y, Lu M (2009) Thermo and pH dual responsive Poly (N-isopropylacrylamide-co-itaconic acid) hydrogels prepared in aqueous NaCl solutions and their characterization. J Polym Res 16(1):29–37
Liu Y, Cao X, Luo M, Le X, Xu W (2009) Self-assembled micellar nanoparticles of a novel star copolymer for thermo and pH dual-responsive drug release. J Colloid Interface Sci 329(2):244–252
Manakker F, Vermonden T, Nostrum C, Hennink W (2009) Cyclodextrin-based polymeric materials: synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules 10(12):3157–3175
Matyjaszewski K, Miller PJ, Pyun J, Kickelbick G, Diamanti S (1999) Synthesis and characterization of star polymers with varying arm number, length, and composition from organic and hybrid inorganic/organic multifunctional initiators. Macromolecules 32(20):6526–6535
Ramkissoon-Ganorkar C, Vaudyš M, Kim SW (2000) Effect of ionic strength on the loading efficiency of model polypeptide/protein drugs in pH-/temperature-sensitive polymers. J Biomater Sci Polym Ed 11(1):45–54
Rwei SP, Ku FH (2001) The dispersion of pigment slurries via incorporation with water-soluble sulfonate poly (ethylene- terephthalate). Colloid Polym Sci 279(3):274–278
Way TF, Chen YT, Chen JJ, and Teng K, US patent application No. 2013/0172490 A1
Zhang J, Ma P (2013) Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev 65(9):1215–1233
Zhao C, Gao X, He P, Xiao C, Zhuang X, Chen X (2011) Facile synthesis of thermo- and pH-responsive biodegradable microgels. Colloid Polym Sci 289(4):447–451
Zhao SP, Zhou F, Li LY (2012) pH- and temperature-responsive behaviors of hydrogels resulting from the photopolymerization of allylated chitosan and N-isopropylacrylamide, and their drug release profiles. J Polym Res 19(9):9944
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 102-2218-E-027-015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rwei, SP., Chuang, YY., Way, TF. et al. Preparation of thermo- and pH-responsive star copolymers via ATRP and its use in drug release application. Colloid Polym Sci 293, 493–503 (2015). https://doi.org/10.1007/s00396-014-3436-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3436-0