Abstract
Aim
In the present study, we evaluated the therapeutic potentiality of S-allylcysteine (SAC) in streptozotocin (STZ)–nicotinamide (NAD)-induced diabetic nephropathy (DN) in experimental rats.
Methods
SAC was orally administered for 45 days to rats with STZ–NAD-induced DN; a metformin-treated group was included for comparison. Effect of SAC on body weight, organ weight, blood glucose, levels of insulin, glycated haemoglobin, and renal biochemical markers was determined. Body composition by total body electrical conductivity (TOBEC) and dual-X ray absorptiometry (DXA), kidney antioxidant analysis, real-time polymerase chain reaction, and western blot analysis of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), nuclear factor kappa B (NF-κB), interleukin (IL)-6, and tumor necrosis factor (TNF)-α; histopathological and scanning electron microscope (SEM) analysis of the kidneys were performed in both control and experimental rats.
Results
SAC treatment showed significantly decreased levels of blood glucose, glycated haemoglobin, creatinine, albumin, AST, ALT, creatinine kinase, lactate dehydrogenase, and expressions of NF-κB, IL-6, and TNF-α compared with DN control rats. Furthermore, SAC administration to DN rats significantly improved body composition and antioxidant defense mechanism which was confirmed by the upregulation of mRNA and protein expressions of antioxidant genes.
Conclusions
Thus, SAC showed adequate therapeutic effect against DN by downregulation of inflammatory factors and attenuation of oxidative stress. Histological and SEM observations also indicated that SAC treatment notably reverses renal damage and protects the kidneys from hyperglycemia-mediated oxidative damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a multifaceted metabolic disorder characterized by defects in the body’s ability to utilize glucose and maintain insulin homeostasis. Persistent hyperglycemia leads to long-term devastation, dysfunction, and eventually malfunction of organs, particularly the eyes, kidneys, nerves, and cardiac system [1]. Approximately 366 million people suffer from DM world, and the incidence of this disease is predicted to be more than double by the year of 2030 [2]. Developing countries, such as India, are to being converted into the diabetic capitals of the world, with over 20 million diabetics, and this measure is set to increase to 57 million by 2025 [3].
Diabetic nephropathy (DN) is the most familiar complication and leading cause of DM-associated mortality. DN is a leading contributor to cases of kidney malfunction in the developed countries, and both type I and type II forms of DM can cause DN [4]. DN is clinically assessed through five-stage criteria, with each stage featuring a discrete set of functional and structural changes and reflected alterations in the standard renal function markers [5]. Several studies evidently point out that both insulin resistance and diabetic state play an essential role in producing oxidative stress. Even though haemodynamic and metabolic factors are the most important causes of DN, the current studies have recommended that DN is an inflammatory process, and immune cells could be implicated in the progression of DN [6]. The foremost pathological conditions of DN comprise mesangial extension, extracellular matrix alterations, tubulointerstitial fibrosis, and glomerular sclerosis [7]. Because the anti-diabetic drugs used for long-term treatment have diverse side effects, the developmental process for innovation in anti-diabetic drugs has shifted its focus towards innate plant sources with negligible side effects [8]. As a breakthrough in new remedial agents against DM, phytoconstituents with a persuasive antioxidant nature are an excellent option, and they have received much consideration as sources of biologically active substances, including antioxidants, and hypoglycemic and hypolipidaemic agents [9].
Garlic, a natural product, has been conservatively used as a food item, folklore medication, and cultural icon, dating back thousands of years. The persuasive remedial limit of garlic and its components has been assessed through many in vivo frameworks [10]. S-Allylcysteine (SAC), a derivative of garlic, is sulphur containing amino acid [3]. Similar to garlic concentrate, SAC has anti oxidative and anticancer properties, and it can also moderate the frequency of stroke [11]. Moreover, SAC has exhibit insulin-like anti hyperglycemic effects in the streptozotocin (STZ)–nicotinamide (NAD)-induced diabetic rats, and has reversed the changes in glucose metabolism in the liver, even though there has been no scientific literature available on the therapeutic action of SAC against DN. Hence, this study aimed to evaluate the effect of SAC on renal damage amelioration of STZ–NAD-induced renal damage in rats.
Materials and methods
Chemicals
SAC ((2R)-2-amino-3-prop-2-enylsulfanylpropanoic acid) (99%) was commercially obtainable and was purchased from LGC Promochem India Pvt. Ltd, Bangalore, India. STZ and NAD were purchased from Himedia, Bangalore, India. All the drugs and biochemicals used in this study were purchased from Sigma Chemical Company Inc., St Louis, MO, USA. All other chemicals used were of analytical grade.
Animal maintenance
Male Wistar/NIN (National Institute of Nutrition) rats of body weight 150–180 g were obtained from the National Centre for Laboratory Animal Science, National Institute of Nutrition, Hyderabad. Animals were housed individually in the standard polycarbonate cages at 22 ± 2 °C, with the top grill containing facilities for holding diet in the form of pellet and drinking water in polycarbonate bottles; hourly air changes, a relative humidity of 50–60%, and a 12 h light/dark cycle were maintained. Before initiating the experiment, the rats were acclimatized to the laboratory environment for 7 days. The protocol of this study was approved by the Institutional animal ethical committee of the National Centre for Laboratory Animal Science, National Institute of Nutrition, Hyderabad (Approval no. P7F/II-IAEC/NIN/2015/GS/WNIN Rats/42M).
Induction of diabetes
The overnight fasted rats were made diabetic by a single intraperitoneal injection of freshly prepared STZ (45 mg/kg body weight) [12] in a citrate buffer (0.1 M, pH 4.5) at a volume of 1 mL/kg, 15 min after the intraperitoneal administration of NAD (110 mg/kg body weight) [3] in 0.9% normal saline. Hyperglycemia was confirmed through the elevated glucose levels (> 250 mg/dL) in the blood, determined at 72 h and then on day 7 after injection. After 15 days of diabetes induction, DN was confirmed through renal markers.
Experimental design
After the successful induction of experimental DN, the rats were divided into four groups comprising a minimum of six rats in each group.
- Group 1:
-
Normal control rats
- Group 2:
-
DN control rats
- Group 3:
-
STZ–NAD-induced DN rats orally treated with SAC (150 mg/kg body weight) in a vehicle solution for 45 days using an intragastric tube [3].
- Group 4:
-
STZ–NAD-induced DN rats orally treated with metformin (25 mg/kg body weight) in a vehicle solution for 45 days using an intragastric tube [13].
The test drug and reference standard drugs were fed orally for 45 days. Groups 1 and 2 rats received 0.9% normal saline solution orally once a day for 45 days. The experiment was terminated at the end of 45 days and the animals were fasted overnight. Blood was collected by retro-orbital sinus puncture method for the analysis.
After blood collection, the animals were anaesthetized using low doses of phenobarbitone and sacrificed by cervical decapitation. Both kidneys were dissected out. Kidney tissues were immediately excised from the rats and stored in ice-cold containers. They were then homogenised with buffer and centrifuged, and the supernatant was collected. Biochemical estimations were performed in the homogenates.
Effect on body and organ weight
Body weight was measured weekly; at the end of the treatment, animals were sacrificed according to the ethical committee (IAEC) guidelines, and the kidney was dissected, washed in ice-cold phosphate-buffered saline, and then weighed.
Body composition determined through total body electrical conductivity (TOBEC)
At the end of the experiment, the body composition parameters, namely lean mass, fat-free mass, fat percentage, total fat (g), total body sodium (Na), potassium (K) levels, and water content, were measured in all experimental groups through TOBEC, using the small animal body composition analysis system (EM-SCAN, Model SA-3000 Multi detector, Springfield, USA), as described by Parim et al. [14].
Estimation of BMC and BMD assessed through dual-X ray absorptiometry (DXA)
At the end of experiment, the body composition parameters of the experimental animals were assessed through DXA, using a body composition analysis system (halogen 1000 series). DXA data were used to compare the values of body adiposity, bone mineral concentration (BMC), and bone mineral density (BMD) between the control and experimental groups [15]; calculations were performed according to manufacturer’s protocols.
Estimation of glucose, insulin, and glycated haemoglobin levels
The effect on the status of hyperglycemic markers, namely, plasma glucose (Stan Bio Laboratory Kits, USA), insulin (Bio-Merieux, RCS, Lyon, France), and glycated haemoglobin (Hitachi 912, Boehringer Mannheim, Germany), was evaluated using the respective kits.
Estimation of biochemical markers
The effect of SAC on nephrotic markers, namely C-reactive protein (CRP), albumin, total protein, serum creatinine, serum uric acid, urea, and blood urea nitrogen (BUN), and biochemical marker enzymes, such as alanine transaminase (ALT), aspartate transaminase (AST), creatinine kinase (CK), and lactate dehydrogenase (LDH), was measured in both the control and experimental rats using the respective kits.
Kidney antioxidant analysis
The antioxidant potentiality of SAC in kidney was measured; the antioxidant parameters such as thiobarbituric acid reactive substances (TBARS) level [16], the hydroperoxide values [17], reduced glutathione (GSH) [18], oxidized glutathione (GSSG) [19], superoxide dismutase (SOD) activity [20], catalase (CAT) level [21], glutathione peroxidase (GPx) activity [22] were measured.
RT-PCR analysis
Total RNA was isolated from the kidney using tri-reagent (Sigma-Aldrich, USA) according to the manufacturer’s protocol and was reverse transcribed to obtain cDNA using the DNA synthesis kit (Applied Bio systems, Foster City, USA); 20 ng of cDNA was used for semi-quantitative PCR. PCR amplification was performed for 38 cycles in the following cycling conditions: 30 s of denaturation at 94 °C, 30 S of annealing at 59 °C, and 1 min of extension at 72 °C with the primers. The sequences of the primers are given in (Table 1). The housekeeping gene β-actin was used for normalization.
Western blot analysis
The kidneys of the rats were collected and lysed using a bead beater, and centrifuged at 7000 rpm for 15 min at 4 °C. The suspension samples were collected, and 20 µL of each sample was loaded on a 6% sodium dodecyl sulfate gel and run for the normal control, DN, and treated groups (SAC and metformin). The bands were transferred the gels to polyvinylidene fluoride membrane using trans-turbo gel transfer equipment. The primary antibodies anti-SOD (ab13498), anti-CAT (ab1673), anti GPx (ab22604), anti-NF-κB (ab16502), anti-IL-6 (ab6672), and anti-TNF-α (ab6671) were added, and the membrane developed blots which were analyzed using chemi doc equipment (ChemiDoc™, Bio-Rad Laboratories, Inc.).
Histopathological study
Kidney tissue was fixed in 10% formalin, routinely processed, and embedded in paraffin wax. Paraffin sections (5 µm) were cut on glass slides, stained with haematoxylin and eosin (H&E), and examined under a light microscope by a pathologist blinded to the evaluated groups.
Scanning electron microscope (SEM) analysis
All the grouped kidney samples were immersed in ice-cold 2.5% glutaraldehyde (buffered with 0.1 M sodium cacodylate buffer, pH 7.4) immediately after their extraction and sectioning, and fixed for 4 h at 4 °C, carefully handling samples to avoid ex vivo artefacts. Sections were repeatedly washed in cacodylate buffer and postfixed in 1% osmium tetroxide for 1 h. Fixed specimens were dehydrated through increasing concentrations of ethanol, starting with 10, 50, 70, and 90% of ethanol. Once the tissues were equilibrated in anhydrous 100% ethanol, they were rinsed with liquid carbon dioxide with a Bal-Tec 030 critical point dryer (BAL-TEC AG, Balzers, Liechtenstein). Samples were mounted on stubs, and coated with a thin layer of atomic gold particles in a sputter coater (Agar Scientific, Stansted, UK). Coated specimens were observed through SEM using secondary electron detection (Supra 55, Zeiss, Oberkochen, Germany). Acceleration voltage was set to 15 kV, working distance to 4–8 mm, and enlargement up to 2 kx.
Statistical analysis
All results for the six animals in each group are expressed as the mean ± SD. All the grouped data were statistically evaluated using SPSS\10.0 software (Genericom, Germany). Hypothesis-testing methods used were the one-way analysis of variance and the least-significant difference test. The significance levels were set at p < 0.05, and 0.01.
Results
Effect of SAC on body and organ weights, blood glucose, insulin, and glycated haemoglobin levels
Figure 1 shows the body weight (Fig. 1a), organ weight (Fig. 1b), blood glucose levels (Fig. 1c), insulin (Fig. 1d), and glycated haemoglobin (Fig. 1e) in both control and experimental animals. A significant decrease in the insulin levels (73.5%) and bodyweight (54.6%) and a concomitant increase in the level of plasma glucose (64.95%) and glycated haemoglobin (68.25%) were observed in STZ–NAD-induced DN rats, and these levels were normalized after treatment with SAC and metformin.
Body composition analysis by TOBEC and DXA
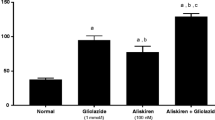
Administration of STZ–NAD produced a substantial change in the body composition of the experimental rats. The results revealed that the body composition parameters were considerably decreased in the DN rats (Table 2). However, oral administration of SAC and metformin for 45 days significantly (p < 0.05, 0.01) increased the body weight, lean mass, total body water, total body Na, total body K, total fat, fat percentage, and fat-free mass of the STZ–NAD-induced DN rats. STZ–NAD produced a substantial decrease in the BMC (43.03%) and BMD (50%) of the experimental rats (Fig. 2.1a–d). However, compared with the untreated DN rats, oral administration of SAC and metformin for 45 days caused a significant (p < 0.05, 0.01) increase in the BMC (Fig. 2.2) and BMD (Fig. 2.3) of the experimental rats.
Effect of SAC on (2.1) body composition by DXA, (2.2) BMC, and (2.3) BMD in control and experimental rats. a Normal control, b DN control, c DN + SAC, d DN + metformin. Values are mean ± SD, n = 6, asignificantly different from normal control, bsignificantly different from DN control, *p < 0.05, **p < 0.01
Effect of SAC on biochemical markers
Table 3 shows the effect of SAC on the levels of CRP, serum creatinine, serum uric acid, blood urea nitrogen, urea, albumin, and total protein in both the control and experimental rats. A noteworthy increase in the levels of CRP, serum creatinine, serum uric acid, urea, BUN, and concomitant decrease in the albumin and total protein was noticed in DN rats, and these levels became normalized in the SAC and metformin-treated animals after 45 days. On the other hand, the increased levels of biochemical marker enzymes such as ALT, AST, CK, and LDH were observed in DN rats, and these levels became diminished after treatment with SAC and metformin (Table 3).
Effect of SAC on kidney antioxidant activities, mRNA, and protein expressions
Figure 3 summarizes the kidney antioxidant profiles of both the control and experimental animals. The levels of TBARS (Fig. 3a) and hydroperoxides (Fig. 3b) in the DN rats were significantly (p < 0.05, 0.01) higher than those in the control rats; in the DN rats treated with SAC and metformin, these altered values were restored to near normal. A decreased concentration (p < 0.05, 0.01) of GSH (Fig. 3c) was observed in the DN rats. Administration of SAC and metformin tended to restore the GSH to near normal levels. Compared with the control rats, a significantly (p < 0.05, 0.01) increased level of GSSG (Fig. 3d) and concomitant decreased level of GSH/GSSG (Fig. 3e) were observed in the DN rats. Oral treatment with SAC and metformin tended to bring the GSSG and GSH/GSSG ratios towards near normal levels. The activities, expressions of mRNA, and protein of SOD, CAT, and GPx in the kidneys were significantly (p < 0.05, 0.01) lower in the DN control rats than in the normal rats (Fig. 4a–f). Treatment with SAC or metformin showed a significant increase in the activities and the levels of mRNA (4D&E) and protein expressions (Fig. 4f) of SOD (Fig. 4a), CAT (Fig. 4b), and GPx (Fig. 4c) in the kidneys of the DN rats.
Effect of SAC on kidney enzymatic antioxidants activities of a SOD, b CAT, c GPx, d mRNA expression percentages, e mRNA expressions, and f protein expressions of SOD, CAT, and GPx in control and experimental rats. I: Normal control; II: DN control; III: DN + SAC; IV: DN + metformin. Values are mean ± SD, n = 6, asignificantly different from normal control, bsignificantly different from DN control, *p < 0.05, **p < 0.01
Effect of SAC on inflammatory markers mRNA and protein expressions
The effect of oral SAC treatment on the mRNA and protein expressions of NF-κB, IL-6, and TNF-α in the renal tissues of the control and experimental rats are shown in Fig. 5a–c. In the DN rats, the mRNA (Fig. 5a, b) and protein expressions (Fig. 5c) of NF-κB, IL-6, and TNF-α indicated significantly enhanced when compared with in the control rats. Moreover, compared with the DN control rats, oral treatment with SAC or metformin to DN rats significantly (p < 0.05, 0.01) restored these levels to near normalcy.
Effect of SAC on inflammatory markers (NF-κB, IL-6, and TNF-α) a mRNA expression percentages, b mRNA expressions, and c protein expressions in control and experimental rats. I: Normal control; II: DN control; III: DN + SAC; IV: DN + metformin. Values are mean ± SD, n = 6, asignificantly different from normal control, bsignificantly different from DN control, *p < 0.05, **p < 0.01
SEM analysis of kidney
Figure 6 depicts the SEM analysis of the control and experimental rat kidneys. SEM analysis of normal control kidneys demonstrated healthy conserved glomerular architecture with normal peripheral capillary loop ultra structure. The outer surfaces of glomerular capillaries were enclosed by vastly divided podocytes exhibiting unbroken cell bodies and primary processes with regimented interdigitating foot processes (Fig. 6a). The examination of the diabetic kidneys displayed a glomerular adaptative growth, with a subsequent disparity between the expansion of the tuft and the hypertrophy of podocytes, which revealed flattened and stretched cell bodies; the filtration barrier was structurally integral in a few areas of the glomerular capillary tuft. The glomerular basement membrane is expansively disjointed and devastated, whereas the fundamental surface displayed a tangle of stretched out fibrils which had replaced the usual glomerular structures, creating a fibrillar collagen network (Fig. 6b). Treatment with SAC or metformin have shown that changes in the diabetic kidneys were significantly restored to near normal and displayed healthy glomerular architecture and normal peripheral capillary loop ultrastructure (Fig. 6c, d).
SEM analysis of kidney in control and experimental rats; a normal control: demonstrated healthy conserved glomerular architecture with normal peripheral capillary loop ultrastructure, b DN control: displayed a glomerular adaptative growth, with a subsequent disparity between the expansion of the tuft and the hypertrophy of podocytes, which revealed flattened and stretched cell bodies; the filtration barrier was structurally integral in a few areas of the glomerular capillary tuft, c DN + SAC and d DN + metformin: SAC or metformin shown that changes in the diabetic kidney were significantly restored to near normal
Histopathological analysis
Figure 7 demonstrates the histology of kidneys in control and experimental rats. Normal control rat kidney exhibits a normocellular glomerulus with mild segmental mesangial expansion and obvious capillary lumina. The adjoining tubular parenchyma does not have any indication of injury (Fig. 7a). Histological analysis of kidneys with H&E staining from the DN rats made distinctly brutal devastation in tubulointerstitial and glomerular lesions including epithelial necrosis and ballooning with focal fibrosis compared with the normal control group (Fig. 7b). Supplementation of SAC or metformin for 45 days ameliorated the histopathological changes in diabetic kidney and changes were brought back to near normal morphology as like normal control kidney when compared to DN group (Fig. 7c, d).
Histopathological analysis of kidney in control and experimental rats, a Normal control: exhibits a normocellular glomerulus with mild segmental mesangial expansion and obvious capillary lumina, b DN control: brutal devastation in tubulointerstitial and glomerular lesions including epithelial necrosis and ballooning with focal fibrosis compared with the normal control group, c DN + SAC and d DN + metformin: supplementation of SAC or metformin for 45 days ameliorated the histopathological changes in diabetic kidney and changes were brought back to near normal morphology as like normal control kidney when compared to DN group. H&E ×40
Discussion
STZ–NAD-induced hyperglycaemia is a suitable animal model for the preliminary screening of agents against DM [23, 24]. In many animal experiments, STZ has produced permanent DM that impersonates the pathological status observed in human DM [25], as reflected by the glycosuria, hyperglycaemia, polyphagia, polydipsia, and weight loss observed in them [26]. STZ is well documented to root pancreatic β-cell destruction, while NAD is administered to rats to moderately guard insulin-secreting cells against STZ. Similarly, in the present study, the intraperitoneal administration of STZ–NAD effectively induced DM in experimental rats, which was reflected by their increased blood glucose levels. The hypoglycemic effectiveness of SAC has been attributed to the sulphur compound [27]. The mechanism of hypoglycemic action, perhaps, involves the direct or indirect stimulation of insulin secretion. This could be a result of the potentiation of the insulin effect of plasma through escalation of the pancreatic secretion of insulin from the existing β cells or liberation from bound insulin [28].
In the present study, total body weight and kidney weight were measured. A significant decrease in body weight was noticed in DN control animals, whereas increased body weight was observed in DN rats treated with SAC. In type 2 DM, the loss of insulin responsiveness prevents the utilization of glucose for energy in the cells. Under these conditions, the body starts burning fat and muscle for energy, which accounts for the loss in body weight observed in DN control rats [29]. In this study, a significant increase in the kidney weight was observed in the DN control group as compared with that in the normal control and SAC-treated rats. This increase in kidney weight can be attributed to glomerular hypercellularity, increased mesangium, and the subsequent closure of glomeruli [30].
DN refers to a characteristic set of structural and functional kidney abnormalities observed in patients with DM. The structural abnormalities include hypertrophy of the kidney, increase in glomerular basement membrane thickness, nodular and diffuse glomerulosclerosis, tubular atrophy, and interstitial fibrosis. Hyperglycaemia and albuminuria are present in STZ–NAD-induced diabetic rats and DN progresses rapidly in this animal model [31]. In our study, increased levels of BUN, urea, albumin, total protein, uric acid, and creatinine were detected in the untreated DN rats, and a significant decrease in these levels were noted in the rats treated with SAC. BUN is commonly known as the major reliable indicator to evaluate renal function. Increment in BUN level is universal in kidney damage or dysfunction. An elevated level of blood urea and blood sugar indicates that hyperglycemia can lead to kidney damage [32]. Hyperglycaemia leads to the destruction of the glomerular filtration barrier, leading to glomerular damage, and which, in turn, causes urinary protein or albumin leakage, thereby exacerbating the progression of DN [33]. Albuminuria is the main pathologic feature of many primary glomerular diseases, including DN [34]. Consistent with this interpretation, our study demonstrated that albuminuria and focal glomerular matrix expansion were markedly increased in the DN rats. The elevated blood glucose; increased kidney weight; altered intraperitoneal insulin tolerance; increased activities of renal AST, ALT, CK, and LDH; diminished creatinine clearance in the DN rats indicate the development of renal hypertrophy, glomerular injury, and renal dysfunction [35]. However, oral treatment with SAC for these rats restored these altered levels to near normalcy, suggesting that SAC effectively ameliorates diabetic renal injury. The increased activities of AST, ALT, CK, and LDH in the plasma may be mainly caused by the leakage of these enzymes from the liver cells into the blood stream [36], indicating the hepatotoxic effect of STZ.
Oxidative damage replicates the inequity between the systemic manifestation of reactive oxygen species and the ability of biological systems to restore the ensuing damage or to willingly detoxify the reactive intermediate. Increased oxidative stress and damaged antioxidant protection system are vital aspects of the evolution and pathogenesis of diseases connected to oxidants [37]. Augmented free radicals detected in DN rats are attributed to the persistent hyperglycemia which damages the defense mechanism against oxidants [38]. Free radicals can also be produced by the auto-oxidation of unsaturated lipids in membrane and plasma lipids. The free radicals produced might react with the polyunsaturated fatty acids in the cell membrane, leading to the peroxidation of lipids [39]. Lipid peroxidation, in turn, contributes to the prominent fabrication of free radicals [40]. Improved lipid peroxidation damages the membrane functions by altering the activity of the membrane-bound enzymes and receptors, and reducing the membrane fluidity [41]. In the current study, a noteworthy rise in tissue TBARS and hydroperoxide levels was detected in STZ–NAD-induced diabetic rats. The amplified TBARS content in the DN rats indicated that the peroxidative damage might be implicated in the progression of diabetic complications. TBARS and hydroperoxide levels in the kidneys of the SAC and metformin-treated rats were markedly lower than those of the DN control rats. These results suggested that SAC has antioxidant activity and defends the tissues from lipid peroxidation.
GSH is a chief endogenous antioxidant that compensates for the damage mediated by free radicals. Studies have revealed that the tissue GSH concentrations in STZ-induced diabetic rats are considerably less than those in control rats [42]. GSH is implicated in the defense of normal cell function and structure through sustenance of the quenching of free radicals, redox homeostasis, and participation in detoxification reactions. Loven et al. [43] reported that a reduction in tissue GSH could be the consequence of increased GSH degradation or lowered synthesis caused by oxidative damage in DM. Improved oxidative stress, ensuing from a momentous boost in the aldehydic products of lipid peroxidation, possibly lowers the tissue GSH [23]. In the present study, elevated GSH levels were detected in the kidneys of the SAC and metformin-supplemented DN rats. This indicates that SAC can either reduce oxidative stress or increase the biosynthesis of GSH, leading to a reduced amount of GSH degradation, or can induce both effects.
In the diabetic state, insulin shortage causes the destruction of glucose consumption, leading to an increased production of oxygen-free radicals. Studies have reported on the decrease in tissue SOD activities in STZ–NAD-induced diabetic rats [44]. SOD is an imperative defense enzyme that catalyses the dismutation reaction in superoxide radicals to produce hydrogen peroxides (H2O2) and molecular oxygen, thereby reversing the toxic effects of this radical. Wohaieb and Godin [45] suggested that reactive oxygen-free radicals can reduce and inactivate the activities of tissue SOD. The noteworthy decrease in SOD activity could result from the inactivation through glycation of enzymes or H2O2 [46]. Oral supplementation of SAC caused a momentous rise in SOD activities in diabetic rats. This indicated that SAC can decrease reactive oxygen-free radicals and improve the activities of the tissue antioxidant enzymes. CAT is a hemeprotein that catalyses the reduction of H2O2 and protects the tissues from highly reactive hydroxyl radicals. The reduction in CAT activity could result from inactivation through glycation of the enzyme [47]. CAT reduces H2O2 produced by the dismutation reaction. It also averts the generation of hydroxyl radicals, thus defending the cellular constituents from peroxisome-induced oxidative damage. The condensed activity of CAT in STZ–NAD-treated rats results in the accretion of H2O2, which produces poisonous effects. In this study, SAC caused a considerable increase in the activity of CAT in diabetic rats. This action is principally due to the antioxidant character of SAC and could involve a mechanism associated with scavenging activity.
GPx, a selenium-containing enzyme present in important concentrations, detoxifies H2O2 to water through reduced GSH oxidation. GPx works jointly with GSH in the putrefaction of H2O2 or other organic hydroperoxides to non-toxic products, at the expense of reduced GSH [42]. Condensed activities of GPx may result from the glycation of the enzyme and radical-induced inactivation. The squat activity of GPx could be explained by the low content of GSH found in diabetic state, because glutathione is a cofactor and substrate of GPx. Decreased activities of GPx in the kidney have been observed during DM and this may lead to numerous lethal effects caused by to the accretion of toxic products. In addition, a reduction in the GPx activity in kidneys of DM rats has been noted [23]. In concurrence with Haliga et al. [48], the DN rats in our study had higher levels of renal TBARS and lower expression of renal antioxidant enzyme (SOD, GPx, and CAT) genes. SAC diminished the renal oxidative stress by the restoring the expression of SOD, CAT, and GPx, and decreasing TBARS in STZ–NAD-induced DN rats. These results were supported those of RT-PCR and western blot analysis, which revealed that of SAC upregulated the expression of SOD, CAT, and GPx genes and proteins in the kidneys of SAC-treated DN rats.
Oxidative stress and inflammation are concerned in multifaceted processes in the expansion and succession of renal disease in diabetic patients. When taking into consideration the task of cytokines in pathophysiological processes underlying disease, it is obligatory to take into description the fact that the activities of these molecules are exceptionally intricate, as reflected by imperative characteristics, including their pleiotropic actions, and therefore, a cytokine may elicit numerous different cellular responses [49]. NF-κB is a family of pleiotropic transcription factors that combine a complex network of signaling pathways and extracellular perturbagens, consequential in the transcriptional regulation of many genes interrelated to immunity, inflammation, cell proliferation, differentiation, and apoptosis [50]. In experimental renal disease, NF-κB triggers in mesangial and podocytes cells in glomerular injury as well as in tubular cells during the line of primary tubulointerstitial diseases including toxic acute kidney injury [51]. IL-6, a cytokine formed by numerous cells. IL-6 plays a major role in the pathogenesis of DN, and there was a well-built relationship between the cruelty of diabetic glomerulopathy and expression of IL-6 mRNA in glomerular cells. More modern studies in type 2 DM patients reveal a noteworthy relationship between IL-6 and glomerular basement membrane thickening, a decisive lesion of DN and a strong forecaster of renal progression [52]. TNF-α is a pleiotropic inflammatory cytokine that is mostly formed by T cells, macrophages, and monocytes. Navarro et al. [53] reported a momentous relationship between TNF-α and proteinuria in DN. TNF-α is cytotoxic to renal cells and capable to persuade direct renal injury, apoptosis, and necrotic cell death [54]. Hence, in the current study, we found that there were significant upregulated mRNA and protein expressions of NF-κB, IL-6, and TNF-α in DN rats when compared to normal rats, and after treatment with SAC or metformin, these expressions were found downregulated. The downregulation of these inflammatory factors by SAC indicates the anti-inflammatory role and it might be playing a significant role in amelioration of renal damage which was caused by inflammation and oxidative stress due to DM.
Conclusion
In conclusion, the present study elucidates the reno-protective nature of SAC in STZ–NAD-induced DN rats and provides evidence that the protective effects are, possibly, due to the decline in oxidant and pro-inflammatory production by the renal tissues. Moreover, SAC treatment for STZ–NAD-induced DN rats exhibited a significant ameliorative potential, probably by attenuating the hyperglycemia, and thereby alleviating the ultra-structural alterations in the proximal and convoluted tubules in kidney which were confirmed by the histopathology and SEM analysis. Hence, this study suggested that SAC has a significant therapeutic action against DN.
References
Tesfaye S, Gill G (2011) Chronic diabetic complications in Africa. Afr J Diabetes Med. 19:4–8
Fernandez Millan E, Ramos S, Alvarez C, Bravo L, Goya L (2014) Microbial phenolic metabolites improve glucose-stimulated insulin secretion and protect pancreatic beta cells against tert-butyl hydroperoxide-induced toxicity via ERKs and PKC pathways. Food Chem Toxicol 66:245–253
Naidu PB, Uddandrao S, Ramavat VV, Naik R, Pothani S, Saravanan G et al (2016) Effects of S-allylcysteine on biomarkers of the polyol pathway in rats with type 2 diabetes. Can J Diabetes 40:442–448
Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F et al (2007) Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int 72:1262–1272
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (2005) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28:164–176
Mima A (2013) Inflammation and oxidative stress in diabetic nephropathy: new insights on its inhibition as new therapeutic targets. J Diabetes Res 2013:1–8
Ziyadeh FN, Wolf G (2008) Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4:39–45
Veerapur VP, Prabhakar KR, Thippeswamy BS, Bansal P, Srinivasan KK, Unnikrishnan MK (2010) Antidiabetic effect of Dodonaea viscose (L). Lacq. aerial parts in high fructose-fed insulin resistant rats: a mechanism based study. Indian J Exp Biol 48:800–810
Saravanan G, Ponmurugan P (2012) Ameliorative potential of S-allylcysteine: effect on lipid profile and changes in tissue fatty acid composition in experimental diabetes. Exp Toxicol Pathol 64:639–644
Hfaiedha N, Muratb JC, Elfekia A (2011) Protective effects of garlic (Allium sativum) extract upon lindane-induced oxidative stress and related damages in testes and brain of male rats. Pestic Biochem Physiol 100:187–192
Kim JM, Chang N, Kim WK, Chun HS (2006) Dietary S-allyl-l-cysteine reduces mortality with decreased incidence of stroke and behavioral changes in stroke-prone spontaneously hypertensive rats. Biosci Biotechnol Biochem 70:1969–1971
Zafar M, Naeem-ul-Hassan Naqvi S, Ahmed M, Kaim Khani ZA (2009) Altered liver morphology and enzymes in streptozotocin induced diabetic rats. Int J Morphol 27:719–725
Sani I, Oche O, Chiaka NG, Samuel NU (2014) Antihyperglycemic and antihyperlipidemic effects of aqueous and ethanolic leaf extracts of Vitex doniana in streptozotocin-induced diabetic rats. Res J Med Plants 8:178–186
Parim B, Harishankar N, Balaji M, Pothana S, Sajjalaguddam RR (2015) Effects of Piper nigrum extracts: restorative perspectives of high fat diet induced changes on lipid profile, body composition, and hormones in Sprague–Dawley rats. Pharm Biol 53:1318–1328
Meriga B, Parim B, Chunduri VR, Naik RR, Nemani H, Suresh P, Ganapathy S, Sathibabu U (2017) Antiobesity potential of Piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr Metab 14:72
Fraga CG, Leibouitz BE, Toppel AL (1988) Lipid peroxidation measured as TBARS in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med 4:155–161
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Annu Rev Biochem 202:384–387
Beutler E, Kelly BM (1963) The effect of sodium nitrate on RBC glutathione. Experientia 19:96–97
Aseni M, Sastre J, Pallardo FV, Lloret A, Lehner M, Garciade-la Asuncion J (1999) Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol 299:267–276
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of SOD. Indian J Biochem Biophys 21:130–132
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Paglia D, Valentine W (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Saravanan G, Ponmurugan P (2011) Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats. Chem Biol Interact 189:100–106
Brahmanaidu P, Sathibabu Uddandrao VV, Sasikumar V, Naik RR, Pothani S, Saravanan G et al (2017) Reversal of endothelial dysfunction in aorta of streptozotocin–nicotinamide-induced type-2 diabetic rats by S-allylcysteine. Mol Cell Biochem 43:225–232
Larson MO, Wilken M, Gotfredsen CF, Carr RD, Svendsen O, Roli B (2002) Mild streptozotocin diabetes in the Gottingen minipig. A novel model of moderate insulin deficiency and diabetes. Am J Physiol Endocrinol Metab 282:1342–1351
Calabresi P, Chabner BA (1991) Antineoplastic agents. In: Goodman A, Gilman's (eds) The pharmacological basis of therapeutics, vol 8. Pergamon Press, New York, pp 1209–1263
Augusti KT, Sheela CG (1996) Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Experientia 52:115–120
Naidu PB, Sathibabu Uddandrao VV, Naik RR, Suresh P, Meriga B, Begum MS, Ganapathy Saravanan (2016) Ameliorative potential of gingerol: promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol 419:139–147
Sathibabu Uddandrao VV, Brahmanaidu P, Saravanan G (2017) Therapeutical perspectives of S-allylcysteine: effect on diabetes and other disorders in animal models. Cardiovasc Hematol Agents Med Chem 15:71–77
Khanra R, Dewanjee S, Dua T, Sahu R, Gangopadhyay M, De Feo V, Zia-Ul-Haq M (2015) Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med 13:1–14
Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC (2006) Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment. BMC Nephrol 7:6
Gyawali P, Shrestha R, Poudel B, Sigdel M, Regmi P (2008) Serum urea and creatinine in diabetic and non-diabetic subjects. J Nepal Assoc Med Lab Sci 9:11–12
Ma ST, Liu DL, Deng JJ, Niu R, Liu RB (2013) Effect of arctiin on glomerular filtration barrier damage in STZ-induced diabetic nephropathy rats. Phytother Res 27:1474–1480
Yamashita H, Nagai Y, Takamura T, Nohara E, Kobayashi K (2002) Thiazolidinedione derivatives ameliorate albuminuria in streptozotocin-induced diabetic spontaneous hypertensive rat. Metabolism 51:403–408
Palsamy P, Subramanian S (2011) Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2–Keap1 signaling. Biochem Biophys Acta 1812:719–731
Navarro CM, Montilla PM, Martin A, Jimenez J, Utrilla PM (1993) Free radicals scavenger and antihepatotoxic activity of Rosmarinus. Plant Med Phytother 59:312–314
Sathibabu Uddandrao VV, Brahmanaidu P, Meriga B, Saravanan G (2016) The potential role of S-allylcysteine as antioxidant against various disorders in animal models. Oxid Antioxid Med Sci 5:79–86
Sathibabu Uddandrao VV, Brahmanaidu P, Nivedha PR, Vadivukkarasi S, Saravanan G (2018) Beneficial role of some natural products to attenuate the diabetic cardiomyopathy through Nrf2 pathway in cell culture and animal models. Cardiovasc Toxicol 18:199–205
Rameshreddy P, Sathibabu Uddandrao VV, Brahmanaidu P, Vadivukkarasi S, Ravindarnaik R, Saravanan G et al (2017) Obesity-alleviating potential of asiatic acid and its effects on ACC1, UCP2, and CPT1 mRNA expression in high fat diet-induced obese Sprague–Dawley rats. Mol Cell Biochem 442:143–154
Lery V, Zaltzber H, Ben-Amotz A, Kanter Y, Aviram M (1999) Carotene affects antioxidant status in non-insulin dependent diabetes mellitus. Pathophysiology 6:157–162
Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–410
Ewis SA, Abdel Rahman MS (1995) Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. J Appl Toxicol 15:387–390
Loven D, Schedl H, Wilson H, Daabees TT, Stegink LD, Diekus M (1986) Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozotocin induced diabetes. Diabetes 35:503–507
Saxena AK, Srivastava P, Kale RK, Baquer NZ (1993) Impaired antioxidant status in diabetic rat liver: effect of vanadate. Biochem Pharmacol 45:539–542
Wohaieb SA, Godin DV (1987) Alterations in free radical tissue-defense mechanisms in streptozotocin-induced diabetes in rat: effect of insulin treatment. Diabetes 36:1014–1018
Sozmen BY, Sozmen B, Delen Y, Onat T (2001) Catalase/superoxide dismutase (SOD) and catalase/paraoxonase (PON) ratios may implicate poor glycemic control. Arch Med Res 32:283–287
Yan H, Harding JJ (1997) Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem J 328:599–604
Haliga R, Mocanu V, Paduraru I, Stoica B, Oboroceanu T, Luca V (2009) Effects of dietary flaxseed supplementation on renal oxidative stress in experimental diabetes. Rev Med Chir Soc Med Nat Iasi 113:1200–1204
Juan Navarro-Gonzalez F, Carmen M-F (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19:433–442
Hayden MS, Ghosh S (2008) Shared principles in NF-kappa-B signaling. Cell 132:344–362
Sanz AB, Sanchez-Nino MD, Izquierdo MC, Jakubowski A, Justo P, Blanco-Colio LM et al (2010) TWEAK activates the non-canonical NFkappa B pathway in murine renal tubular cells: modulation of CCL21. PLoS One 5:8955
Dalla Vestra M, Mussap M, Gallina P, Brueghin M, Cernigoi AM, Saller A. Plebani M, Fioretto P (2005) Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol 16:78–82
Navarro JF, Mora C, Maca M, Garca J (2003) Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis 42:53–61
Boyle JJ, Weissberg PL, Bennett MR (2003) Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol 23:1553–1558
Acknowledgements
The authors thank the Department of Science and Technology (DST-SERB), Government of India (Ref no. DST/SERB/SR/SO/HS/0227/2012) and Innovation in Science Pursuit for Inspired Research (INSPIRE), Department of Science and Technology (DST). Government of India (Grant no. DST/INSPIRE/04/2016/000893) for providing the financial assistance, and also thank for the management of K. S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, for providing facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
The protocol of this study was approved by the Institutional animal ethical committee of the National Centre for Laboratory Animal Science, National Institute of Nutrition, Hyderabad (Approval no. P7F/II-IAEC/NIN/2015/GS/WNIN Rats/42M) and experiments were carried out according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Rights and permissions
About this article
Cite this article
Sathibabu Uddandrao, V.V., Brahmanaidu, P., Ravindarnaik, R. et al. Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin–nicotinamide-induced diabetic rats. Eur J Nutr 58, 2425–2437 (2019). https://doi.org/10.1007/s00394-018-1795-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1795-x