Abstract
Objectives
The global population is undergoing rapid aging, and the consequential decline in skeletal muscle mass with age presents substantial health risks, underscoring the importance of precise identification and gender-specific measurements. This study aimed to investigate the prevalence of low muscle mass among individuals aged > 60 years and identifying potential risk factors.
Methods
This study, conducted within the framework of Phase VII of the Tehran Lipid and Glucose Study (TLGS), included 860 participants selected through meticulous inclusion and exclusion criteria, focusing on good health. Various demographic, anthropometric, and metabolic measurements were collected, and bioelectrical impedance analysis (BIA) was employed for skeletal muscle mass assessment. Multiple logistic regression analysis was conducted to evaluate associations between low muscle mass and various factors.
Results
The prevalence of low skeletal muscle mass was 16.4% (CI: 14.06–19.03), with gender-based variations (20.24% (CI: 16.66–24.36) in males vs. 12.73% (CI: 9.92–16.19) in females). Multiple logistic regression analysis revealed associations between low muscle mass and increasing age, male gender, smoking, low physical activity, and higher HDL levels (P < 0.05). Protective effects were observed with higher BMI, weight, height, waist and hip circumference, and serum triglyceride levels (P < 0.05).
Conclusion
This study provides valuable insights into the prevalence and related factors of low skeletal muscle mass among older adults. Addressing modifiable risk factors and promoting healthy lifestyle behaviors are crucial steps in preventing and managing sarcopenia. Further longitudinal research is recommended to explore causal pathways and inform targeted interventions for optimizing muscle health across the lifespan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, the population is experiencing an increase in age. Projections indicate that by 2050, the number of elderly individuals will triple, rising from the 2000 figure of 600 million to surpass two billion [1]. Accordingly, enhancing the health and independence of seniors has emerged as a key priority for public health systems, both at present and in the future [2].
The aging process is linked to a physiological decline in skeletal muscle mass, which can amplify the impact on overall health and function, potentially increasing the risk of mortality in the long run [1]. After the age of 30, there is an approximate decline in muscle mass, ranging from 3 to 8% per decade. This rate of decrease becomes even more pronounced after reaching the age of 60 [3]. It is acknowledged that women generally have lower muscle mass than men [4]. Due to age-related muscle decline in both women and men, women continue to have lower muscle mass than men as they age [5]. Hence, sex-specific measurements and thresholds are crucial for a more precise identification of low muscle mass.
Various factors contribute to the susceptibility of older adults to poor muscle health, including detrimental lifestyle choices such as physical inactivity [6] and smoking [7]. Additionally, inadequate nutritional intake, underlying chronic diseases, and a low socioeconomic status, which encompasses low income and education levels [5], are also predisposing factors.
Older adults displaying evidence of low muscle mass face an elevated risk of adverse health outcomes, including diminished mobility, impaired ability to carry out daily activities, reduced quality of life, injuries related to falls, susceptibility to infections, increased likelihood of hospitalization, and a greater need for long-term care [8, 9]. This can potentially initiate a vicious cycle, leading to additional muscle mass loss, heightened disability, deteriorating health, and, ultimately, an increased risk of mortality [5, 8]. In a recent narrative review, it was consistently observed that low muscle mass or sarcopenia serves as a predictive factor for increased healthcare expenditure in various settings, including community, perioperative, and general hospital contexts [10].
Given the profound implications of age-related muscle loss on the health and well-being of the elderly, it is crucial to further investigate the prevalence of low muscle mass and its associated risk factors. Thus, this study aims to address this gap by investigating the prevalence of low muscle mass among individuals aged 60 and above who participated in Phase VII of the Tehran Lipid and Glucose Study (TLGS). By examining this specific cohort, we seek to shed light on the extent of muscle loss within this population and identify potential risk factors that may contribute to or exacerbate the condition.
Methods and materials
Study population
The study participants were recruited from the TLGS, a long-term community-based research initiative with the primary goal of identifying and preventing non-communicable disorders. It is situated in district No. 13, encompassing an area of approximately 13 km², in the eastern part of Tehran City. The study was performed under the coverage of Shahid Beheshti University of Medical Sciences and Health Services. In this area, three medical health centers were chosen, each with comprehensive field data on over 90% of all families in the region. Initial measurements were recorded, and the participants underwent follow-up studies over the course of three years. A preliminary sample of 15,005 participants aged ≥ 3 years was selected using a multistage stratified cluster sampling method [11]. Participants aged over 60 years, who had demographic, anthropometric, metabolic, and BIA data in phase VII of the TLGS (2019–2021), were selected through a simple random sampling method.
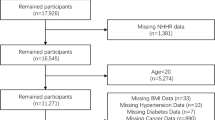
Participants aged > 60 years were selected based on meticulous inclusion and exclusion criteria, taking into consideration diverse factors, including health status. We performed a comprehensive evaluation of demographic information to gain a thorough understanding of the overall health and well-being of the participants. Additionally, priority was given to including only individuals in good health while excluding those with any underlying health conditions that could potentially introduce confounding factors into the results. Conversely, participants were excluded from the study if they had missing data; suffered from conditions such as diabetes, heart failure, or renal failure; had a history of cancer; were pregnant or lactating; or had a history of using diuretics or glucocorticoids. Ultimately, the final analysis comprised 860 participants (Fig. 1). Approval for this study was obtained from the Ethics Committee of the Research Institute for Endocrine Sciences (RIES) at the Shahid Beheshti University of Medical Sciences (code: IR.SBMU.ENDOCRINE.REC.1401.083). All participants provided written informed consent prior to participating in the study.
Measurements
Assessment of demographic factors
Trained interviewers gathered participant data, including age, sex, marital status, education, history of medications, physical activity, and smoking status (yes vs. no) using a predefined questionnaire.
Physical activity level was assessed using a questionnaire that covered all three activities over the past year: leisure time, job-related activities, and household activities over the past year. The results were expressed as metabolic equivalents of hours per week (METs/week) [12].
Measurements of anthropometric and body composition indices
Following the invitation to participate in the TLGS, individuals who expressed interest were directed to trained physicians after completing and signing an informed consent form. For anthropometric measurements, the participants were dressed in light clothing and without shoes. Weight and height were measured using a digital electronic weighing scale (Seca 707; range 0.1–150 kg; Seca, Hanover, MD) with a precision of up to 100 g and a tape meter stadiometer, respectively. Body Mass Index (BMI) was calculated by dividing the weight (in kilograms) by the square of the height (in meters). Waist circumference (WC) was assessed in centimeters at the level of the umbilicus. The hip circumference measurement involved encircling the broadest region of the buttocks, encompassing the iliac crest and greater trochanter, while ensuring that the tape was aligned parallel to the floor.
Body composition was evaluated using a portable multifrequency bioelectrical impedance analyzer (BIA) device (Model: InBody 570, InBody Co., Ltd. Seoul, KOREA). The BIA technique provides a straightforward, safe, and reliable means of assessing skeletal muscle mass and has been validated for the measurement of appendicular skeletal muscle mass (ASM) in extensive study populations [13]. InBody 570 is widely recognized for its credibility and consistency across different populations, although its precision may be affected by variables including hydration status, body temperature, and traits specific to certain populations. Participants adhered to specific preparation guidelines, which included a 2-hour fasting period, abstaining from caffeine for 2 h, refraining from exercise for 4–6 h, and wearing lightweight clothing without shoes and socks. The participants were instructed to commence the test after an overnight fasting period and remain in a seated position for 5 min before the measurement.
It is crucial to emphasize that BIA is not recommended for individuals with heart pacemakers, platinum or metal prostheses, or Holter devices implanted in their bodies. Participants were instructed to remove any metallic items or jewelry in their possession prior to undergoing the BIA measurement. This precautionary step was taken to reduce potential interference and improve the reliability of BIA results. After cleaning the palm and sole, each participant was instructed to stand barefoot, with their feet evenly placed on the foot electrodes in an upright stance. Simultaneously, they were instructed to keep their arms straight down, gripping the hand electrodes, as indicated by the instrument. Bioelectrical impedance analysis with eight electrodes assesses different segmental impedances (i.e., the trunk, right and left arms, and right and left legs) at 5, 50,500 kHz employing eight electrodes in a tetrapolar arrangement, and the device output included parameters composed of fat mass, fat free mass, ASM, trunk muscle mass, protein, mineral, total body water, intracellular water, extracellular water, and visceral fat area. The Skeletal Muscle Index (SMI) was calculated by dividing the Appendicular Skeletal Muscle Mass (ASM) by the square of height in meters. Furthermore, additional data such as sex, height, weight, and age were documented.
The intraclass correlation coefficient (ICC) was employed to evaluate the reproducibility of the measurements acquired by the BIA device within each group [14]. ICC is a statistical metric that gauges the consistency or reproducibility of measurements, encompassing both technical reproducibility and daily biological variations. A sample consisting of 15 women and 16 men was selected based on the relevant criteria. Body composition analyses for each group were performed twice by the same operator, with a three-day interval between sessions. The average age of men was 24 ± 6.4 years, whereas women had a mean age of 35 ± 10.8 years. The ICC values and 95% confidence intervals (CI) were calculated using the SPSS software version 20. The ICCs and 95% CIs calculated for PBF and FFM were 0.996 (0.991–0.998) and 0.998 (0.997–0.999), respectively. The mean differences for the two measurements of FM and FFM were (0.04 ± 1.11) and (0.10 ± 1.04), respectively. The proximity of these values to zero indicates reliability.
Predictive models, considering impedance, age, sex, height, and weight, were used to estimate body composition parameters. These models were created through regression analysis, utilizing data from various populations, including those assessed using more accurate methods such as Dual-Energy X-ray Absorptiometry (DXA). The precision of BIA is contingent on the device’s quality, accuracy of the reference data, and characteristics of the population being studied. Basal Metabolic Rate (BMR) values were estimated using BIA software, employing prediction equations based on factors such as age, weight, height, and sex. It is crucial to acknowledge that these values are calculated estimates and should not be interpreted as direct measurements of BMR.
Measurements of metabolic indices
Blood samples were gathered from every participant in the study during the time frame of 7:00 am to 9:00 am, after a fasting period of 12–14 h overnight. Fasting glucose levels were assessed by glucose oxidase and enzymatic colorimetry. The levels of serum total cholesterol (TC) and triglycerides (TGs) were determined using an enzymatic colorimetric method utilizing cholesterol esterase, cholesterol oxidase, and glycerol phosphate oxidase. The quantification of high-density lipoprotein cholesterol (HDL-C) was carried out by precipitating apolipoprotein B-containing lipoproteins with phosphotungstic acid. On the day of sample collection, all biochemical tests were conducted using commercial kits obtained from Pars Azmoon, Inc. (Tehran, Iran). The analysis was carried out with a Selectra 2 auto-analyzer from Vital Scientific, located in Spankeren, The Netherlands. Analyses were performed on all samples after ensuring quality control. LDL-C was computed from the concentrations of serum TC, TGs, and HDL-C, expressed in mg/dl, using the Friedewald formula [15]. Both inter- and intra-assay coefficients of variation (CVs) were < 2.3% for glucose, < 2% for TC, < 2.1% for TG, and < 3% for HDL-C.
All measurements were concurrently conducted at the RIES Research Laboratory.
Following a 15-minute rest period, a certified physician measured the systolic blood pressure (SBP) and diastolic blood pressure (DBP) of the subjects twice while they were seated. The first measurement was used to determine the peak inflation level by using a mercury sphygmomanometer. In this study, the participants’ blood pressure was calculated as the mean of two measurements.
Definition of low muscle mass
Appendicular skeletal muscle mass (ASM) was evaluated using BIA and was determined as the overall lean soft tissue mass in the arms and legs [16]. Then, the skeletal muscle index (SMI) was calculated by dividing ASM by the square of the individual’s height in meters. Low muscle mass was defined as low muscle mass in accordance with the AWGS criteria. Low muscle mass was identified as SMI values falling below 7.0 kg/m² in men and below 5.7 kg/m² in women, as determined by BIA [13].
Statistical analysis
For data exhibiting a normal distribution, the mean and standard deviation were used, whereas for skewed distributions, the median (interquartile range [IQR]) was employed. Categorical variables were presented as frequencies (%). Differences were assessed using the independent t-test for quantitative variables with a normal distribution, Mann-Whitney U test for quantitative variables without a normal distribution, and chi-square test for qualitative variables. Multiple logistic regression models were used to independently assess the factors associated with different stages of sarcopenia. All statistical analyses were conducted using SPSS (Statistical Package for the Social Sciences, Windows version 27.0; SPSS, Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
General characteristics
The general characteristics of the study are presented in Table 1. A total of 860 participants (420 males and 440 females) were recruited. The mean age was 67.98 ± 6.97 years. The mean BMI and ASM for the total population were 27.93 ± 4.64 and 7.13 ± 1.02, respectively. The majority of participants were non-smokers (88.1%), had education levels below the 12th grade (83.8%), and were married (80.8%). Additionally, over half of the participants engaged in physical activity exceeding 600 MET/week (55.5%), and the majority did not have hypertension (62.1%).
Comparison between genders
In comparing the two genders, male participants exhibited significantly higher age, weight, height, waist-to-hip ratio, fat-free mass, ASM, and BMR (P < 0.001). The frequency of marriage and smoking was also significantly higher among male participants. Females had higher levels of BMI, waist circumference, hip circumference, percent body fat, body fat mass, education, triglycerides, HDL, LDL, and total cholesterol (P < 0.05). However, there were no significant differences observed between males and females in physical activity, hypertension, and FBS (P > 0.05).
Prevalence of low skeletal muscle Mass
The prevalence of low skeletal muscle mass in the total population was 16.4% (Table 2). This prevalence was higher in males compared to females (20.24% vs. 12.73%) (P < 0.01).
Characteristics of participants according to muscle mass status
Table 3 presents the characteristics of participants according to normal or low muscle mass. Participants with low muscle mass were found to have a higher age (73.13 vs. 66.97 years) and higher HDL levels (51.71 vs. 48.64 mg/dl), and lower BMI (23.07 vs. 28.89 kg/m²), weight (57.67 vs. 75.13 kg), height (158.40 vs. 161.51 cm), waist circumference (87.48 vs. 98.86 cm), hip circumference (91.86 vs. 103.43 cm), SMMI (8.28 vs. 9.71), fat-free mass (39.28 vs. 46.91 kg), percent body fat (31.65 vs. 37.46), body fat mass (18.54 vs. 28.48 kg), BMR (1218.6 vs. 1383.2 kcal/day), and triglyceride levels (99 vs. 123 mg/dl). Furthermore, participants with low muscle mass had a higher percentage of males (60.3% vs. 46.6%) and engaged in lower physical activity (52.7% vs. 42.9%) compared to those with normal muscle mass. No significant differences were observed in waist-to-hip ratio, smoking status, education, marital status, hypertension, and FBS according to muscle mass status (P > 0.05).
Factors associated with low skeletal muscle mass
Factors associated with low skeletal muscle mass were evaluated using multiple logistic regression (Table 4). Low skeletal muscle mass was found to be associated with increasing age (OR 1.12 [95% CI 1.09, 1.15]), male gender (OR 1.74 [95% CI 1.20, 2.51]), smoking (OR 1.67 [95% CI 1.02, 2.76]), low physical activity (OR 1.48 [95% CI 1.01, 2.15]), and higher HDL levels (OR 1.02 [95% CI 1.01, 1.04]). Conversely, low skeletal muscle mass was associated with higher BMI (OR 0.62 [95% CI 0.57, 0.67]), weight (OR 0.8 [95% CI 0.77, 0.83]), height (OR 0.96 [95% CI 0.94, 0.98]), waist circumference (OR 0.88 [95% CI 0.85, 0.90]), hip circumference (OR 0.82 [95% CI 0.79, 0.85]), and serum triglyceride levels (OR 0.995 [95% CI 0.991, 0.998]). Waist-hip ratio, education status, marital status, hypertension, FBS, LDL, and total cholesterol were not found to be associated with low skeletal muscle mass.
Discussion
The present study provides valuable insights into the prevalence of low skeletal muscle mass among a cohort of participants and sheds light on some various associated risk factors. Our findings indicate that 16.4% of the total population exhibited low skeletal muscle mass, with gender-based differences. Notably, the prevalence was higher among males (20.24%) compared to females (12.73%), emphasizing the need for gender-specific interventions and awareness campaigns addressing muscle health. The observed associations between low muscle mass and certain demographic and health-related factors warrant careful consideration. The analysis unveiled a multifaceted interplay of factors influencing skeletal muscle mass. Notably, advancing age, male gender, smoking, low physical activity, and higher HDL levels were associated with increased risk, while higher BMI, weight, height, waist and hip circumference, and serum triglyceride levels demonstrated a protective effect.
Given the scarcity of studies solely addressing muscle wasting among older adults, comparing our findings with research on sarcopenia becomes crucial. Muscle wasting, which lays the groundwork for the initiation of sarcopenia, encompasses diverse factors that affect muscle health. The well-defined criteria and focus on the progressive decline in muscle mass and strength in sarcopenia studies provide a valuable benchmark. This comparison not only enriches our discussion by aligning with an established context but also underscores the unique contribution of our study in highlighting the multifaceted dynamics of muscle health in older adults, where dedicated investigations into muscle wasting are limited.
The findings of our research align with a prior epidemiological study conducted in Iran [17], which involved 300 individuals aged > 55 years, randomly chosen from the 6th district of Tehran. It reported a prevalence of pre-sarcopenia (defined as low ASM) based on the EWGSOP definition. The reported rates were 52.7% in men and 25.3% in women. In our study, the AWGS criteria was used for defining low muscle mass, while Heshmat et al. [17] employed the EWGSOP algorithm. Additionally, our study excluded participants with diabetes, whereas they did not exclude participants with diabetes. These differences in the sarcopenia definition and the inclusion/exclusion criteria, particularly regarding diabetes, could lead to variations in the prevalence rates observed between our findings and the one by Heshmat et al. [17]. In a study conducted in Singapore [5] to ascertain the prevalence of low ASMI (calculated as ASM/height2) and identify factors linked to low ASMI, 1211 community-dwelling adults aged 65 or older were included. The overall cohort exhibited a 59.9% prevalence of low ASMI, with rates of 57.0% among males and 61.8% among females. Those with low ASMI tended to be older and had lower physical activity scores, with all differences being statistically significant (all P < 0.0001). Low ASMI was associated with older age and smoking, (P ≤ 0.0328) [5]. Low muscle mass is commonly reported in community-dwelling older adults across Asia, with prevalence rates ranging from 20 to 63% [9, 18,19,20,21].
Muscular atrophy tends to occur at a higher rate in older men than in older women. This discrepancy may be influenced by the role of insulin-like growth factor 1 (IGF-1), a key mediator in muscle growth and repair, suggesting its potential significance in this phenomenon. Among older individuals, men generally exhibit lower IGF-1 levels than women. Sex differences in sarcopenia prevalence may be influenced by variations in IGF-1 levels [17, 22]. In our study, the higher prevalence of low skeletal muscle mass among males (20.24%) compared to females (12.73%) can be attributed to several probable interconnected factors identified in the study. Firstly, the significantly higher age of male participants aligns with age-related muscle decline, known as sarcopenia. Additionally, the observed higher prevalence of smoking among males, a known contributor to muscle wasting, may further explain this gender-based difference. Lower physical activity levels among males, as indicated in the study, align with existing literature associating physical inactivity with muscle loss. Anthropometric variations, such as lower BMI in males, may contribute to the higher muscle mass loss in males. The interplay of these factors, including potential disparities in health-seeking behavior and nutritional habits, contributes to the nuanced understanding of why the prevalence of low muscle mass is higher among males in this population.
Fewer studies have delved into the exploration of risk factors for low muscle mass or sarcopenia. Aging has emerged as a critical factor for predicting a low ASMI. Prior research indicated that each year beyond the age of 65, there was a 6% increase in the likelihood of experiencing low ASMI [5]. Although age is a non-modifiable risk factor, it serves as a crucial indicator of low ASMI. The correlation between age and a low ASMI was similarly evident in the relationship between age and sarcopenia [23, 24]. This is due to the fact that reduced muscle mass is one of the diagnostic criteria for sarcopenia. Indeed, researchers have linked the greatest probability of sarcopenia to the oldest age group [18, 25], with individuals aged 70 years and above reportedly experiencing a muscle mass decline of up to 15% per decade [26]. Furthermore, individuals aged 80 years or older were six times more likely to be at risk of sarcopenia than those in the 60–80 years age group [23].
An inverse association was observed between being overweight or obese, as measured by BMI, and the risk of sarcopenia [27,28,29]. Nevertheless, this inverse association could be influenced by muscle mass, which is positively correlated with BMI [27]. Upon adjusting for muscle quantity, higher BMI was associated with an elevated risk of sarcopenia [27].
Our study also revealed a significant association between higher HDL levels and low skeletal muscle mass. While the mechanistic underpinnings of this relationship remain unclear, it is plausible that alterations in lipid metabolism may influence muscle protein turnover and function. Further investigation is warranted to elucidate the complex interplay between lipid profiles and skeletal muscle health.
Similarly, earlier studies have indicated that lifestyle-related factors contributing to low muscle mass include physical inactivity [6] and smoking [7]. Insufficient physical activity leads to muscle decline due to the disuse of atrophy [5]. Smoking can hinder muscle protein synthesis [5]. As both factors are potentially modifiable, the risk can be mitigated by increasing physical activity and smoking cessation.
To the best of our knowledge, this study is the first to assess the prevalence of low ASMI, using AWGS criteria, and its associated factors among older adults residing in the community in Iran. Our study findings indicated a high prevalence of low ASMI in this cohort. Furthermore, we identified factors associated with an elevated risk of a low ASMI. These findings can be utilized to develop public health programs aimed at assisting older adults in restoring and maintaining physical function, thereby fostering independent living within the community. The limitations of our study include its cross-sectional design, which restricts our ability to establish causal inferences and to examine temporal relationships.
Conclusion
In conclusion, our study provides valuable insights into the prevalence, determinants, and correlates of low skeletal muscle mass among older adults. Addressing modifiable risk factors and promoting healthy lifestyle behaviors are pivotal steps in preventing and managing sarcopenia, thereby fostering optimal aging trajectories and reducing the burden of musculoskeletal disorders in aging populations. Further longitudinal research is warranted to elucidate the causal pathways linking demographic, clinical, and lifestyle factors to skeletal muscle mass and function, thereby informing targeted interventions and personalized approaches to enhance muscle health across the lifespan.
Data availability
The data that support the findings of this study are available on request from the corresponding author, FH.
References
Loenneke JP, Pujol TJ. Sarcopenia: an emphasis on occlusion training and dietary protein. Hippokratia. 2011;15:132–7.
Hashemi R, Heshmat R, Motlagh AD, Payab M, Esmaillzadeh A, Baigy F, Pasalar P, Siassi F. Sarcopenia and its determinants among Iranian elderly (SARIR): study protocol. J Diabetes Metab Disord. 2012;11(1):23. https://doi.org/10.1186/2251-6581-11-23. PMID: 23497567; PMCID: PMC3598166.
Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7:405–10.
Janssen I, Heymsfeld SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 year. J Appl Physiol. 2000;89:81–8. https://doi.org/10.1152/jappl.2000.89.1.81
Tey SL, Huynh DTT, Berde Y, Baggs G, How CH, Low YL, Cheong M, Chow WL, Tan NC, Chew STH. Prevalence of low muscle mass and associated factors in community-dwelling older adults in Singapore. Sci Rep. 2021;11(1):23071. https://doi.org/10.1038/s41598-021-02274-3. PMID: 34845250; PMCID: PMC8630119.
Kwon Y-J, Kim HS, Jung D-H, Kim J-K. Cluster analysis of nutritional factors associated with low muscle mass index in middle-aged and older adults. Clin Nutr. 2020;39:3369–76. https://doi.org/10.1016/j.clnu.2020.02.024
Bai H-J, et al. Age-related decline in skeletal muscle mass and function among elderly men and women in Shanghai, China: a cross sectional study. Asia Pac J Clin Nutr. 2016;25:326–32. https://doi.org/10.6133/apjcn.2016.25.2.14
Prado CM, et al. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med. 2018;50:675–93. https://doi.org/10.1080/07853890.2018.1511918
Kim M, Kim J, Won CW. Association between involuntary weight loss with low muscle mass and health-related quality of life in community-dwelling older adults: nationwide surveys (KNHANES 2008–2011). Exp Gerontol. 2018;106:39–45. https://doi.org/10.1016/j.exger.2018.02.027
Norman K, Otten L. Financial impact of Sarcopenia or low muscle mass—a short review. Clin Nutr. 2019;38:1489–95. https://doi.org/10.1016/j.clnu.2018.09.026
Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II. Trials. 2009;10:5. https://doi.org/10.1186/1745-6215-10-5
Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ). —Short and Long Forms. https://www.researchgate.net/file.PostFileLoader.html?id=5641f4c36143250eac8 b45b7&assetKey = AS%3A29423741606593%401447163075131. Published November 2005.
Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q, Hu X, Tao R, Li L, Qian F, Yu L. Type 2 diabetes mellitus is associated with increased risks of Sarcopenia and Pre-sarcopenia in Chinese elderly. Sci Rep. 2016;6:38937. https://doi.org/10.1038/srep38937. PMID: 27958337; PMCID: PMC5153616.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. PMID: 4337382.
Hwang YC, Cho IJ, Jeong IK, Ahn KJ, Chung HY. Differential association between Sarcopenia and metabolic phenotype in Korean young and older adults with and without obesity. Obes (Silver Spring). 2017;25(1):244–51. https://doi.org/10.1002/oby.21694. Epub 2016 Nov 22. PMID: 27874274.
Hashemi R, Shafiee G, Motlagh AD, Pasalar P, Esmailzadeh A, Siassi F, Larijani B, Heshmat R. Sarcopenia and its associated factors in Iranian older individuals: results of SARIR study. Arch Gerontol Geriatr 2016 Sep-Oct;66:18–22. doi: 10.1016/j.archger.2016.04.016. Epub 2016 Apr 28. PMID: 27176487.
Pang BWJ, et al. Prevalence and associated factors of Sarcopenia in Singaporean adults - Te Yishun Study. J Am Med Dir Assoc. 2021;22(885). https://doi.org/10.1016/j.jamda.2020.05.029. e881-885.e810.
Tey SL, et al. Factors associated with muscle mass in community-dwelling older people in Singapore: fndings from the SHIELD study. PLoS ONE. 2019;14:e0223222. https://doi.org/10.1371/journal.pone.0223222
Xu H-Q, et al. Sarcopenia-related features and factors associated with low muscle mass, weak muscle strength, and reduced function in Chinese rural residents: a cross-sectional study. Arch Osteoporos. 2019;14:2. https://doi.org/10.1007/s11657-018-0545-2
Zhang Y, et al. Prevalence and risk factors governing the loss of muscle function in elderly sarcopenia patients: a longitudinal study in China with 4 years of follow-up. J Nutr Health Aging. 2020;24:518–24. https://doi.org/10.1007/s12603-020-1361-0
Yamada M, Nishiguchi S, Fukutani N, Tanigawa T, Yukutake T, Kayama H, et al. Prevalence of Sarcopenia in community-dwelling Japanese older adults. J Am Med Dir Assoc. 2013;14(12):911–5.
Sato PHR, Ferreira AA, Rosado EL. Te prevalence and risk factors for Sarcopenia in older adults and long-living older adults. Arch Gerontol Geriatr. 2020;89:104089. https://doi.org/10.1016/j.archger.2020.104089
Terakomen V, Petchlorlian A, Lakananurak N. Prevalence and risk factors of primary Sarcopenia in community-dwelling outpatient elderly: a cross-sectional study. Sci Rep. 2020;10:19551. https://doi.org/10.1038/s41598-020-75250-y
Martone AM, et al. Treating Sarcopenia in older and oldest old. Curr Pharm Des. 2015;21:1715–22. https://doi.org/10.2174/13816
Grimby G, Saltin B. Te ageing muscle. Clin Physiol. 1983;3:209–18. https://doi.org/10.1111/j.1475-097x.1983.tb00704.x
Liu C, Wong PY, Chung YL, Chow SK, Cheung WH, Law SW, et al. Deciphering the obesity paradox in the elderly: a systematic review and meta-analysis of sarcopenic obesity. Obes Rev. 2022;24(2):e13534.
Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F et al. Associated factors of Sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients 2021;13.
Zhang JZ, Shi W, Zou M, Zeng QS, Feng Y, Luo ZY, et al. Diagnosis, prevalence, and outcomes of Sarcopenia in kidney transplantation recipients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;14(1):17–29.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
F.H., B.A., and M.V. conceived and designed the study. F.H., B.A., M.M., and A.M. extracted data. B.A., AH.RA, A.V., F.H. and M.M. contributed to the interpretation of the results and wrote the first draft of the manuscript. F.H., M.V., F.A., and F.H. critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
None.
Ethics approval and consent to participate
Approval for this study was obtained from the Ethics Committee of the Research Institute for Endocrine Sciences (RIES) at the Shahid Beheshti University of Medical Sciences (code: IR.SBMU.ENDOCRINE.REC.1401.083). All participants provided written informed consent prior to participating in the study.
Consent for publication
All authors have given consent for the paper to be published by the corresponding author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abiri, B., Ahmadi, A.R., Nikoohemmat, M. et al. Prevalence and some related factors of low muscle mass in older adults: findings from the Tehran lipid and glucose study. J Diabetes Metab Disord (2024). https://doi.org/10.1007/s40200-024-01457-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40200-024-01457-9