Abstract
Introduction
Muscle mass is vital for physical activity and fundamental physiological processes supporting long-term health. While aging is inevitable, certain modifiable factors positively influence muscle preservation and overall well-being. However, the relationship between the consumption of ultra-processed foods (UPF) and muscle mass is not yet clear.
Methods
This study included 7,173 men and nonpregnant women aged 20–59 years with valid 24-hour dietary recalls and accessible whole-body dual-energy x-ray absorptiometry (DXA) scans from NHANES 2011–2018. UPFs were identified through the NOVA classification system, and the percentage of energy derived from UPF consumption was evaluated in quintiles. Muscle mass measures were derived from DXA scans and quantified by the total and regional muscle mass index (MMI, kg/m²) and appendicular muscle mass index (AMMI, kg/m²). Multivariable-adjusted generalized linear regression models were applied to investigate the association between consumption of UPFs and muscle mass measures overall and by sociodemographic subgroups.
Results
The multivariable-adjusted differences of total MMI from the lowest to highest quintile of UPF consumption were 0 (reference), -0.03 (95% CI, -0.13, 0.07), -0.13 (95%CI, -0.24, -0.04), -0.12 (95% CI, -0.23, -0.01), and − 0.17 (95% CI, -0.27, -0.08) (P for trend < 0.001). Subtotal MMI followed a similar magnitude of associational pattern as total MMI. For trunk MMI, corresponding values from the lowest to highest quintiles of UPF consumption were 0 (reference), -0.02 (95% CI, -0.07, 0.02), -0.05 (95%CI, -0.11, 0.00), -0.07 (95% CI, -0.13, -0.01), and − 0.07 (95% CI, -0.12, -0.01). For AMMI, corresponding values from the lowest to highest quintiles of UPF consumption were 0 (reference), -0.004 (95% CI, -0.07, 0.06), -0.08 (95%CI, -0.14, -0.02), -0.05 (95% CI, -0.11, 0.02), and − 0.10 (95% CI, -0.16, -0.04) (All P for trend < 0.001). While most subgroups maintained similar overall patterns, heterogeneous findings were also observed. For example, the multivariable-adjusted differences in total MMI between the lowest and highest quantile of UPF consumption were − 0.19 (95% CI, -0.32, -0.06) for non-Hispanic Whites, 0.18 (95% CI, 0.01, 0.36) for non-Hispanic Blacks, -0.25 (95%CI, -0.45, -0.04) for Hispanics, -0.25 (95% CI, -0.51, 0.05) for non-Hispanic Asians and − 0.32 (95% CI, -0.75, 0.12) for others (P for interaction < 0.001).
Conclusion
Higher consumption of UPFs was significantly associated with lower values of total and regional muscle mass. Specifically, comparing the highest quantile of UPF consumption to the lowest, total MMI decreased by 0.93%, trunk MMI decreased by 0.76%, and AMMI decreased by 1.25%. The differences in associational patterns between UPF consumption and muscle mass across sociodemographic subgroups require further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle mass is a critical component of body composition, constituting approximately 40% of total mass [1]. It plays an important role in facilitating physical activity and many essential physiological processes that are fundamental for maintaining long-term health and an enhanced quality of life [2]. Robust muscle mass plays a pivotal role in maintaining bone health, enhancing physical performance, elevating metabolic rates, and improving blood sugar regulation, among other vital functions [3, 4]. While the amount of muscle mass can differ among individuals, advancing age is the primary driver for the progressive and general loss of muscle mass. It is estimated that approximately 35-40% of muscle mass could be lost between the ages of 20 and 80 years [5, 6]. Though aging is an inherent aspect of the life course, there are well-established modifiable factors known to positively influence the preservation of muscle mass and promotion of overall well-being [7, 8]. A substantial body of research underscores the profound impact of a healthy diet on maintaining muscle mass and its related functions [9,10,11].
In recent decades, ultra-processed foods have become increasingly prevalent, accounting for more than half of daily US calories due to their convenience and extended shelf life [12]. These food products are defined as items that have undergone multiple industrial processes and typically contain substantial amounts of added sugar, salt, unhealthy fats, and various additives [13, 14]. A growing body of studies has documented the adverse health impacts associated with UPFs such as obesity, diabetes, cardiovascular diseases, cancer, and various chronic conditions [15,16,17,18,19,20]. The consumption of food has been demonstrated to have a significant impact on muscle mass, particularly through the intake of protein, which notably enhances muscle protein synthesis [21]. However, research on the association of UPF consumption with body composition remains relatively limited. Our prior study examined associations of UPF consumption with whole and regional body fat distribution among U.S. adults [22]. Several studies have investigated the relationship between UPF consumption and muscle mass, mainly among Brazilian adults and adolescents [23,24,25]. In addition, potential differences in the association of UPFs with muscle mass across socioeconomic status are unknown.
To address those research gaps, this study aimed to examine the associations of UPF consumption and muscle mass among nationally representative U.S. adults, overall and across sociodemographic strata.
Methods
Study population
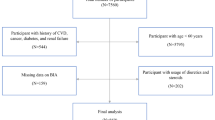
The National Health and Nutrition Examination Survey (NHANES) is a series of cross-sectional surveys designed to assess the health and nutritional status of Americans. It is typically conducted in a two-year cycle by the Centers for Disease Control and Prevention. To ensure a nationally representative sample of U.S. non-institutionalized civilians, NHANES employs a complex survey design including stratified, multi-stage, and probability sampling. Details on the study design, protocol, and data collection have been documented elsewhere [26]. Written informed consent was obtained from all participants. The present analysis included data from four NHANES cycles (2011–2012, 2013–2014, 2015–2016, 2017–2018). Dietary information was obtained through 1–2 24-hour dietary recalls, collected by trained staff using the computer-assisted multiple-pass method during interviews [27]. Body composition was measured by anthropometry and dual energy X-ray absorptiometry (DXA) using Hologic QDR 4500 A fan-beam bone densitometers. The DXA whole-body scan was performed on individuals aged 8 to 59 years, with pregnant women being excluded from the scan [28]. The final analytical sample comprised 7,173 adults including men and non-pregnant women aged 20–59 years with available information on dietary intake and whole-body DXA scans (Appendix Fig. 1).
Measures
Each food item reported by the participants was classified according to the extent of industrial processing using the Nova classification system [29]. Nova has 4 groups. Group 1 is unprocessed or minimally processed foods including foods that have undergone no processing or processing like grinding, roasting, pasteurization, and freezing. Group 2 is processed culinary ingredients including sugar, oils, fats, salt, and other substances that have been extracted, pressed, or centrifuged from group 1 foods or from nature, and are used to make culinary preparations. Group 3 is processed foods that are manufactured by adding salt or sugar or other processed culinary ingredients to unprocessed or minimally processed foods to prolong their durability and modify their palatability. Group 4 is UPFs that are industrial formulations containing no or small amounts of whole foods, and typically contain food cosmetic additives such as flavors, colors, sweeteners, emulsifiers, and other substances to disguise undesirable qualities of the final products. Further details of the Nova classification are presented elsewhere [29]. In this study, the exposure of interest was the percentage of daily energy intake consumed from UPFs. This measure adjusted for total energy intake using the nutrient density approach [30]. The percentage of energy intake from macronutrients, such as protein, carbohydrates, and fats, was determined by multiplying their respective weights by the energy coefficient assigned to each nutrient per gram.
Whole-body DXA scans were conducted by certified radiologists in the NHANES mobile examination center. These scans measured the amounts of fat, bone, and lean tissue (like muscle) for the total body and body regions. The outcome of interest is muscle mass which is quantified by the total and regional muscle mass index (MMI, kg/m²) and appendicular muscle mass index (AMMI, kg/m²). These included total MMI (whole body MMI), subtotal MMI (whole body except head MMI), upper limb MMI (right and left arms MMI), lower limb MMI (right and left legs MMI) and AMMI (upper and lower limb MMI). This way, we can minimize the potential impact of height on the distribution and overall amount of muscle mass [31]. Specifically, MMI (kg/m²) was calculated by dividing muscle mass (kg) by the square of height (m) [32].
Sociodemographic and lifestyle factors, including biological sex, age, race and ethnicity, education, income, smoking status, and physical activity were self-reported during household interviews. Race and ethnicity were grouped based on fixed categories provided by the CDC as non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic and others. Education was categorized into four levels: less than high school, high school graduate, some college, college graduate or above. Income was measured by the ratio of family income to poverty at the household level and classified into < 1.30, 1.30–3.49, and ≥ 3.50 according to Supplemental Nutrition Assistance Program eligibility [33]. Smokers were categorized as never, former, current smokers, and current smokers were further categorized according to the number of cigarettes smoked as some days, < 20 cigarettes per day, and ≥ 20 cigarettes per day. Physical activity was calculated as METs-min using self-reported weekly hours of moderate- and vigorous-intensity recreational and work activity, and presented as quartiles [34].
Statistical analysis
All analyses incorporated NHANES sampling weights, stratification, and clustering of the complex survey design to produce nationally representative estimates of the non-institutionalized U.S. population. Participant characteristics were presented by quintiles of UPF contribution to total energy intake in the diet and their differences were tested using chi-square statistic for categorical variables and ANOVA for continuous variables. To evaluate the relationship of UPF consumption with each outcome, multivariable linear regression models were conducted adjusting for potential confounders, including age, sex, race and ethnicity, education, income, smoking status, and physical activity. Predicted means with 95% confidence interval (CI) of outcomes of interest were estimated across quantiles of UPF contribution. Tests for linear trend across quintiles were conducted using the P for trend analysis.
This study further conducted stratified analyses to explore potential effect modifications by age, sex, race and ethnicity, education, and income. The interactions between those sociodemographic subgroup variables and quintiles of UPF consumption were examined using the Wald F statistic. To account for the large number of tests being performed, the Bonferroni correction was used to adjust for the P-values and statistical significance was set at P < 0.003 to minimize the risk of type I error.
All statistical analyses were survey-weighted accounting for survey complex sampling design and conducted using R, version 4.3.0 and Stata, version 18.0. The statistical significance was set at a 2-tailed P < 0.05.
Results
A total of 7,173 adults (mean [SD] age, 39.18 [11.81] years; 48.40% female) were included in the present analysis. Table 1 displays the characteristics of study participants according to quintiles of UPF consumption. Participants with a higher level of UPF consumption were more likely to be younger, non-Hispanic White or non-Hispanic Black; to have lower educational level and income level; to be current and more frequent smokers. In contrast, participants with a lower level of UPF consumption tended to be older, non-Hispanic Asian or Hispanic, non-smokers and had higher levels of education and income. There was a U-shaped relation between physical activity and UPF consumption. Moreover, a higher intake of UPFs correlates with reduced protein consumption and elevated carbohydrate intake (Appendix Table 1).
Muscle mass was significantly correlated with consumption of UPF (Appendix Table 2). Following multivariable-adjusted linear regression analysis, muscle mass was significantly associated with consumption of UPF (Table 2). The multivariable-adjusted differences of total MMI from the lowest to highest quintile of UPF consumption were 0 (reference), -0.03 (95% CI, -0.13, 0.07), -0.13 (95%CI, -0.24, -0.04), -0.12 (95% CI, -0.23, -0.01), and − 0.17 (95% CI, -0.27, -0.08) (P for trend < 0.001). Subtotal MMI followed similar associational patterns as total MMI with consumption of UPF. The multivariable-adjusted differences of trunk MMI from the lowest to highest quintiles of UPF consumption were 0 (reference), -0.02 (95% CI, -0.07, 0.02), -0.05 (95%CI, -0.11, 0.00), -0.07 (95% CI, -0.13, 0.01), and − 0.07 (95% CI, -0.12, -0.01). The multivariable-adjusted differences of AMMI from the lowest to highest quintiles of UPF consumption were 0 (reference), -0.004 (95% CI, -0.07, 0.06), -0.08 (95% CI, -0.14, -0.02), -0.05 (95% CI, -0.11, 0.02), and − 0.10 (95% CI, -0.16, -0.04) (All P for trend < 0.001). Compared to the lowest quintile, the muscle mass in the highest quintile was reduced by 0.93% for the total MMI, 0.76% for trunk MMI, and 1.25% for the AMMI.
Findings from stratified analyses were presented in Figs. 1 and 2 and Appendix Tables 3–8. After adjusting for type I error due to multiple testing, statistically significant interactions were detected between UPF consumption and race/ethnicity for all MMI and AMMI outcomes (P for interactions < 0.001) except trunk MMI (P for interactions = 0.056). For example, the multivariable-adjusted differences in total MMI between the lowest and highest quantile of UPF consumption were − 0.19 (95% CI, -0.32, -0.06) for non-Hispanic Whites, 0.18 (95% CI, 0.01, 0.36) for non-Hispanic Blacks, -0.25 (95%CI, -0.45, -0.04) for Hispanics, -0.25 (95% CI, -0.51, 0.05) for non-Hispanic Asians and − 0.32 (95% CI, -0.75, 0.12) for others (P for interaction < 0.001). The association of UPF consumption with total MMI was also significantly modified by education (P for interaction < 0.001) with more notable decreases across quintiles observed in low-educational levels and by income (P for interaction = 0.002) with more notable decreases observed at high-income levels. Similar interactional patterns were observed for AMMI and upper limb MMI with education (P for interactions < 0.001) and lower limb MMI with income (P for interaction = 0.002).
Adjusted predicted means of muscle mass index (total, subtotal, trunk, upper and lower limb) and AMMI (appendicular muscle mass index) across quintiles of ultra-processed food consumption among U.S. adults (aged 20 − 59 years) by (A) sex and (B) age groups, NHANES 2011 − 2018. Analyses were adjusted for age (years), Sex (male, female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian and others), education (less than high school, high school graduate or GED, some college, or college graduate or above), the ratio of family income to poverty (< 1.30, 1.30 − 3.49, and ≥ 3.5), smoking status (never smoker, former smoker, and smokers smoking for some days and smokers smoking daily < 20 cigarettes per day and equal or larger than 20 cigarettes per day), and physical activity (METs-Min)
Adjusted predicted means of muscle mass index (total, subtotal, trunk, upper and lower limb) and AMMI (appendicular muscle mass index) across quintiles of ultra-processed food consumption among U.S. adults (aged 20 − 59 years) by (A) education, (B) race/ethnicity and (C) income, NHANES 2011 − 2018. Analyses were adjusted for age (years), Sex (male, female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian and others), education (less than high school, high school graduate or GED, some college, or college graduate or above), the ratio of family income to poverty (< 1.30, 1.30 − 3.49, and ≥ 3.5), smoking status (never smoker, former smoker, and smokers smoking for some days and smokers smoking daily < 20 cigarettes per day and equal or larger than 20 cigarettes per day), and physical activity (METs-Min)
Discussion
In this nationally representative sample of U.S. men and nonpregnant women aged 20–59 years, we found that higher consumption of UPF was significantly associated with lower values of muscle mass including total MMI, subtotal MMI, trunk MMI, AMMI, and limb (lower and upper) MMI. These inverse associational patterns remained consistent across most sociodemographic subgroups, including age, sex, race and ethnicity, education, and income. In exploratory stratified analyses, the associational patterns were less pronounced among non-Hispanic Blacks and non-Hispanic Asian as compared to non-Hispanic Whites and Hispanics. Additionally, they were less prominent among individuals with high education and low income compared to their respective counterparts.
Our findings provide important information about UPF consumption and muscle mass directly measured by DXA scans. While the detrimental health effects of consuming UPFs on conditions such as obesity, diabetes, cardiovascular disease, cancer, and cognitive functions have been increasingly recognized [16, 35, 36], research on the relationship between UPF consumption and muscle mass remains limited. To the best of our knowledge, this is the first study that extensively examined the relationship of UPF consumption with objectively measured total and regional muscle mass in a nationally representative U.S. adult sample. The results aligned consistently with findings from the limited existing research in this area. One cross-sectional study focused on 490 adults aged 20 years and older living in Teresina, Brazil and found an inverse association of UPF consumption with arm circumference (ß: −0.02; 95% CI: −0.03/−0.01) and corrected arm muscle area (ß: −0.07; 95% CI: −0.12/−0.02) among those aged 36 to 59 years [23]. Another cross-sectional study conducted among Brazilian adolescents 18 to 19 years of age found that a 1% increase in percent contribution of UPFs to total energy intake was associated with a 0.04 kg decrease in muscle mass and a 0.01 kg/m2 decrease in lean body mass [24]. One cohort study followed 815 Brazilian women aged 23–25 years to 37–39 years and found that higher UPF consumption associated with a longitudinal decrease in muscle mass (β = −0.07; 95% CI, − 0.11, − 0.03) [25]. Another study conducted in Augusta, Georgia, USA, among 640 European American or African American adolescents aged 14 to 18 years found that DXA-scanned based skeletal muscle mass index was negatively associated with consumption of sugar-sweetened beverages (β = −0.12, P = 0.001) [37].
Our study also provides critical insights through sociodemographic subgroup analyses. We found a suggestion of associational differences related to health determinant social factors such as race and ethnicity, education, and income. For example, we found the associational patterns of UPF consumption with muscle mass were less pronounced among non-Hispanic Asians, non-Hispanic Blacks, and individuals with higher educational attainment or lower income levels. These differences across race/ethnicity were consistent with findings from another study which observed no association between UPF consumption with percentage fat (total, android, and gynoid) among non-Hispanic Blacks and non-Hispanic Asians [21].Cultural factors including dietary traditions, preferences, and culinary practices might influence food choices and consumption patterns [38,39,40]. Furthermore, our findings suggested that MMI was notably higher in individuals aged 40–59 compared to those aged 20–39. This observation can be attributed to middle-aged individuals having a higher average BMI compared to younger adults, a pattern that aligns with prior research [41, 42]. Our study corroborates this, showing that middle-aged adults (40–59 years) possess a higher BMI of 29.1 kg/m2 in contrast to young adults (20–39 years), who have an average BMI of 27.87 kg/m2. Additionally, the specific types and formulations of UPFs may vary across cultural contexts, potentially leading to divergent exposures to substances, contaminants and additives that might alter the absorption or metabolism of nutrients that influence the associations of UPF consumption and muscle mass [43].
The association between higher UPF consumption and lower levels of muscle mass can be attributed to several underlying mechanisms. First, population consuming higher amounts of UPF tend to have lower protein intake, which is significant given that dietary protein is crucial for muscle mass development by enhancing the availability of plasma amino acids [21, 44]. Our exploratory analysis reflects similar findings. Future studies are warranted to validate these observations. In addition, some research has linked UPF intake to elevated intramuscular fat content, characterized by heightened lipid droplet count and size within muscle cells [45]. This phenomenon could trigger adaptive responses including lipolysis and heightened autophagy, which may contribute to the loss of muscle mass. Moreover, the accrual of body fat resulting from UPF consumption can impair mitochondrial function and biogenesis [46]. Dysfunctional mitochondria can generate higher levels of reactive oxygen species, fostering inflammation and inhibiting the mammalian target of rapamycin (mTOR) signaling pathway, a key regulator of muscle protein synthesis [11, 47, 48]. Furthermore, UPFs have a proclivity to induce an inflammatory response and promote insulin resistance, both of which could adversely affect muscle protein metabolism [49]. Alterations in gut microbiota composition, hormonal imbalances, and the absence of bioactive compounds found in minimally processed foods add additional layers of complexity to this relationship [50, 51].
Strengths of our study include the use of objective measures such as DXA scans for accurate body composition assessment, detailed dietary information, and a nationally representative sample of U.S. adults. Limitations of our study include reliance on self-reported estimates of UPF consumption, which is subject to measurement errors. However, the 24-hour dietary recalls were collected using the computer-assisted system by trained dietary interviewers and results were further adjusted for total energy, both of which reduce measurement error. Detailed information, including brand name/list of ingredients, is in some cases required though not always available for accurate classification of food items according to Nova. However, independent classification by two researchers was conducted for all food items and discrepancies regarding the degree of processing were resolved by opting for the lesser degree, potentially resulting in a conservative estimate of UPF consumption. Social desirability bias may further underestimate UPF consumption. Moreover, the temporality of the association cannot be established due to the nature of cross-sectional study design. Finally, despite careful adjustment for multiple potential confounding factors, the possibility of residual or unmeasured confounding factors could not be entirely ruled out.
Conclusion
Higher consumption of UPFs was significantly associated with lower values of total and regional muscle mass. Specifically, comparing the highest quantile of UPFs consumption to the lowest, total MMI decreased by 0.93%, trunk MMI decreased by 0.76%, and AMMI decreased by 1.25%. The differences in associational patterns between UPF consumption and muscle mass across sociodemographic subgroups require further investigation.
References
Janssen I, Heymsfield SB, Wang ZM, Ross R (Jul 2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 year. J Appl Physiol (1985) 89(1):81–88. https://doi.org/10.1152/jappl.2000.89.1.81
Distefano G, Standley RA, Zhang X et al (2018) Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle Apr 9(2):279–294. https://doi.org/10.1002/jcsm.12272
Ross SA, Wakeling JM (2021) The energy of muscle contraction. IV. Greater mass of larger muscles decreases contraction efficiency. J R Soc Interface Sep 18(182):20210484. https://doi.org/10.1098/rsif.2021.0484
Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB (2017) Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle Apr 8(2):245–250. https://doi.org/10.1002/jcsm.12160
Proctor DN, Balagopal P, Nair KS (1998) Age-related Sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J Nutr Feb 128(2 Suppl):351S–355S. https://doi.org/10.1093/jn/128.2.351S
Volpi E, Nazemi R, Fujita S (2004) Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care Jul 7(4):405–410. https://doi.org/10.1097/01.mco.0000134362.76653.b2
Distefano G, Goodpaster BH (2018) Effects of Exercise and Aging on skeletal muscle. Cold Spring Harb Perspect Med Mar 1(3). https://doi.org/10.1101/cshperspect.a029785
Goodpaster BH, Chomentowski P, Ward BK et al (2008) Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) Nov 105(5):1498–1503. https://doi.org/10.1152/japplphysiol.90425.2008
Carbone JW, Pasiakos SM (2019) Dietary protein and muscle Mass: translating Science to Application and Health Benefit. Nutrients May 22(5). https://doi.org/10.3390/nu11051136
Antonio Paoli A, Mancin L, Caprio M et al (2021) Effects of 30 days of ketogenic diet on body composition, muscle strength, muscle area, metabolism, and performance in semi-professional soccer players. J Int Soc Sports Nutr Sep 16(1):62. https://doi.org/10.1186/s12970-021-00459-9
Chen L, Ming J, Chen T et al (2022) Association between dietary inflammatory index score and muscle mass and strength in older adults: a study from National Health and Nutrition Examination Survey (NHANES) 1999–2002. Eur J Nutr Dec 61(8):4077–4089. https://doi.org/10.1007/s00394-022-02941-9
Liu J, Steele EM, Li Y et al (2022) Consumption of Ultraprocessed Foods and Diet Quality among U.S. children and adults. Am J Prev Med Feb 62(2):252–264. https://doi.org/10.1016/j.amepre.2021.08.014
Juul F, Vaidean G, Parekh N (2021) Ultra-processed Foods and Cardiovascular diseases: potential mechanisms of action. Adv Nutr Oct 1(5):1673–1680. https://doi.org/10.1093/advances/nmab049
Monteiro CA, Cannon G, Levy RB et al (2019) Ultra-processed foods: what they are and how to identify them. Public Health Nutr Apr 22(5):936–941. https://doi.org/10.1017/S1368980018003762
Rousham EK, Goudet S, Markey O et al (2022) Unhealthy Food and Beverage Consumption in children and risk of overweight and obesity: a systematic review and Meta-analysis. Adv Nutr Oct 2(5):1669–1696. https://doi.org/10.1093/advances/nmac032
Gomes Goncalves N, Vidal Ferreira N, Khandpur N et al (2023) Association between Consumption of Ultraprocessed Foods and Cognitive decline. JAMA Neurol Feb 1(2):142–150. https://doi.org/10.1001/jamaneurol.2022.4397
Srour B, Fezeu LK, Kesse-Guyot E et al (2019) Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). Bmj May 29:365:l1451. https://doi.org/10.1136/bmj.l1451
Samuthpongtorn C, Nguyen LH, Okereke OI et al (2023) Consumption of Ultraprocessed Food and Risk of Depression. JAMA Netw Open Sep 5(9):e2334770. https://doi.org/10.1001/jamanetworkopen.2023.34770
Askari M, Heshmati J, Shahinfar H, Tripathi N, Daneshzad E (2020) Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes (Lond) Oct 44(10):2080–2091. https://doi.org/10.1038/s41366-020-00650-z
Chen Z, Khandpur N, Desjardins C et al (2023) Ultra-processed food consumption and risk of type 2 diabetes: three large prospective U.S. Cohort studies. Diabetes Care Jul 1(7):1335–1344. https://doi.org/10.2337/dc22-1993
Gorissen SH, Rémond D, van Loon LJ (2015) The muscle protein synthetic response to food ingestion. Meat Sci 109:96–100. https://doi.org/10.1016/j.meatsci.2015.05.009
Liu J, Steele EM, Li Y, Yi SS, Monteiro CA, Mozaffarian D (Sep 2023) Consumption of Ultraprocessed Foods and Body Fat distribution among U.S. adults. Am J Prev Med 65(3):427–438. https://doi.org/10.1016/j.amepre.2023.03.012
Monteles Nascimento L, de Carvalho Lavor LC, Mendes Rodrigues BG et al (2023) Association between Consumption of Ultra-processed Food and Body composition of adults in a Capital City of a Brazilian region. Nutrients Jul 15(14). https://doi.org/10.3390/nu15143157
Viola P, Carvalho CA, Braganca M, Franca A, Alves M, da Silva AAM (2020) High consumption of ultra-processed foods is associated with lower muscle mass in Brazilian adolescents in the RPS birth cohort. Nutr Nov-Dec 79–80. https://doi.org/10.1016/j.nut.2020.110983
Rudakoff LCS, Magalhaes E, Viola P et al (2022) Ultra-processed food consumption is associated with increase in fat mass and decrease in lean mass in Brazilian women: a cohort study. Front Nutr 9:1006018. https://doi.org/10.3389/fnut.2022.1006018
US Centers for Disease Control and Prevention; National Center for Health Statistics (2023) National Health and Nutrition Examination Survey data. Available at, https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Accessed on November 16
Moshfegh AJ, Rhodes DG, Baer DJ et al (2008) The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr Aug 88(2):324–332. https://doi.org/10.1093/ajcn/88.2.324
National Health and Nutrition Examination Survey (NHANES) (2023) Body Composition Procedures Manual. Available at, https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/Body_Composition_Procedures_Manual_2018.pdf. Accessed on November 16
Steele EM, O’Connor LE, Juul F et al (2023) Identifying and Estimating Ultraprocessed Food Intake in the US NHANES according to the Nova classification system of Food Processing. J Nutr Jan 153(1):225–241. https://doi.org/10.1016/j.tjnut.2022.09.001
Willett W (2012) Nutritional epidemiology. Oxford University Press
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing Jul 1(4):601. https://doi.org/10.1093/ageing/afz046
Cawthon PM, Peters KW, Shardell MD et al (2014) Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol Biol Sci Med Sci May 69(5):567–575. https://doi.org/10.1093/gerona/glu023
Johnson CL, Paulose-Ram R, Ogden CL et al (2013) National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2 Sep ;(161):1–24
Ainsworth BE, Haskell WL, Whitt MC et al (Sep 2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32(9 Suppl):S498–504. https://doi.org/10.1097/00005768-200009001-00009
Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F (2021) Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr Feb 14(3):308–318. https://doi.org/10.1017/S0007114520002688
Isaksen IM, Dankel SN (2023) Ultra-processed food consumption and cancer risk: a systematic review and meta-analysis. Clin Nutr Mar 30(6):919–928. https://doi.org/10.1016/j.clnu.2023.03.018
Hao G, Pollock NK, Harris RA, Gutin B, Su S, Wang X (2019) Associations between muscle mass, physical activity and dietary behaviour in adolescents. Pediatr Obes Mar 14(3):e12471. https://doi.org/10.1111/ijpo.12471
Poggiogalle E, Kiesswetter E, Romano M et al (2021) Psychosocial and cultural determinants of dietary intake in community-dwelling older adults: a determinants of Diet and physical activity systematic literature review. Nutr May 85:111131. https://doi.org/10.1016/j.nut.2020.111131
Bach-Faig A, Berry EM, Lairon D et al (2011) Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr Dec 14(12A):2274–2284. https://doi.org/10.1017/S1368980011002515
Guillaume D (2011) The Mediterranean diet: a cultural journey. Lancet. Aug 27. ;378(9793):766-7; author reply 767. https://doi.org/10.1016/S0140-6736(11)61370-6
Akazawa N, Kishi M, Hino T et al (2022) Higher body Mass Index in hospitalized older patients is related to higher muscle quality. J Nutr Health Aging 26(5):495–500. https://doi.org/10.1007/s12603-022-1785-9
Kim NH, Park Y, Kim NH et al (2021) Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing 50(3):780–786. https://doi.org/10.1093/ageing/afaa208
Baker P, Machado P, Santos T et al (2020) Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev 21(12):e13126
Christian P, Sacco J, Adeli K, Autophagy (2013) Emerging roles in lipid homeostasis and metabolic control. Biochim Biophys Acta Apr 1831(4):819–824. https://doi.org/10.1016/j.bbalip.2012.12.009
Kalinkovich A, Livshits G (2017) Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev May 35:200–221. https://doi.org/10.1016/j.arr.2016.09.008
Smith CD, Lin CT, McMillin SL et al (2021) Genetically increasing flux through β-oxidation in skeletal muscle increases mitochondrial reductive stress and glucose intolerance. Am J Physiol Endocrinol Metab May 1(5):E938–e950. https://doi.org/10.1152/ajpendo.00010.2021
Bonnard C, Durand A, Peyrol S et al (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest Feb 118(2):789–800. https://doi.org/10.1172/jci32601
Handakas E, Chang K, Khandpur N et al (2022) Metabolic profiles of ultra-processed food consumption and their role in obesity risk in British children. Clin Nutr Nov 41(11):2537–2548. https://doi.org/10.1016/j.clnu.2022.09.002
Illiano P, Brambilla R, Parolini C (2020) The mutual interplay of gut microbiota, diet and human disease. FEBS J 287(5):833–855
Leitao AE, Roschel H, Oliveira-Junior G et al Association between ultra-processed food and flavonoid intakes in a nationally representative sample of the US population. Br J Nutr Nov 8 2023:1–10. https://doi.org/10.1017/S0007114523002568
Aggarwal R, Bains K (2022) Protein, lysine and vitamin D: critical role in muscle and bone health. Crit Rev Food Sci Nutr 62(9):2548–2559. https://doi.org/10.1080/10408398.2020.1855101
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest/financial disclosure
Not applicable for all authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, W., Liu, J., Steele, E.M. et al. Association of ultra-processed food consumption with muscle mass among young and middle-aged US adults. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03437-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03437-4