Abstract
In the present study, a simple but effective strategy was introduced to synthesize pH and thermo double responsive poly(N-isopropylacrylamide-co-itaconic acid) P(NIPAAm-co-IA) hydrogels with improved properties through preparation in aqueous sodium chloride solutions. With the increase of concentration of NaCl, the phase separations of the gels became more and more pronounced, thus induced different chain conformations and network structures. The equilibrium swelling ratios and deswelling/reswelling kinetics of the hydrogels were investigated under conditions of different pH, temperature and ionic strength. The result showed these hydrogels were able to absorb large amounts of water and exhibit improved temperature and pH sensitivities, which may be ascribed to the formed porous network structures. Their chemical structures, lower critical solution temperatures (LCSTs) and surface morphologies were also checked by Fourier transform infrared spectroscopy (FTIR), Differential scanning calorimetry (DSC) and scanning electron microscopy (SEM).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(N-isopropylacrylamide) (PNIPAAm) hydrogel is the best known temperature sensitive polymeric network [1], which exhibits a lower critical solution temperature (LCST) at about 32–34 °C. Below the LCST, the PNIPAAm gel is swollen in water. As the temperature is increased above LCST, it undergoes abrupt changes in volume and shrinks quickly [2–4].
During the last decades, many works have been carried out, mainly focusing on the acceleration of response kinetics of the PNIPAAm hydrogels and several successful strategies have been proposed. For example, employing high temperature techniques [5, 6], introduction of the dangly comb-chains [7, 8], formation of macro-porous [9, 10]/super-porous [11, 12] structures, use of mixed solvents [13, 14], addition particles in gel networks [15, 16], cold-treating [17] or freeze-drying [18] and very recently invented RAFT polymerization [19, 20].
However, the researches on the improvement of properties of temperature and pH dual responsive hydrogels have seldom been reported. That is due to the introduction of pH sensitivities to temperature responsive hydrogels often results in fading even elimination of thermal sensitivities [21]. But in the practical, high water-absorbent, pH and temperature dual responsive hydrogels [22, 23] have a wide variety of applications, such as, devices for drug delivery systems [24, 25], water absorption and treatment [26, 27] and bioengineering applications [28, 29].
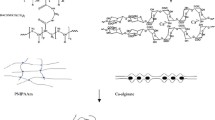
To solve the dilemma, in this work, an attempt was made by copolymerizing the pH sensitive monomer, itaconic acid with NIPAAm in aqueous sodium chloride solutions. The temperature and pH sensitivities of the resultant hydrogels were examined through swelling ratios, deswelling/reswelling kinetics experiments. The chemical structures, lower critical solution temperatures (LCSTs) and surface morphologies of the resultant hydrogels were characterized by Fourier transform infrared spectroscopy (FTIR), Differential scanning calorimetry (DSC) and Scanning electron microscopy (SEM) as well.
Experimental
Materials
N-isopropylacrylamide (NIPAAm, Acros) purified by recrystallization from a mixture of toluene and n-hexane (v/v = 3:2) and itaconic acid (IA, purity>99%) purchased from Aldrich were used as monomers. N,N′-methylenebisacrylamide (BIS) purified by recrystallization from methanol were used as a cross-linking agent, potassium persulfate (KPS), N,N,N′,N′-tetramethylethylenediamine (TEMED) were used as the polymerization initiator and accelerator. Sodium chloride (NaCl) was analytical grade and was used as received without further purification.
Potassium tetroxalate dehydrate (KH3(C2O4)2·2H2O), potassium hydrogen tartrate (KHC4H4O6), potassium hydrogen phthalate (C6H4CO2HCO2K), Disodium hydrogenorthophosphate (Na2HPO4), potassium dihydrogen phosphate (KH2PO4) and sodium tetraborate decahydrate (Na2B4O7·10H2O) were analytical grade and used as received to prepare pH buffer solutions.
Preparation of hydrogel
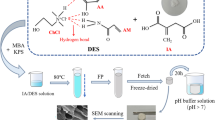
The polymerization of the P(NIPAAm-co-IA) hydrogels was carried out in aqueous NaCl solutions of different concentrations at room temperature (25 °C) for 24 h, using KPS and TEMED as a pair of redox initiator. BIS was used as the cross-linker. The feed compositions of the monomers, cross-linker and the concentrations of salt solutions are listed in Table 1. After the polymerization, the resultant hydrogels were cut into discs (10 mm in diameter, 0.2 mm in thickness). Then these samples were immersed in cold distilled water for a week and the water was refreshed every day in order to leach out the Na+, Cl−, unreacted monomers and linear polymers. The produced hydrogels are designate as G000, G005, G010, G015, G020, G030 and G040 corresponding to their respective salt concentrations.
Preparation of pH buffer solutions
Standard pH buffer solutions were prepared according to IUPAC STANDARDS AND PROCEDURES [30]. The pH and Compounding way are listed in Table 2. Then the pH buffer solutions were adjusted to uniform ionic strength (I = 0.05 mol kg−1) using NaCl.
Instrumentation
Optical images
All the optical images were recorded using Canon A510 cameras.
FT-IR
For FT-IR measurements an RFX-65A spectrometer (Analect, USA) was used. The hydrogel samples were dried in vacuum at 50 °C for 48 h till constant weight. The samples were embedded in KBr disks after grinded into powder. The scanning wave number ranged from 4,000 to 500 cm−1
DSC measurements
Thermal studies were performed from 20 to 65 °C at a heating rate of 2 °C/min on the swollen gels using a differential scanning calorimeter (PE-7) under a nitrogen atmosphere (20 ml/min).
Surface morphology observation
Scanning electron microscopy (SEM, JSM-5910, Japan) was used to study the surface morphologies of the hydrogels. To prepare samples for SEM, the swollen hydrogels were freeze-dried and then sputter coated with gold.
Temperature dependence of the swelling ratio
The temperature dependence of swelling ratio of hydrogels was measured gravimetrically after carefully blotting surface water with moistened filter paper in the temperature range from 25 to 60 °C. Hydrogel samples were immersed in distilled water for at least 24 h at each predetermined temperature. The average value of three measurements was taken for each sample, and the equilibrium swelling ratio (ESRT) is defined as follows:
where W s is the weight of swollen hydrogel at the particular temperature and W d is the dry weight of the hydrogel.
The equilibrium swelling ratio in pH buffer solutions
The swelling ratio of hydrogels was measured gravimetrically after carefully blotting surface water with moistened filter paper at ambient temperature (25 °C). Hydrogel samples were immersed in various pH buffer solutions for at least 24 h. The average value of three measurements was taken for each sample, and the equilibrium swelling ratio (ESRpH) is defined as follows:
where W s is the weight of swollen hydrogel at ambient temperature and W d is the dry weight of the hydrogel.
Measurement of deswelling kinetics
The deswelling kinetics of hydrogels was measured gravimetrically at 60 °C. The hydrogel samples were first immersed in distilled water at 25 °C until equilibrium was reached. Then, the equilibrated hydrogels were quickly transferred to distilled water or pH buffer solutions of 60 °C. At specified time intervals, the samples were removed from the hot water and weighted after wiping off the excess surface water with moistened filter paper. The average value of three measurements was taken for each sample, and the water retention capacity (W r) is calculated from equation:
where W x, W t and W e are the weight of xerogel, gel at time t, and gel at initial swollen equilibrium, respectively.
Measurement of reswelling kinetics
The kinetics of reswelling of gels was measured gravimetrically at 25 °C. Before the measurement, the hydrogel samples were freeze-dried for 24 h. The weight changes of gels were recorded during the course of reswelling in distilled water after blotting the excess surface water at regular time intervals. The water uptake capacity (W u) is calculated from equation:
where W x, W t and W e are the weight of xerogel, gel at time t, and gel at final swollen equilibrium, respectively.
Results and discussion
Preparation of hydrogels
The chemical structures and the process of preparation of the hybrid hydrogels are illustrated in Fig. 1. G040 and G030 formed gels half an hour later, while the other gels need several hours. The physical appearances of the formed hydrogels are shown in Fig. 2. As can be seen, with the increase of NaCl concentrations, gels undergo transparent–translucent–opaque transitions. G000 is almost completely transparent; G015 and G020 are translucent, while G030 and G040 are opaque. This difference in appearances should be attributed to phase separation which originated from the effect of NaCl on the conformations of P(NIPAAm-co-IA) chains. After purification in water, G030 turns to turbid (Fig. 3) due to high water absorbency, while G040 breaks into pieces due to its poor strength.
In fact, in our experiments the synthesis temperature has a significant impact on the appearances of the formed gels. For instance, if the reaction temperature was lowered to 15 °C without changing other conditions in the experiments, the formed G040 could remain robust and the formed G030 was even translucent, as revealed in Fig. 4. The reasons are as follows. First, increasing temperature will accelerate the decomposition of initiator [31] and the growth of polymer chains. Second, in the high temperature range, the hydrophilicity of the P(NIPAAm-co-IA) chains decreases. As a result, the polymer chains can not fully extend and are forced to take twist conformations [29, 32], finally leading to phase separation.
During the synthesis of the P(NIPAAm-co-IA) hydrogels in distilled water, P(itaconic acid) were ionized, the electrostatic repulsion forces between polymer chains were strong and lead to expansion of networks. However, in the aqueous NaCl solutions, the repulsion forces were shielded somewhat by the added Na+ and Cl− ions, the P(NIPAAm-co-IA) chains were forced to take twist conformations and probably elastic strain energies were stored. After purification in deionized water, Na+ and Cl− were leached out, the strong electrostatic repulsions between carboxylate anions (–COO−) of P(NIPAAm-co-IA) chains drove the polymer chains to disentangle and extend. So the expansion networks and high water uptakes were obtained. With the increase of the NaCl concentration, this twist effect (phase separation phenomenon) became more pronounced (Fig. 2) and the resultant hydrogels could absorb more water (Fig. 3). But if NaCl was added too much, the osmotic pressures were so high that the coil-globule transition took place resulting almost completely shrinkage of the P(NIPAAm-co-IA) chains. Under this condition, even after purification, the transitions of chain conformations were still partly unrecoverable which may be due to the viscoelastic characteristics of the polymer chains [33].
The conversion efficiency of the hydrogels were determined from weight measurements and found it was above 95% and with very little change, except that of G030 and G040, which was about 92% and 89%, respectively. The high conversion efficiency observed in this study indicated that the presence of NaCl did not prevent the formation of gel remarkably and the polymerization and cross-linking reactions were close to completion. Furthermore, the P(NIPAAm-co-IA) hydrogels were subjected to acid–base analysis after extraction [34]. The results showed that the percentage of PIA in these P(NIPAAm/IA) hydrogels were close to each other, except G030 which was slightly lower.
FT-IR
The FT-IR spectra of the P(NIPAAm-co-IA) hydrogels are shown in Fig. 5. The IR spectra of all hydrogels were similar to each other. Each spectrum shows a broad IR band in the range of 3,700–3,100 cm−1 (band a) which is the evidence of the OH stretching vibrations of carboxylic acid groups of IA and NH stretching vibration of NIPAAm. The peak at approximately 1,730 cm−1 (band b) can be attributed to the typical carbonyl vibration in IA [34, 35]. The typical amide-I band and amide-II band of NIPAAm are obvious at 1,650 cm−1 (band c) and 1,550 cm−1 (band d), respectively.
DSC
Figure 6 shows the DSC thermograms of P(NIPAAm-co-IA) hydrogels in the temperature range from 20 to 65 °C [36]. Broad peaks appeared in each curve, distinct from those pure PNIPAAm hydrogels which exhibited in the form of narrow peaks. This may be owing to the introduction of highly hydrophilic itaconic acid. As been reported [21], the introduction of hydrophilic monomer often results in the broadening and right shifting of the lower critical solution temperatures. From Fig. 6, it can be observed that the enthalpy values of the swollen hydrogels increased from G000 to G020 and then decreased. For example, the ΔH of G000, G005, G010, G015, G020 and G030 is 2.3479, 3.4125, 4.6231, 5.7128, 7.8453 and 6.4427, respectively. This order agrees very well with the equilibrium swelling ratios of the corresponding gels presented below. As for the lower critical solution temperature (LCST), it lays in the vicinity of 35–55 °C. And with the increase of NaCl concentration, the peak shifted to the left slightly. For instance, the peak of G000, G005, G010, G015, G020 and G030 is 54.43, 54.04, 53.25, 52.09, 50.01 and 50.51, respectively.
SEM
Figure 7 shows the SEM images of the surfaces of the hydrogels lyophilized from equilibrium swelling conditions at 25 °C. Macro pores were observed in the hydrogels which prepared in brine, whereas hydrogels prepared in the absence of NaCl had a dense, smooth and homogeneous surface [37, 38]. Furthermore, we may find that with the increase of the concentration of NaCl, the polymers chains become looser and networks become more porous after purification in distilled water for several days.
Temperature dependence of the swelling ratio
To detect the thermal-sensitivities of the hydrogels, equilibrium swelling ratios (ESRT) of the gels in distilled water are plotted as a function of temperature from 25 to 60 °C in Fig. 8. It can be seen that at room temperature the ESRT at first increases from G000 to G020, and then decreases as the NaCl concentration increased further. For example, the ESRT of G000, G005, G010, G015, G020 and G030 is 65.2, 116.0, 137.5, 149.8, 161.3 and 143.8, respectively. The result is in accordance with their respective network structures as discussed above, for more porous structures can accommodate more water. With increasing temperature, all curves display the same tendency, i.e. a fall in swelling ratios. For all the gels, no significant loss of thermal-sensitivity was observed. Finally, in the high temperature regime above 55 °C there is almost complete deswelling for all systems. The difference between the swelling ratios of the hydrogels tends to be less significant.
The equilibrium swelling ratio in pH buffer solutions
To investigate pH sensitivities, the hydrogels were immersed into pH buffer solutions ranged from 1.67 to 9.18. Figure 9 shows the equilibrium swelling ratios (ESRpH) in various pH buffer solutions after a 24 h immersion. As shown, swelling ratios of all samples increase with the increase of pH. For example, the swelling ratio of G020 in pH 1.67, 3.55, 4.01, 6.86 and 9.18 is 15.8, 20.7, 21.5, 33.2 and 68.8, respectively. At lower pH conditions the carboxyl groups were difficult to ionize and in the protonated form. The hydrogen bonding between carboxyl groups (of the IA units) with the amide groups appeared, so lower swelling ratios were exhibited. As pH increased, carboxyl groups ionized and provoked the dissociation of inter-polymer hydrogen bonds. The resulting electrostatic forces repulsed the hydrogels to swell [39]. From the Fig. 9, it can be also found that at every pH condition, the swelling ratios also agree well with the relationship which existed in the distilled water, i.e. at first increased from G000 to G020 and then dropped. For instance, the swelling ratio of G000, G005, G010, G015, G020 and G030 in pH 9.18 buffers is 32.3, 45.4, 50.5, 57.9, 68.6, and 54.7, respectively. Of course, a remarkable decrease in equilibrium swelling ratios can be observed when compared with the ones in distilled water, which may be ascribed to the screening effect [40].
Deswelling kinetics
Figure 10a shows the time course of the swelling ratios of nonporous and porous hydrogels in distilled water with temperature jumping from 25 to 60 °C. Surprising to find that all the samples from G000 to G030 need about 120 min to shrink to equilibrium and the difference in deswelling rates is not so apparent. Ordinarily speaking, hydrogels with expanded and porous structures should exhibit fast deswelling kinetics [13–15]. However, in our systems, the acceleration effect of deswelling is not such obvious. The possible reason for this result is that the incorporation of highly hydrophilic monomer, itaconic acid. According to the literature [41], the presence of P(itaconic acid) linear chains is effective in facilitating deswelling, presumably because the P(itaconic acid) linear chains increased the hydrophilicity of the gel matrix, making it difficult to form a skin layer in distilled water. So both nonporous and porous P(NIPAAm-co-IA) hydrogels exhibit fast deswelling behavior when compared with pure PNIPAAm hydrogels. On the other hand, hydrophobic polymer aggregation forces in the phase transition are apparently weakened because hydrated charged itaconic acid units disrupt regular aggregation of isopropylamide groups in polymer chains. As a result, the acceleration effect induced by the porous structures of the network is shielded and similar deswelling profiles are observed. However, these porous hydrogels are superior to the nonporous hydrogels when the water-release amount was taken into account. Because within the 2 h, the water-loss of G030, G020, G015, G010 and G005 was 139.9, 152.0, 143.4, 131.9 and 112.2, respectively, while that of G000 is only 61.8. That is to say the water-loss of gels fabricated in sodium chloride solutions is much larger than conventional P(NIPAAm-co-IA) hydrogels. This feature could also be particularly beneficial in controlled drug release applications because gels prepared in these NaCl solutions can release more water or drugs, in other words, it means less amount of gels are needed to achieve the same drug release.
To verify the above hypothesis and better understanding the thermal sensitivity upon heating, the shrinking rates of P(NIPAAm-co-IA) hydrogels were further performed in pH 1.67 buffer solutions and the data were illustrated in Fig. 10b. It can be seen that the porous hydrogels exhibit much faster response rates than that of the corresponding conventional hydrogel. Gels prepared in various NaCl solutions quickly shrunk to equilibrium within an hour. While in the same time period the conventional gel G000 only released about 40% water and need about 24 h to reach the deswelling equilibrium. At pH 1.67, the carboxyl groups of P(itaconic acid) polymer chains were protonated after transferring from distilled water to pH buffers and the extensive hydrogen bonding between polymer chains produced correspondingly which constrained the network [42]. More important, the thick, dense layer formed on its surface, which restricted water molecules from being squeezed out and finally resulted in a slow response rate. However, gels prepared in NaCl solutions could shrink quickly due to the formed macro-pores structures. And it was surprised to find that the G030 sample prepared with NaCl content of 0.30 g exhibited the most rapid deswelling rate. It lost more than 90% water within 20 min. This is maybe owing to the shape memory effect. As discussed above, the G030 polymer chains took global conformations in the preparation. When it was placed into hot pH 1.67 buffer solutions, the environment was similar to where it was born, since in both of the conditions, the polymer chains were forced to take global conformations. So it remembered its original global conformations and shrunk quickly.
Reswelling kinetics
The water absorption of the P(NIPAAm-co-IA) hydrogels was determined gravimetrically as a function of time in distilled water at room temperatures(25 °C) and the water uptake-time curves were represented in Fig. 11. It was found that all the gels prepared in brine had faster swelling rates than G000 which prepared in pure water, since all the gels prepared in brine reached equilibrium within 80 min while G000 need about 110 min. Usually, the reswelling process is involved three successive steps [14, 15]: (1) the diffusion of water molecules into the matrixes, (2) the hydrated polymer chains gets relaxation, and (3) the expansion of the polymer network. Due to the freeze-dried treatment before reswelling, the porous structures, the relaxation conformation and expanded structures of the matrixes can be preserved to some extent. All these factors exert effect on the swelling cooperatively. So porous gels G030, G020, G015, G010 and G005 exhibit faster swelling rates that that of G000.
Conclusions
In the paper, a simple but effective strategy was proposed to prepare pH and temperature double responsive P (NIPAAm-co-IA) hydrogels through copolymerizing in aqueous sodium chloride solutions. It was found that the presence of NaCl could lead to improved responses to changes in pH and temperature. The finding may provide some useful information for the preparation of superior pH and temperature dual sensitive hydrogels which have the potential to be used in the drug delivery systems.
References
Okamura H, Maruyama T, Masuda S et al (2002) J Polym Res 9:17
Lee WF, Hsu CH (1997) J Polym Res 4:233
Lee WF, Yuan WY (2000) J Polym Res 7:29
Lee WF, Chiu RJ (2002) J Polym Res 9:141
Kabra BG, Gehrke SH (1991) Polym Commun 32:322
Wu XS, Hoffman AS, Yager P (1992) J Polym Sci A: Polym Chem 30:2121
Yoshida R, Uchida K, Okano T et al (1995) Nature 374:240
Kaneko Y, Sakai K, Okano T et al (1995) Macromolecules 28:7717
Oxley HR, Corkhill PH, Fitton JH et al (1993) Biomaterials 14:1064
Zhang XZ, Yang YY, Chung TS et al (2001) Langmuir 17:6094
Chen J, Park H, Park K (1999) J Biomed Mater Res 44:53
Dorkoosha FA, Brusseeb J, Verhoefa JC et al (2000) Polymer 41:8213
Zhang XZ, Yang YY, Chung TS (2002) Langmuir 18:2538
Zhang XZ, Zhuo RX, Yang YY (2002) Biomaterials 23:1313
Zhang JT, Cheng SX, Zhuo RX et al (2003) Macromol Rapid Commun 24:447
Serizawa T, Wakita K, Akashi M (2002) Macromolecules 35:10
Mori Y, Tokura H, Yoshikawa M (1997) J Mater Sci 32:491
Xue W, Hamley IW, Huglin MB (2002) Polymer 43:5181
Liu QF, Zhang P, Lu MG (2005) J Polym Sci A: Polym Chem 43:2615
Liu QF, Li SD, Lu MG et al (2007) J Polym Res 14:397
Yoo MK, Sung YK, Lee YM et al (2000) Polymer 41:5713
Atta AM, Maysour NE, Arndt KF (2006) J Polym Res 13:53
Lee WF, Shieh CH (1999) J Polym Res 6:41
Changag WC, Chang SJ, Wang YJ (2005) J Polym Res 11:317
Lee WF, Lin WJ (2003) J Polym Res 10:31
Demirel G (2007) J Polym Res 14:23
Atta AM, Arndt KF (2005) J Polym Res 12:77
Üzüm ÖB, Karadağ E (2007) J Polym Res 14:483
Uzgören A, Rzaev ZMO, Okay G (2007) J Polym Res 14:329
Buck RP, Rondinni S, Covington AK (2002) Pure Appl Chem 74:2169
Chu HH, Lin CS (2003) J Polym Res 10:283
Dincer S, Rzaev ZMO, Piskin E (2006) J Polym Res 13:121
Ma D, Zhang LM, Yang C et al (2008) UV photopolymerized hydrogels with b-cyclodextrin moieties. J Polym Res (in press) DOI 10.1007/s10965-007-9171-1
Mudassir J, Ranjha NM (2007) Dynamic and equilibrium swelling studies: crosslinked pH sensitive methyl methacrylate-co-itaconic acid (MMA-co-IA) hydrogels. J Polym Res (in press) doi:10.1007/s10965-007-9159-x
Liu GQ, Guan CL, Zou WJ et al (2007) J Polym Res 14:461
Kuo SM, Liou CC, Chang SJ et al (2001) J Polym Res 8:169
Zahra M, Mohammad ZM, Kourosh K et al (2007) Tragacanth gum-graft-polyacrylonitrile: synthesis, characterization and hydrolysis. J Polym Res (in press) doi:10.1007/s10965-007-9156-0
Gohil JM, Bhattacharya A, Ray P (2006) J Polym Res 13:161
Wu RL, Xu SM, Wang JD et al (2006) J Polym Res 13:33
Li F, Zhao Y, Tan TW (2006) J Polym Res 13:145
Ebara M, Aoyagi T, Sakai K et al (2000) J Polym Sci A: Polym Chem 39:335
Chao GT, Deng HX, Qian ZY et al (2006) J Polym Res 13:349
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ling, Y., Lu, M. Thermo and pH dual responsive Poly (N-isopropylacrylamide-co-itaconic acid) hydrogels prepared in aqueous NaCl solutions and their characterization. J Polym Res 16, 29–37 (2009). https://doi.org/10.1007/s10965-008-9199-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9199-x