Abstract
Purpose
Despite adaptive thermogenesis (AT) being studied as a barrier to weight loss (WL), few studies assessed AT in the resting energy expenditure (REE) compartment after WL maintenance. The aim of this study was twofold: (1) to understand if AT occurs after a moderate WL and if AT persists after a period of WL maintenance; and (2) if AT is associated with changes in body composition, hormones and energy intake (EI).
Methods
Ninety-four participants [mean (SD); BMI, 31.1(4.3)kg/m2; 43.0(9.4)y; 34% female] were randomized to intervention (IG, n = 49) or control groups (CG, n = 45). Subjects underwent a 1-year lifestyle intervention, divided in 4 months of an active WL followed by 8 months of WL maintenance. Fat mass (FM) and fat-free mass (FFM) were measured by dual-energy X-ray absorptiometry and REE by indirect calorimetry. Predicted REE (pREE) was estimated through a model using FM, FFM. EI was measured by the “intake-balance” method.
Results
For the IG, the weight and FM losses were − 4.8 (4.9) and − 11.3 (10.8)%, respectively (p < 0.001). A time–group interaction was found between groups for AT. After WL, the IG showed an AT of -85(29) kcal.d−1 (p < 0.001), and remained significant after 1 year [AT = − 72(31)kcal.d−1, p = 0.031]. Participants with higher degrees of restriction were those with an increased energy conservation (R = − 0.325, p = 0.036 and R = − 0.308, p = 0.047, respectively). No associations were found between diet adherence and AT. Following a sub-analysis in the IG, the group with a higher energy conservation showed a lower WL and fat loss and a higher initial EI.
Conclusion
AT in REE occurred after a moderate WL and remained significant after WL maintenance. More studies are needed to better clarify the mechanisms underlying the large variability observed in AT and providing an accurate methodological approach to avoid overstatements. Future studies on AT should consider not only changes in FM and FFM but also the FFM composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite lifestyle interventions aimed weight loss (WL) being abundant in the literature, there is a lack of information regarding one’s ability to maintain their new and lower weight. Indeed, most people struggle with maintaining a weight-reduced state, often regaining their lost weight over time [1, 2].
During WL, changes in energy expenditure (EE) components are expected to occur as a consequence of changes in FM and FFM [3], such as decreases in resting and non-resting energy expenditure [4, 5]. However, it has been shown that some changes in components of EE occur to a greater extent than would be predicted based on changes in body composition stores [6]. This mass-independent decrease in any of the EE components, such as resting EE (REE), physical activity EE (PAEE), and thermic effect of food (TEF), beyond what we predicted from changes in FM and FFM is defined as adaptive thermogenesis (AT) [7, 8].
While AT after WL has been widely studied and discussed [6], the lack of concordance among methodologies employed to assess AT and/or how REE is predicted was recently highlighted [9]. AT has been studied as a possible barrier especially in WL maintenance, contributing to weight regain [10,11,12]. Moreover, its influence on long-term weight management has been recently questioned, as some authors found that this “phenomenon” seems to be attenuated or even disappeared after a period of weight stabilization [13,14,15,16,17]. Regarding moderate WL, while some studies suggest that a disproportionate decrease in REE appears during WL and may persist during the weight-reduced state [10, 18], others have found no evidence of AT in any of the EE components [19, 20]. In addition, the limited number of studies available assessing AT during a WL maintenance typically employs weak-to-moderate designs, being mostly observational studies or controlled trials without a control group [6].
Therefore, the aims of this study were: (1) to understand if AT remains significant during a WL maintenance period, i.e., under a neutral energy balance (EB), comparing with a control group; and (2) if the degree of energy conservation is related with changes in body composition, weight-related hormones, or the percentage of energy restriction.
Methodology
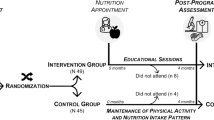
This is a secondary analysis of the Champ4life project [21], a 1-year lifestyle intervention that consisted of a 4-month WL intervention and an 8-month WL maintenance period. All participants were former elite athletes, aged 18–65 years old, inactive (< 20 min/day of vigorous physical activity intensity for at least 3 days per week or < 30 min/day of moderate intensity physical activity for at least 5 days per week [22]) and with a body mass index (BMI) \(\ge\) 24.9 kg/m2. They also needed to be ready to modify their diet and physical activity habits and be available to attend the educational sessions at the study site. A detailed description of the protocol study (including inclusion and exclusion criteria) and its main results and are presented elsewhere [21, 23].
A total of 94 participants were included in this study (clinicaltrials.gov ID: NCT03031951) and were randomly assigned to one of the two groups: intervention (IG) or control group (CG). Randomization was performed according to an automated computer-generated randomization scheme managed by the principal investigator (A.M.S.). The study was single-blinded, as the research team who assessed all outcomes were blinded to participant group assignment. Also, all outcome data were kept blinded until the final data entry for the entire study was completed.
The study was approved by the Ethics Committee of the Faculty of Human Kinetics, University of Lisbon (Lisbon, Portugal, CEFMH Approval Number: 16/2016) and was conducted in accordance with the Declaration of Helsinki for human studies from the World Medical Association [24]. Prior to participants’ recruitment, the trial was registered at www.clinicaltrials.gov (clinicaltrials.gov ID: NCT03031951). Measures of body weight and composition, REE, and EB-related blood biomarkers were measured at baseline, post WL (4 months) and post WL maintenance (1 year).
The Champ4life intervention
The Champ4life was a 1-year intervention SDT-based [25], divided in 4 months of active WL and 8 months of follow-up (WL maintenance). For the active WL, participants from IG had a nutritional appointment with a registered dietitian to discuss their eating patterns and to induce a moderate caloric deficit (~ 300–500 kcal.d-1). Additionally, the IG underwent 12 educational sessions (1 per week) aimed to promote behavioral changes possible to be integrated in participants’ daily lives and contexts, including educational content and practical application in the areas of PA and exercise, diet and eating behavior as well as behavior modification [21]. Also, participants had their weight tracked weekly. After the active WL phase, participants underwent an 8-month weight maintenance period, aimed to understand if participants were able to maintain the reduced weight state at a long term. During this phase, the IG underwent nutritional appointments to adjust their caloric intake to create a neutral EB (maintenance calories). When needed, participants were able to contact with the project team throughout the follow-up period to clarify any doubts or to readjust their caloric intake. Participants from the CG were placed in a waiting list. After completing the 3 assessments (baseline, 4 months post-intervention, and after the follow-up period—1 year), they were provided with the Champ4Life intervention. A detailed description of the Champ4life program is provided elsewhere [21].
Body composition
Participants had their weight and height measured wearing a bathing suit and without shoes to the nearest 0.01 kg and 0.1 cm, respectively, with a scale and stadiometer (Seca, Hamburg, Germany). Body mass index was calculated using the formula [weight(kg)/height2(m2)]. Dual-energy X-ray absorptiometry (DXA) (Hologic Explorer-W, Waltham, USA) was used to assess total FM (kg and %), FFM (kg) and sub-total lean soft tissue (LST) (kg) [26]. FM and LST were also presented for sub-regions, namely the trunk and appendicular (arms + legs) regions. When a participant did not fit within the active scan area (given the superior width dimensions), and to avoid overlapping of body parts, a partial scan was performed and the left arm was left outside the scan area [27]. Therefore, in 6 participants, this technique was considered for the body composition analysis.
Measured resting energy expenditure (mREE)
Assessment of REE was performed in the morning when fasted (8.00–10.00 a.m.). All measurements will be performed in the same room at an environmental temperature and humidity of approximately 22 ℃ and 40–50%, respectively. The MedGraphics CPX Ultima indirect calorimeter (MedGraphics Corporation, Breezeex Software, Italy) was used to measure breath-by-breath oxygen consumption (V̇O2) and carbon dioxide production (V̇CO2) using a face mask, for 30 min. Before the measurement, participants lay in a supine position for 15 min covered with a blanket. The first and the last 5 min of data collection were discarded and the mean V̇O2 and V̇CO2 of 5 min steady states was used in Weir equation [28] and the period with the lowest REE was considered for data analysis. Steady state was defined as a 5 min period with ≤ 10% CV for V̇O2 and V̇CO2 [29]. Based on test–re-test of 7 participants, the technical error of measurement (TEM) for REE was 56.4 kcal. A more detailed description of the procedures is presented in the protocol paper [21].
Predicted resting energy expenditure (pREE)
To predict REE (pREE), a predictive equation using measured body composition values for FM and FFM for all participants as the independent predictors was created. The following prediction model was created:
The equation was used to predict pREE at baseline and after 4 (WL) and 12 months (WL maintenance) using the body composition values measured at each respective time point.
Physical activity energy expenditure (PAEE) and total daily energy expenditure (EE)
PAEE was objectively measured using a tri-axial accelerometer (ActiGraph GT3X + , Pensacola, FL) as described elsewhere [23]. EE was estimated as the sum of REE, PAEE and thermic effect of food (TEF):
The TEF component was assumed as 10% of TDEE [30].
Adaptive thermogenesis (AT)
AT was assessed after 4-month WL and 8-month follow-up based on the difference between predicted and measured REE, after accounting for baseline differences in these parameters:
After 4 months of WL:
After 8 months of follow-up:
Negative values indicate a lower-than-expected decrease in REE considering the changes in body composition (measured REE lower than predicted REE) and positive values represent a change in REE equal to or greater than the predicted REE (measured REE higher than predicted REE) [31].
Energy balance (EB)
To assure the EB state for each time point, the EB equation was applied to quantify the average rate of changed body energy store or lost in kilocalories per day.
The EB equation is denoted as follows:
A negative EB is considered when the EE surpasses the EI, while EB is positive when EI is larger than EE. A neutral EB represents the average rate of energy deficit or surplus expressed in kilocalories per day. EB can be calculated from the changed body energy stores from the beginning to the end of the WL intervention. Hence, using the established energy densities for FM [32] and FFM [33], the following equation was applied:
where ∆FM and ∆FFM represent the change in grams of FM and FFM from the beginning to end of the intervention and ∆t is the time length of the intervention in days.
Energy intake (EI)
EI was estimated by the “intake-balance method” [34]. This method has been previously validated [35, 36] and has been shown to provide valid estimation of EI through changes in body energy stores such FM and FFM (please check the EB section), together with EE. The following equation was used:
where EE represents the total daily energy expenditure measured by accelerometry and the EB (calculated through changes in FM and FFM). For the baseline EI, as participants were weight-stable during at least 3 months (inclusion criteria), we considered an EB = 0, and therefore EI = EE.
This equation was used not only to determine EI at each time point, but also to calculate the degree of energy restriction during the WL phase.
Adherence to the diet
In the Champ4life project, rather than having a fixed diet plan, participants were asked to change some of their eating patterns to induce a caloric restriction between 300 and 500 kcal.d−1 (previously calculated by a registered dietitian). Therefore, the prescribed caloric restriction varied among participants and was calculated as:
where C represents the number of calories that were taken out from the initial EI (between 300 and 500 kcal).
Adherence was assessed through the following equation proposed by Racette et al. [36]:
Blood samples
Blood samples were collected according to the standard procedures by venipuncture from the antecubital vein into ethylene-diamine-tetra-acetic acid tubes (EDTA) and dry tubes with accelerated for serum separation. Whole blood was used directly, or sample treatment was performed, including centrifugation at 500g at 4 C for 15 min. Serum was frozen at – 80 ℃ for posterior analyses.
The thyroid panel [including Thyroid-Stimulating Hormone (TSH) free tri-iodothyronine (FT3) and free thyroxine (FT4)] and insulin were assessed by immuno-quimio-luminescence in a different automated analyzer (Cobas e411, Roche Diagnostics, Portugal). Serum levels of leptin were assessed by ELISA (enzyme-linked immunosorbent assay) using commercial kits (DIAsource ImmunoAssays, Belgium).
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics version 25.0 (IBM, Chicago, Illinois, USA). The Kolmogorov–Smirnov test was performed to examine whether variables followed a normal distribution. Baseline differences between the intervention and control groups were assessed by independent two sample t test. Changes in body composition and EB-related hormones were previously assessed through linear mixed models. To assess the effect of time, group and time–group interaction in AT, linear mixed models using group (intervention vs control group) and time (baseline vs 4 months and vs 12 months) as fixed effects were performed. The covariance matrix for repeated measures within subjects over time was modeled as compound symmetry.
The one-sample t test was performed to test the significance for AT (if it is different from zero). Pearson’s correlation was performed to examine the association between AT and body composition, blood samples and adherence to the diet. The analysis was intention-to-treat, as none of the participants were excluded whether they completed or not the 1-year intervention and missing data were treated through maximum likelihood (by linear mixed models). The typical error (TE) for AT was calculated from the SD of AT for the control group divided by \(\sqrt{2}\), representing the technical error of measurement as well as the within-subject variability [37]. Statistical significance was set at a two-sided p < 0.05.
Results
Ninety-four participants [mean (SD): BMI = 31.1 (4.3)kg/m2, age = 43.0 (9.4)y, 34% females] were initially included in this study and randomized to either intervention [IG, n = 49, mean (SD): BMI = 31.7 (3.9)kg/m2, age = 42.4 (7.3)y, 35% females] or control groups [CG, n = 45, mean (SD): BMI = 30.5 (4.7)kg/m2, age = 43.6 (11.3)y, 33% females]. A detailed description of the results of the Champ4life intervention is presented elsewhere [23]. Values of body composition, blood biomarkers, and changes between time points are presented in (Table 1).
Eleven participants (IG: 8; CG: 4) were lost to follow-up after 4 months and a further fourteen during the 8-month follow-up (IG: 6, CG: 8). The drop-out rate was ~ 27.7% and was similar between groups (28.6% and 26.7% for the IG and CG, respectively).
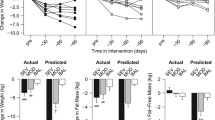
After 4 months, the IG showed significant decreases for weight, BMI, and FM (kg and %) (p < 0.001). These alterations remained significant at the end of the intervention (p < 0.001). The IG also showed a larger trunk and appendicular FM loss when compared with the CG at 4 months (trunk FM: ED = − 2.1, [95% CI: − 2.9 to − 1.3], p < 0.001; appendicular FM: ED = − 1.5, [95% CI: − 2.0 to − 0.9], p < 0.001) and 12 months (trunk FM: ED = − 2.4, [95% CI: − 3.2 to − 1.5], p < 0.001; appendicular FM: ED = − 1.6, [95% CI: − 2.2 to − 1.0], p < 0.001). For the blood biomarkers, insulin decreased for the IG after the 1-year intervention (Estimated difference (ED) = − 4.9, [95% CI: − 8.0 to − 1.8], p = 0.006) when compared with the CG. Leptin levels decreased more in the IG than in the CG at 4 months (ED = − 3.8, [95% CI: − 5.9 to − 1.7], p < 0.001) and 12-month (ED = − 4.2, [95% CI: − 6.5 to − 2.0], p < 0.001) time points. No differences were found for the thyroid panel (TSH, T3 and T4).
Considering within group differences, the IG showed decreases in body composition variables (weight, BMI, FM, FFM, trunk FM and appendicular FM), insulin and leptin after 4- and 12-month time points (compared with baseline, p < 0.05). No differences were found between after 4 months and after 12 months for the IG. The CG increased insulin from 4 months’ time point to after 12 months (p < 0.001).
After the intervention (4 months), the IG underwent a negative EB (EB = − 269.7 ± 289.1 kcal.d−1, p < 0.001), while the CG was at a neutral EB (EB = 14.0 ± 129.4 kcal.d−1, p = 0.489). At the end of the program, both groups were at a neutral EB (15.6 ± 72.3 kcal.d−1, p = 0.204 for IG; 21.5 ± 98.7 kcal.d−1, p = 0.219 for CG).
The values for REE and AT are presented in (Table 2).
A group–time interaction was found for mREE, pREE and AT estimated using both equations (p < 0.05). Participants from the IG decreased mREE and pREE estimated from both equations after 4 months and 1 year, when compared with the baseline values (within group, p < 0.05). After 1 year of intervention, the CG increased pREE using both equations (within group, p < 0.05).
A time-by-group interaction was found for AT assessment (p = 0.012). After 4 months, AT occurred for the IG (statistically different from zero, p = 0.002) and remained significant after 1 year (p = 0.031). On the other hand, the CG showed an energy dissipation (with a positive value for AT) after 1 year (p = 0.047).
No correlations (adjusted for group) were found between AT and WL (kg and %), \(\Delta\) trunk (FM and LST), \(\Delta\) appendicular (FM and LST) and blood biomarkers, except for AT and \(\Delta\) trunk FM (%) at the end of the intervention (12 months) (R = 0.294, p = 0.031).
Changes in body composition stores (FM and FFM) and in REE (measured and predicted) are displayed in (Fig. 1).
Comparison between adherence and AT
Diet adherence was ~ 89% (95%IC: 40 to 137%), with a calculated CR of 13.6% (95%IC: 6.4 to 20.9%) compared with 17.5% (95%IC: 16.3 to 18.7%) prescribed. The calculated CR was negatively associated with AT (kcal/d and %), where participants with higher degrees of restriction were those who showed an increased energy conservation (R = − 0.325, p = 0.036 and R = − 0.308, p = 0.047, respectively). No associations were found between adherence (%) and AT.
AT variability: differences between thrifty and spendthrift individuals
A sub-analysis comparing changes in body composition and blood samples dividing the IG in those who showed an energy conservation (negative value for AT, thrifty) with those who dissipate energy (positive value for AT, spendthrift) is presented in (Table 3).
The TE for AT was 103 kcal/d and individuals with an energy conservation < − 103 kcal/d were considered “thrifty” and those with positive values for AT as “spendthrift”.
Differences were found between groups for weight, BMI, FM (kg and %), trunk FM and appendicular FM (p < 0.05). The group with a higher energy conservation showed a lower WL and fat loss. These thrifty individuals showed a lower initial EI [mean difference = − 396 (174) kcal/d, 95%IC (− 754, − 39), p = 0.031] when compared to the spendthrift group. No differences were found between groups for the adherence (%) nor the measured CR (%) (p > 0.05).
Discussion
The main finding of this study was the presence of AT in REE after a moderate WL (~ 5%), which remained significant after an 8-month WL maintenance period in which body weight was maintained. These results indicated that energy is conserved via adaptive mechanisms both during active WL and in the weight-reduced state.
The existence of AT and its clinical relevance has been widely debated in the literature [6]. However, the findings are not consistent, as some studies suggest that AT exists and works as a barrier to WL and its maintenance [38], while others indicate that AT is not a predictor of weight regain [17, 39].
Recently, Martins et al. [39] found an AT of ~ − 90 kcal.d−1 after a 9-week WL period, which halved to ~ − 38 kcal.d−1 after a 4-week period of weight stabilization. It should also be noted that the used approach to calculate AT differed between studies, as AT was assessed by subtracting the pREE to the mREE, without taking into consideration the baseline residuals (baseline measured REE minus baseline predicted REE). However, despite decreasing its magnitude, AT was still significant even under a period of an “assumed” neutral EB. Nevertheless, despite participants being weight-stable during this period, the authors did not assess the “real” EB at each time point and, consequently, a true neutral EB cannot be assured. According to the authors, 4 weeks of weight stabilization may not be sufficient to return to a neutral EB, especially if participants underwent a very-low-calorie diet (~ 800 kcal.d−1), which may explain why AT remained significant after this phase [17]. In our study, participants underwent a moderate caloric restriction (300 to 500 kcal/d of CR) and a longer WL maintenance period (~ 32 weeks). Nevertheless, AT remained significant at the end of the intervention. Moreover, although a neutral EB was calculated at the end of the program, it is important to consider that this calculation is integrated over several months, which may raise some concerns regarding the weight stabilization (i.e., if participants were able to maintain the WL steadily or suffered significant weight fluctuations marked by periods of WL followed by periods of weight regain). Though body weight was not tracked by our team, the last educational session lectured to the participants from the IG aimed at discussing strategies to foster weight loss maintenance and a healthy lifestyle [21], such as the regular self-weighting [40]. Moreover, participants were allowed to contact our team members if they were struggling to maintain their reduced weight state, to clarify any rising doubts, ask for advice and, if necessary, to readjust their maintenance diet. Lastly, before the measurements, participants were asked to provide some details regarding their WL maintenance period. Therefore, despite we did not track weight between month 4 and month 12, mean weight changes were below 3% of the weight loss observed in the IG [23].
Apart from the aforementioned recent manuscripts, few studies have assessed AT after WL and after a period of WL maintenance [10, 41,42,43]. Participants of The Biggest Loser competition [10], after a massive WL (~ − 58 kg), showed an AT of ~ − 275 kcal.d−1. Additionally, after 6 years of follow-up, AT’s magnitude increased to ~ − 500 kcal.d−1, with a huge variability among participants in terms of the regained weight and AT’s magnitude. Nevertheless, as participants are considered a very specific group (TV show participants) and the sample size was small (n = 14), the results cannot be generalized to our study. Consistent with our data, Karl et al. [41] showed similar AT after 12 weeks of a diet intervention (~ − 54 kcal.d−1). However, after 1 year of ad libitum diet (follow-up periods), some participants regained part of their weight and AT was attenuated [41]. In our study, participants were able to maintain the WL during 8 months of follow-up (with a neutral EB) and, thus, this may be the reason why AT remained significant.
In fact, the existence of a relationship between the degree of AT and the magnitude of weight loss has been postulated by some authors [11, 44]. However, some studies have reported contradictory results [17, 45]. Also, if a relationship between WL and AT exists, it would be expected that studies with higher WL (for example, bariatric surgery) would lead to a greater energy conservation. However, in our recent systematic review aimed to understand if AT occurs after WL (induced by different types of interventions) [6], considering the surgical interventions, only Tam et al. reported higher values for AT (> 300 kcal/d) [46]. Interestingly, despite their higher amount of WL (~ 20%), two studies did not report AT [47, 48].
Our study included the analysis of weight-related hormones. No differences were found for thyroid hormones but participants from the IG showed a decrease in leptin throughout time. We could state that the lack of an association between AT and changes in leptin or thyroid hormones might be due to the moderate amount of WL (~ 5%); however, other authors also did not find any associations between these WL-related hormones and the degree of AT. Muller et al. [49], whose study included participants that presented a WL of ~ 8% after a lifestyle intervention, did not find any association between AT and hormones. Additionally, participants from the Johannsen et al. study [11] who showed a WL of ~ 10 and ~ 38% after 6 and 30 weeks, a significant larger WL when compared with our findings, did not observe associations between AT and changes in the aforementioned hormones. Moreover, Bettini et al. study [50] who studied participants that underwent a sleeve gastrectomy and lost ~ 30% of BW, did not find a relationship between AT and weight-related hormones. Therefore, our findings extend the results observed from the aforementioned studies [11, 49, 50].
Although no correlations were found between AT and WL, a sub-analysis comparing those who conserved energy versus those who dissipated energy (IG only) showed that the thrifty phenotype presented a lower WL and FM loss compared to the spendthrift phenotype (p < 0.05). As no differences were found regarding the %CR nor the %adherence, we may hypothesize that those who showed a higher energy conservation may struggle to remain in a weight-reduced state. Nevertheless, the role of metabolic adaptations in other EE components and behavioral compensations (decreases in physical activity) were not analyzed and may have also influenced the magnitude of WL. Therefore, more studies are needed to better address the observed large inter-individual variability in AT, including the use of accurate methodologies for assessing metabolic and behavioral compensations during WL and WL maintenance.
Although the reported AT values in the present paper were statistically significant, it is important to consider their clinical importance during WL and WL maintenance. Similar to Martins et al., the magnitude of AT values reported was small. Also, the reliability of the used instrument to assess REE must be taken into account. In our laboratory, the coefficient of variation (CV) and the technical error of measurement (TEM) for REE were 4% and ~ 60 kcal/day, respectively [51], where the TEM was similar to our AT values at the end of the intervention (~ 60–70 kcal/d). Therefore, the precision of the AT assessment may be affected by the reliability of the used instrument to assess REE (indirect calorimetry).
Though AT may play a role in WL and its maintenance, these findings suggest that AT is unlikely to be a major barrier for WL and its maintenance, especially due to its limited magnitude [17]. In fact, a recent systematic review showed that AT seems to be attenuated or non-existent after a period of weight stabilization/neutral EB [6]. Moreover, the role of behavioral compensations as possible barriers to WL is unquestionably more impactful than AT, whereas behavior is 100% of EI and 20–60% of EE [52]. During a lifestyle intervention, new healthy habits aimed to reduce weight are presented and expected to be adopted. However, only a small percentage of people adopt and maintain these new behaviors that promote a reduced body weight long-term [53] and thus, long-term success rates for WL maintenance are low, as participants often report weight regain [5]. In fact, a decrease in physical activity after a period of caloric restriction has already been showed by Redman et al. [43]. In our main paper regarding Champ4life’s results, during the active WL phase (4 months), participants showed a slight tendency to decrease their sedentary behavior and to increase ~ 10 min/day of moderate-to-vigorous physical activity (MVPA), although this was not statistically significant (data not shown in this paper). However, at the end of the program (1 year), participants from the IG increased their sedentary time (compared with baseline) and returned to the baseline values of MVPA. Therefore, the lack of a successful WL and its maintenance may be mostly due to behavioral issues, such as increasing food intake and/or decreasing physical activity. Nevertheless, metabolic adaptations can also contribute to the difficulty in maintaining the reduced weight by increasing the “energy gap” [53]. Under a negative EB, this concept is characterized by a discordance between appetite (by increasing hunger) and energy requirements (by decreasing EE), resulting in a desire for more calories than are actually required [53]. This response, together with the behavioral compensations, may force an individual to re-establish a positive EB and to retake the body weight set point [53].
It should be mentioned that comparisons among studies should be interpreted carefully due to the discrepancy among methodologies to assess AT, also dependent on how REE and body composition are assessed. A recent systematic review showed that studies with stronger methodologies are those who observed lower or non-significant values for AT [6]. Moreover, when participants are measured during a neutral EB, the degree of AT is reduced or even non-significant [17, 49, 54]. Another methodological issue that should be addressed is the precision of the measurements involved in the calculation of AT, such as the REE, as these errors must be below changes between two longitudinal measurements to represent a “true” difference. Indeed, the technical error of measurement of our REE method is 56 kcal/day, a value that is way below the decrease in REE observed in the IG [estimated changes (SE)], that is – 115 (28) kcal/d and 117 (31) kcal/d after 4 and 12 months, respectively. Thus, we expect that changes in REE were “true” differences that could be biologically explained rather than artifacts resulting from the measurement error.
Additionally, the inclusion of a control group is also important to understand if AT occurs as a result of the WL intervention rather than other external factors. Moreover, the calculation of the typical error for AT, that takes into account the standard deviation for control group (where the outcomes of interest are not expected to change), will allow us to better clarify which AT values are likely to be meaningful in practice [55].
Taking into account the aforementioned methodological issues, there is a need to standardize the calculation of AT and to include precise and accurate methods for body composition and REE determination to fully understand whether a meaningful energy conservation in the REE occurs during and/or after WL when designing future studies [9]. Lastly, measurements of EE should be conducted in a neutral EB, not only to assure a similar condition to the baseline but also to eliminate the potential influence of an acute state of energy deficit.
One of the major strengths of this study was that it was conducted as a randomized controlled trial, with a CG who did not receive the lifestyle intervention. Also, we collected data not only after a period of WL (negative EB) but also after 8 months of WL maintenance in which (neutral EB). However, some limitations need to be addressed. First, our findings need to be interpreted carefully, as the Champ4life was a lifestyle intervention targeting former elite athletes with overweight/obesity and inactive. While a non-athletic population with obesity may have been sedentary all their life’s, when it comes to athletes, they generally experienced a weight gain and a transition to a sedentary state throughout adulthood. Although former athletes tend to adopt healthier lifestyles after their retirement, if that is not carried throughout their lives, they do not seem to have health-related benefits when compared to a non-athletic population [56, 57]. In fact, a study that aimed to analyze 25-year trends in weight gain showed that after an athletic retirement the weight gain reported was of a similar magnitude to that observed in studies with non-athletic populations [58]. Also, the same study showed that former football athletes appear to have similar risk factors for developing cardiovascular disease when compared to the general U.S. population [58]. It may be expected that athletes gain weight with a different body composition, characterized by a higher percentage of lean muscle mass, in comparison to that seen in other cohorts [56, 59]. However, as we used not only BMI but also %FM to characterize this sample, we believe that the results are not strictly useful for this specific population, but also for non-athletic populations that were highly active in their youth and with similar levels of %FM. Nevertheless, most of the studies have been conducted in non-athletes. It is also important to mention that our intervention was not designed to prescribe a standardized diet or physical activity to each participant which may have contributed to the large WL variability and, consequently AT. This large variability within subjects is widely reported in studies that determined changes in body composition stores (FM and FFM) [6]. Also, tracking changes in body composition by DXA does not assess the changes in FFM composition (i.e., molecular and anatomical composition) [60]. Therefore, possible changes in the FFM contribution to REE were not taken into account. Moreover, it is known that a particular limitation of the DXA equipment is the reduced width of the active scanning area, which compromises the measurement in individuals who surpass the scan width. In this study, 6 participants had their body composition measured with a technique called “Reflection scan”, where their left arm was placed outside the scan window and data from the right arm were “reflected” to the left upper limb, validated elsewhere [27]. Though a small impact was observed in whole-body bone measurement using this approach, no differences were found in assessing soft tissues [27]. Nevertheless, this technique affects the weight measured by DXA, as the left upper limb is not included. In the scan area and therefore it is not being correctly weighted which may have contributed to so a certain degree of discrepancy between weight measured by DXA vs scale. Regardless, a Pearson’s correlation was performed between weight measured by DXA vs scale and an almost perfect association was found between measurements (R = 0.999, p < 0.001). It is also important to address that we used method to assess EB did not account for the daily variations related to food intake. Lastly, AT was just calculated for the REE compartment. It is known that AT may occur in all EE components and it might be of a larger magnitude at the level of non-resting EE [4]. Therefore, it would be interesting to calculate AT in all EE components to better understand its magnitude.
To conclude, AT occurred after 4 months of a moderate WL and persisted during the 8-month WL maintenance. Nevertheless, researchers should be aware of the lack of standardization among the techniques and of a huge variability within-studies. Future studies on AT should consider not only changes in FM and FFM but also the FFM composition. Results from studies examining AT should be interpreted carefully according to the used methodology, avoiding overstatements and academic clickbait about its existence and/or influence of AT in WL and its maintenance.
References
Aronne LJ, Hall KD, Jakicic MJ, Leibel RL, Lowe MR, Rosenbaum M, Klein S (2021) Describing the weight-reduced state: physiology, behavior, and interventions. Obesity 29(S1):S9–S24. https://doi.org/10.1002/oby.23086
Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC (2015) Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health 105(9):e54-59. https://doi.org/10.2105/ajph.2015.302773
Muller MJ, Enderle J, Bosy-Westphal A (2016) Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep 5(4):413–423. https://doi.org/10.1007/s13679-016-0237-4
Leibel RL, Rosenbaum M, Hirsch J (1995) Changes in energy expenditure resulting from altered body weight. N Engl J Med 332(10):621–628. https://doi.org/10.1056/nejm199503093321001
Maclean PS, Bergouignan A, Cornier MA, Jackman MR (2011) Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol 301(3):R581-600. https://doi.org/10.1152/ajpregu.00755.2010
Nunes CL, Casanova N, Francisco R, Bosy-Westphal A, Hopkins M, Sardinha LB, Silva AM (2021) Does adaptive thermogenesis occur after weight loss in adults? A systematic review. Br J Nutr. https://doi.org/10.1017/S0007114521001094
Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A (2007) Clinical significance of adaptive thermogenesis. Int J Obes (Lond) 31(2):204–212. https://doi.org/10.1038/sj.ijo.0803523
Dulloo AG, Jacquet J, Montani JP, Schutz Y (2012) Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obesity Rev 13(Suppl 2):105–121. https://doi.org/10.1111/j.1467-789X.2012.01041.x
Nunes CL, Jesus F, Francisco R, Matias CN, Heo M, Heymsfield SB, Bosy-Westphal A, Sardinha LB, Martins P, Minderico CS, Silva AM (2021) Adaptive thermogenesis after moderate weight loss: magnitude and methodological issues. Eur J Nutr. https://doi.org/10.1007/s00394-021-02742-6
Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD (2016) Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring, Md) 24(8):1612–1619. https://doi.org/10.1002/oby.21538
Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD (2012) Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab 97(7):2489–2496. https://doi.org/10.1210/jc.2012-1444
Tremblay A, Royer MM, Chaput JP, Doucet E (2013) Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int J Obes (Lond) 37(6):759–764. https://doi.org/10.1038/ijo.2012.124
Gomez-Arbelaez D, Crujeiras AB, Castro AI, Martinez-Olmos MA, Canton A, Ordoñez-Mayan L, Sajoux I, Galban C, Bellido D, Casanueva FF (2018) Resting metabolic rate of obese patients under very low calorie ketogenic diet. Nutr Metab (Lond) 15:18. https://doi.org/10.1186/s12986-018-0249-z
Marlatt KL, Redman LM, Burton JH, Martin CK, Ravussin E (2017) Persistence of weight loss and acquired behaviors 2 y after stopping a 2-y calorie restriction intervention. Am J Clin Nutr 105(4):928–935. https://doi.org/10.3945/ajcn.116.146837
Novaes Ravelli M, Schoeller DA, Crisp AH, Shriver T, Ferriolli E, Ducatti C, Marques de Oliveira MR (2019) Influence of energy balance on the rate of weight loss throughout one year of Roux-en-Y gastric bypass: a doubly labeled water study. Obes Surg 29(10):3299–3308. https://doi.org/10.1007/s11695-019-03989-z
Wolfe BM, Schoeller DA, McCrady-Spitzer SK, Thomas DM, Sorenson CE, Levine JA (2018) Resting metabolic rate, total daily energy expenditure, and metabolic adaptation 6 months and 24 months after bariatric surgery. Obesity (Silver Spring) 26(5):862–868. https://doi.org/10.1002/oby.22138
Martins C, Gower BA, Hill JO, Hunter GR (2020) Metabolic adaptation is not a major barrier to weight-loss maintenance. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqaa086
Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL (2008) Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 88(4):906–912. https://doi.org/10.1093/ajcn/88.4.906
Hopkins M, Gibbons C, Caudwell P, Hellstrom PM, Naslund E, King NA, Finlayson G, Blundell JE (2014) The adaptive metabolic response to exercise-induced weight loss influences both energy expenditure and energy intake. Eur J Clin Nutr 68(5):581–586. https://doi.org/10.1038/ejcn.2013.277
Bosy-Westphal A, Schautz B, Lagerpusch M, Pourhassan M, Braun W, Goele K, Heller M, Glüer CC, Müller MJ (2013) Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (Lond) 37(10):1371–1377. https://doi.org/10.1038/ijo.2013.1
Silva AM, Nunes CL, Matias CN, Jesus F, Francisco R, Cardoso M, Santos I, Carraça EV, Silva MN, Sardinha LB, Martins P, Minderico CS (2020) Champ4life study protocol: a one-year randomized controlled trial of a lifestyle intervention for inactive former elite athletes with overweight/obesity. Nutrients. https://doi.org/10.3390/nu12020286
Riebe D, Ehrman JK, Liguori G, Magal M American College of Sports M (2018) ACSM’s guidelines for exercise testing and prescription
Silva AM, Nunes CL, Jesus F, Francisco R, Matias CN, Cardoso M, Santos I, Carraça EV, Finlayson G, Silva MN, Dickinson S, Allison D, Minderico CS, Martins P, Sardinha LB (2021) Effectiveness of a lifestyle weight-loss intervention targeting inactive former elite athletes: the Champ4Life randomised controlled trial. Br J Sports Med 56:394–402. https://doi.org/10.1136/bjsports-2021-104212
World Medical Association (2013) Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310(20):2191–2194
Marques M, Hagger M (2019) Classification of techniques used in self-determination theory-based interventions in health contexts: an expert consensus study. Motiv Sci. https://doi.org/10.31234/osf.io/z9wqu
Park YW, Heymsfield SB, Gallagher D (2002) Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord 26(7):978–983. https://doi.org/10.1038/sj.ijo.0801982
Sherman M, Fan B, Borrud LG, Powers CL, Shepherd JA (2011) Accuracy and precision of the hologic reflection technique for obese whole body scan analysis. J Clin Densitom 14:165
Weir JB (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109(1–2):1–9
Compher C, Frankenfield D, Keim N, Roth-Yousey L (2006) Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106(6):881–903. https://doi.org/10.1016/j.jada.2006.02.009
Weststrate JA (1993) Resting metabolic rate and diet-induced thermogenesis: a methodological reappraisal. Am J Clin Nutr 58(5):592–601. https://doi.org/10.1093/ajcn/58.5.592
Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, Martin CK, Silva AM, Vossen M, Westerterp K, Heymsfield SB (2012) Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev 13(10):835–847. https://doi.org/10.1111/j.1467-789X.2012.01012.x
Merril A, Watt B (1973) Energy value of foods, basis and derivation. Agriculture Handbook No 74 Washington, DC, ARS United States Department of Agriculture 2
Dulloo AG, Jacquet J (1999) The control of partitioning between protein and fat during human starvation: its internal determinants and biological significance. Br J Nutr 82(5):339–356. https://doi.org/10.1017/s0007114599001580
Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, Heymsfield SB, Hirsch J, Leibel RL (1996) A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol 270(3 Pt 2):R496-504. https://doi.org/10.1152/ajpregu.1996.270.3.R496
Shook RP, Hand GA, O’Connor DP, Thomas DM, Hurley TG, Hébert JR, Drenowatz C, Welk GJ, Carriquiry AL, Blair SN (2018) Energy intake derived from an energy balance equation, validated activity monitors, and dual X-ray absorptiometry can provide acceptable caloric intake data among young adults. J Nutr 148(3):490–496. https://doi.org/10.1093/jn/nxx029
Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, Redman LM (2012) Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab 302(4):E441-448. https://doi.org/10.1152/ajpendo.00290.2011
Bonafiglia JT, Nelms MW, Preobrazenski N, LeBlanc C, Robins L, Lu S, Lithopoulos A, Walsh JJ, Gurd BJ (2018) Moving beyond threshold-based dichotomous classification to improve the accuracy in classifying non-responders. Physiol Rep 6(22):e13928. https://doi.org/10.14814/phy2.13928
Martins C, Roekenes J, Gower BA, Hunter GR (2021) Metabolic adaptation is associated with less weight and fat mass loss in response to low-energy diets. Nutr Metab 18(1):60. https://doi.org/10.1186/s12986-021-00587-8
Martins C, Roekenes J, Salamati S, Gower BA, Hunter GR (2020) Metabolic adaptation is an illusion, only present when participants are in negative energy balance. Am J Clin Nutr 112(5):1212–1218. https://doi.org/10.1093/ajcn/nqaa220
Painter SL, Ahmed R, Hill JO, Kushner RF, Lindquist R, Brunning S, Margulies A (2017) What matters in weight loss? An in-depth analysis of self-monitoring. J Med Internet Res 19(5):e160. https://doi.org/10.2196/jmir.7457
Karl JP, Roberts SB, Schaefer EJ, Gleason JA, Fuss P, Rasmussen H, Saltzman E, Das SK (2015) Effects of carbohydrate quantity and glycemic index on resting metabolic rate and body composition during weight loss. Obesity (Silver Spring) 23(11):2190–2198. https://doi.org/10.1002/oby.21268
Byrne NM, Sainsbury A, King NA, Hills AP, Wood RE (2018) Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. Int J Obes (Lond) 42(2):129–138. https://doi.org/10.1038/ijo.2017.206
Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E (2009) Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE 4(2):e4377. https://doi.org/10.1371/journal.pone.0004377
McNeil J, Schwartz A, Rabasa-Lhoret R, Lavoie JM, Brochu M, Doucet É (2015) Changes in leptin and peptide YY do not explain the greater-than-predicted decreases in resting energy expenditure after weight loss. J Clin Endocrinol Metab 100(3):E443-452. https://doi.org/10.1210/jc.2014-2210
Müller MJ, Enderle J, Bosy-Westphal A (2016) Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep 5(4):413–423. https://doi.org/10.1007/s13679-016-0237-4
Tam CS, Rigas G, Heilbronn LK, Matisan T, Probst Y, Talbot M (2016) Energy adaptations persist 2 years after sleeve gastrectomy and gastric bypass. Obes Surg 26(2):459–463. https://doi.org/10.1007/s11695-015-1972-4
Coupaye M, Bouillot JL, Coussieu C, Guy-Grand B, Basdevant A, Oppert JM (2005) One-year changes in energy expenditure and serum leptin following adjustable gastric banding in obese women. Obes Surg 15(6):827–833. https://doi.org/10.1381/0960892054222768
Browning MG, Rabl C, Campos GM (2017) Blunting of adaptive thermogenesis as a potential additional mechanism to promote weight loss after gastric bypass. Surg Obes Relat Dis 13(4):669–673. https://doi.org/10.1016/j.soard.2016.11.016
Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Glüer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A (2015) Metabolic adaptation to caloric restriction and subsequent refeeding: the minnesota starvation experiment revisited. Am J Clin Nutr 102(4):807–819. https://doi.org/10.3945/ajcn.115.109173
Bettini S, Bordigato E, Fabris R, Serra R, Dal Pra C, Belligoli A, Sanna M, Compagnin C, Foletto M, Prevedello L, Fioretto P, Vettor R, Busetto L (2018) Modifications of resting energy expenditure after sleeve gastrectomy. Obes Surg 28(8):2481–2486. https://doi.org/10.1007/s11695-018-3190-3
Silva AM, Santos DA, Matias CN, Minderico CS, Schoeller DA, Sardinha LB (2013) Total energy expenditure assessment in elite junior basketball players: a validation study using doubly labeled water. J Strength Cond Res 27(7):1920–1927. https://doi.org/10.1519/JSC.0b013e31827361eb
Blundell J, King N, Bryant E (2005) Interactions among physical activity food choice and appetite control: health message in physical activity and diet. Taylor & Francis, London
Melby CL, Paris HL, Foright RM, Peth J (2017) Attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up? Nutrients. https://doi.org/10.3390/nu9050468
de Jonge L, Bray GA, Smith SR, Ryan DH, de Souza RJ, Loria CM, Champagne CM, Williamson DA, Sacks FM (2012) Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity (Silver Spring) 20(12):2384–2389. https://doi.org/10.1038/oby.2012.127
Swinton PA, Hemingway BS, Saunders B, Gualano B, Dolan E (2018) A Statistical framework to interpret individual response to intervention: paving the way for personalized nutrition and exercise prescription. Front Nutr. https://doi.org/10.3389/fnut.2018.00041
Laine MK, Eriksson JG, Kujala UM, Kaprio J, Loo BM, Sundvall J, Bäckmand HM, Peltonen M, Jula A, Sarna S (2016) Former male elite athletes have better metabolic health in late life than their controls. Scand J Med Sci Sports 26(3):284–290. https://doi.org/10.1111/sms.12442
Griffin JR, Maxwell TM, Griffin L (2016) The prevalence and consequences of obesity in athletes. Curr Orthop Pract 27(2):129–134. https://doi.org/10.1097/bco.0000000000000346
Dutton GR, Kim Y, Jacobs DR Jr, Li X, Loria CM, Reis JP, Carnethon M, Durant NH, Gordon-Larsen P, Shikany JM, Sidney S, Lewis CE (2016) 25-year weight gain in a racially balanced sample of U.S. adults: the CARDIA study. Obesity (Silver Spring) 24(9):1962–1968. https://doi.org/10.1002/oby.21573
Provencher MT, Chahla J, Sanchez G, Cinque ME, Kennedy NI, Whalen J, Price MD, Moatshe G, LaPrade RF (2018) Body mass index versus body fat percentage in prospective national football league athletes: overestimation of obesity rate in athletes at the national football league scouting combine. J Strength Cond Res 32(4):1013–1019. https://doi.org/10.1519/jsc.0000000000002449
Müller MJ, Heymsfield SB, Bosy-Westphal A (2021) Are metabolic adaptations to weight changes an artefact? Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqab184
Acknowledgements
The authors express their gratitude to all the participants involved in this study.
Funding
The Champ4life program was financially supported by the Portuguese Institute of Sports and Youth and by the International Olympic Committee, under the Olympic Solidarity Promotion of the Olympic Values Unit (Sports Medicine and Protection of Clean Athletes Programme). The program was also supported by national funding from the Portuguese Foundation for Science and Technology within the R&D units UIDB/00447/2020. C.L.N., R.F. and F.J. were supported with a PhD scholarship from the Portuguese Foundation for Science and Technology (SFRH/BD/143725/2019 and 2020.05397.BD and 2021.07122.BD, respectively).
Author information
Authors and Affiliations
Contributions
CLN: participated in the conceptualization, methodology, formal analysis, data curation and writing the first draft; FJ, RF: contributed to preparation, visualization and reviewing and editing. MH, LBS, PM and CSM: supervised and contributed to reviewing and editing, Supervision; AMS: participated in the conceptualization, methodology, supervision, funding acquisition, reviewing and editing. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors reported no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nunes, C.L., Jesus, F., Francisco, R. et al. Effects of a 4-month active weight loss phase followed by weight loss maintenance on adaptive thermogenesis in resting energy expenditure in former elite athletes. Eur J Nutr 61, 4121–4133 (2022). https://doi.org/10.1007/s00394-022-02951-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02951-7