Abstract
Purpose
Lutein’s role on chronic hyperglycemia-induced oxidative stress and associated glucose homeostasis in heart and kidney is limited. Purpose of the study is to investigate the effect of lutein on cardiac and renal polyol pathway enzymes and oxidative stress markers under hyperglycemia-induced oxidative stress condition using streptozotocin (STZ)-injected rat model.
Methods
STZ-induced hyperglycemic (fasting blood glucose ≥11 mM) male Wistar rats were divided into two groups (n = 11/group). Group 1 received micellar lutein (39 nmol/day/rat) and group 2 (negative control) received micelle without lutein for 8 weeks. A separate group (no STZ injected) served as a positive control (n = 11/group). Oral glucose tolerance test (OGTT), biweekly urine glucose and activities of aldose reductase (AR) and sorbitol dehydrogenase (SDH) enzymes were assessed. Activities of antioxidant enzymes and antioxidant level were also evaluated.
Results
Lutein-administered hyperglycemic rats showed better glucose tolerance as evidenced with OGTT and biweekly urine glucose when compared to negative control. Activities of AR and SDH were decreased in heart and kidney of lutein-fed hyperglycemic rats. Also, they had significantly (p < 0.05) decreased malondialdehyde levels (66, 34, and 33 %) and increased reduced glutathione level (81, 18 and 92 %) in serum, heart and kidney, respectively. Altered antioxidant enzyme activities such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase and glutathione transferase were also affected in serum, heart and kidney of lutein-fed diabetic group.
Conclusion
Lutein prevented cardiac and renal injury in STZ-induced hyperglycemic rats due to potential amelioration of altered activities in polyol pathway and oxidative stress markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a syndrome characterized by hyperglycemia due to insulin resistance or non-secretion or both. Chronic hyperglycemia impairs glucose metabolism and develops secondary complications of diabetes [1]. Under such conditions, to maintain glucose homeostasis, metabolism of glucose deviates from glycolysis to polyol pathway resulting in fructose formation that is less catabolic than glucose [2]. Accumulation of fructose leads to increased generation of advanced glycation end products (AGEs) and reactive oxygen species (ROS) in tissue [2]. Excess ROS contributes to oxidative stress at the cellular level and is controlled by antioxidant defense system, essential for regulating normal physiological functions. ROS such as superoxide anion, hydroxyl radical and hydrogen peroxide overwhelms the antioxidant system in diabetic condition [3]. In addition, increased oxidative stress induces lipid peroxidation by striking double bonds in polyunsaturated fatty acids, resulting in more oxidative cellular damage [4]. Excessive redox active state reported to damage DNA, protein and other macromolecules [1]. Endogenous and dietary antioxidant defense molecules prevent such damage by scavenging the ROS [5]. Moreover, an inadequate intake of antioxidant nutrients may compromise antioxidant potential, thus compounding overall stress. Unfortunately, under the hyperglycemia condition, the activity of endogenous antioxidants is so limited that oxidative stress and its mediated cellular damage can become cumulative and debilitating. Increased oxidative insult paves a foundation for various organ failure including kidney and heart apart from eye and brain [6].
Polyol pathway enzymes, aldose reductase and sorbitol dehydrogenase, require NADPH and NAD+, respectively, as cofactors and compete with other NADPH and NAD+ requiring antioxidant enzymes. Increase in activity of these enzymes under chronic hyperglycemia depletes NADPH and NADP+ due to glucose flux through polyol pathway [2]. Subsequently, glutathione reductase lacks sufficient NADPH to regenerate GSH. To add up more impaired glutathione metabolism along with NAD+ and NADPH depletion disturbs the antioxidant enzyme activities and contributes to the redox imbalance [7]. All such complications observed with diabetes are reported mainly due to the altered oxidative status and glucose metabolism.
Lutein, an essential carotenoid, is well known for its protective effect against nitro-oxidative stress, inflammation and angiogenesis [8, 9]. Lutein undergoes oxidative metabolism in the body to produce several physiologically active compounds that may exhibit its own antioxidant properties [10]. Many observations state that the level of lutein in serum is low in diabetic patients, accounted for its lesser bioavailability [11]. However, biologically available antioxidants such as lutein can prevent increase in fasting blood glucose (FBG) because symptoms observed with diabetes are mainly due to chronic hyperglycemia-induced oxidative stress. Therefore, increased bioavailability of lutein using an appropriate lipid carrier can improvise the insulin sensitivity and decrease hyperglycemic aftereffects. Studies on the effect of lutein on diabetic condition have been limited to only diabetic retinopathy and neuropathy, but very scarce information on heart and kidney [12]. Therefore, we have suspended isolated pure lutein in mixed micelles to increase its bioavailability [13, 14]. Also, we have investigated whether the increased lutein bioavailability has a protective effect on oxidative imbalance and glucose flux through polyol pathway in diabetic rats. Our attempt was to establish the effect of lutein on structural abnormalities, antioxidant enzymes and polyol pathway enzymes (aldose reductase and sorbitol dehydrogenase) in kidney and heart of STZ-induced diabetic rats.
Materials and methods
Chemicals and reagents
Bovine serum albumin, butylated hydroxyl toluene (BHT), 1-chloro 2, 4-dinitrobenzene (CDNB), cholesterol, 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), lutein standard (>97 %), lysophosphatidyl choline (lysoPC), monooleoyl glycerol, oleic acid, sodium taurocholate, streptozotocin (STZ), trichloroacetic acid (TCA), TRIReagent, thiobarbituric acid (TBARS) and 1,1,3,3-tetramethoxypropane (TMP) were purchased from Sigma-Aldrich (St. Louis, USA). Analytical and HPLC—grade organic solvents and other solvents were obtained from Rankem Laboratories (Mumbai, India). Other chemicals mentioned elsewhere in the text were procured from Sisco Research Laboratories (Mumbai, India). Marigold flower and refined groundnut oil were obtained from a local super market (Mysore, India). Casein was procured from Nimesh Corporation (Mumbai, India), and all other chemicals for experimental diet preparation were obtained from Himedia Laboratories (Mumbai, India).
Lutein isolation and mixed micelles preparation
Marigold flower petals were used to extract lutein as per the protocol of Lakshminarayana et al. [15]. In brief, petals of marigold flower were cleaned in distilled water, air-dried under shade and ground into fine powder using commercial mixer. Ground powder was squashed well in ice-cold acetone with 0.1 % BHT using mortar and pestle. The extract was shaken at 100 rpm in shaking water bath (Scigenics Orbitek, India) at 40 °C till it became colorless (approximately 2 h) to isolate total carotenoids. Total extract was saponified with potassium hydroxide (30 %) at dark for 3 h. Saponified extract was phase separated with hexane and condensed using flash evaporator (Hahn-Shin, HS-2005V-N, Korea) to a volume of 5 mL. Condensed volume was subjected to open column chromatography (OCC, 20 cm 1.5 cm) packed with activated silica gel (particle size 60–120 mesh), and lutein fraction was eluted using dichloromethane:methanol (DCM:MeOH, 1:1 v/v).
Lutein was clarified by HPLC as per Lakshminaryana et al. [16]. In brief, lutein extract dissolved in mobile phase (acetonitrile:MeOH:DCM; 60:20:20, v/v/v) was detected at 444 nm on C-30 (4.6 mm × 250 mm, Princeton, USA) column with photodiode array detector (SPD-M20A, Shimadzu) by reverse phase HPLC (LC-10 Avp, Shimadzu, Japan) under isocratic condition with a flow rate of 1 mL/min.
Mixed micelles with and without lutein were used for oral intubation studies [14]. Mixed micelles with lysophosphatidyl choline (lysoPC) have been previously shown to increase the bioavailability of lutein, so used as lipid source for micelle preparation [13]. In brief, 200 µM lutein, 0.5 mM cholesterol, 3 mM lysoPC, 2.5 mM monooleoyl glycerol and 7.5 mM oleic acid in hexane were nitrogen-evaporated and vigorously mixed with PBS (pH 7.4) containing 12 mM sodium taurocholate to form micellar lutein. Mixed micelles without lutein served as a vehicle control.
Experimental design
Male Wistar rats (8 weeks old) weighing 120–130 g were kept in a room on a 12-h light–dark cycle and received ad libitum water and AIN 93G diets throughout the experiment [17]. All investigations were conducted in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) implemented through the Institutional Animal Ethical Committee of CSIR-CFTRI.
Diabetes induction
Hyperglycemia was stimulated in 12-h fasted rats (n = 22) by streptozotocin injection (36 mg/kg wt) intraperitoneally. Age-matched healthy animals (group I, n = 11 rats) were injected with vehicle control (10 mM citrate buffer, pH 4.5). After 7 days of streptozotocin injection, diabetic-confirmed rats (fasting blood glucose >11.1 mmol/L) were divided into two groups viz., diabetic control group (group II, n = 11 rats) and diabetes + lutein group (group III, n = 11 rats), respectively. Group III rats were orally intubated with 200 µL of mixed micelles containing lutein (200 µM), whereas Group I and II rats received mixed micelles alone (no lutein) for 8 weeks.
Weight gain, feed intake and urine glucose
Food and water consumption (daily) and gain in body weight (weekly) were monitored during the 8-week experiment. Also, biweekly urine was collected, and glucose was estimated using glucose oxidase method [18].
Oral glucose tolerance test
On day 48 of treatment, animals were fasted for 12 h and subjected to oral glucose tolerance test with an oral glucose load (10 g/kg body wt). Thereafter, glucose was measured at 0, 30, 60, 90, 120 and 180 min in blood drawn from caudal vein using glucose strips (Accu-Chek Active Glucose Monitor). This study did not measure insulin level since STZ injection leads to pancreatic β cell destruction i.e., in other words no plasma insulin level, and our scope is to understand effect of lutein on hyperglycemia irrespective of type I (no insulin secretion) or type II (insulin insensitivity) diabetes.
Tissue processing
Animals were killed under isofluorane anesthesia after 8 weeks (post diabetic). Cardiac puncture was done for blood collection. Blood was permitted to clot at 4 °C for 2 h. Clotted blood was centrifuged at 2600 rpm for 20 min at 4 °C, and the supernatant (serum) was collected. Kidney and heart were harvested by snap freezing. Serum and tissue samples were stored at −80 °C until analysis. Kidney and liver were homogenized with 10 volumes (w/v) of normal saline, and the supernatant was used for analysis of polyol pathway enzymes and antioxidant enzymes, whereas homogenate was used for lipid peroxidation and glutathione estimation.
Histopathology
Left kidney and heart tissue sections were H&E-stained for histopathology evaluation. In brief, tissues were fixed in 4 % formaldehyde (v/v, in PBS) overnight at 4 °C and embedded in paraffin wax. Transverse sections (10 micron) were cut using microtome (Leica Microsystems, India). Prior to H&E staining, sections were deparaffinized using xylene, dehydrated in decreasing concentration of alcohol (100, 75, 50, 30 and 10 %) followed by staining for 30 s. Stained sections were rehydrated with increasing concentrations of alcohol (10, 30, 50, 75 and 100 %) and mounted with DPX.
MDA, GSH:GSSG and ROS analysis
Malondialdehyde (MDA) level in serum, kidney and heart homogenates was measured by thiobarbituric acid reacting substance (TBARS) method [19]. Briefly, the reaction mixture consists of 200 µL biological sample in 1.5 mL acetic acid (pH 3.5, 20 %, v/v), 1.5 mL of thiobarbituric acid (0.8 %, v/v) and 200 µL of sodium dodecyl sulfate (8 %, v/v). The mixture was incubated in boiling water bath for 1 h and purified the TBARS (pink complex) with 5 mL of n-butanol (upper phase). The complex was read spectrofluorometrically (Hitachi, F-2000, Japan) with 515 nm excitation and 553 nm emission. Readings were quantified as malondialdehyde equivalents using TMP as a standard.
Reduced glutathione was monitored by the rate of formation of 5-dithio-2-nitrobenzoic acid at 412 nm [20]. In brief, serum and tissue homogenates (10 %, w/v in PBS, pH 7.4) were de-proteinized using tricarboxylic acid (10 %, v/v). De-proteinized sample (100 µL) was reacted with 50 µL of 10 mM DTNB in 4.75 mL of sodium phosphate buffer (0.1 M, pH 8.0). Formation of 5-dithio-2-nitrobenzoic acid was read spectrophotometrically (Shimadzu Corporation, UV-1800, Japan) using standard reduced glutathione at 412 nm. Oxidized glutathione was measured fluorometrically with excitation at 345 nm and emission at 425 nm as per the protocol of Hissin and Hilf [21] with slight modification. In brief, serum and tissue sample supernatant as described above for GSH was reacted with 100 µL of 40 mM N-ethylmaleimide (NEM) mL for 30 min followed by addition of 1.4 mL of 0.1 N NaOH and 100 µL OPT and incubation for 45 min at RT and taken. Standard oxidized glutathione standard is used as a reference.
ROS level was determined by the rate of conversion of DCFH-DA to fluorescent DCF as per the method of Driver et al. [22] with slight modifications. In brief, heart and kidney homogenates in ice-cold Locke’s buffer (154 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 5 mM HEPES, 2 mM CaCl2, 50 µM FeSO4 and 10 mM d-glucose; pH 7.4) incubated with 5 mM DCFH-DA for 30 min at room temperature to allow the DCFH-DA to be incorporated into any membrane-bound vesicles and the diacetate group cleaved by esterases. The conversion of DCFH to DCF was measured using a fluorescence spectrophotometer with excitation at 485 nm and emission at 530 nm. The ROS formed was calculated in relation to a DCF standard curve and expressed in pmol DCF/min/mg protein.
Polyol pathway enzyme assays
Aldose reductase (AR) activity was measured spectrophotometrically according to Kim and Oh [23]. Assay mixture contained 0.32 mM NADPH and enzyme source in phosphate buffer (0.135 M; pH 7.0). The reaction was initiated by the addition of 5.5 mM dl-glyceraldehyde and monitored by following the decrease in absorbance at 340 nm. Sorbitol dehydrogenase (SDH) activity was measured using fructose as substrate according to Gerlach and Hiby [24].Briefly, reaction initiated by adding 1.19 mM fructose to solution containing 1.2 µM NADH, 0.2 M triethanolamine buffer (pH 7.4) and sample (enzyme source).
Antioxidant enzymes
Antioxidant enzyme activities were measured in serum, kidney and heart samples. Total superoxide dismutase (SOD) activity was determined by cytochrome c reduction method [25]. Total catalase (CAT) activity was analyzed by reduction of hydrogen peroxide [26]. Glutathione peroxidase (GPx) activities were quantified by reduction of t-butyl hydroperoxide solution by NADP+ [27]. Glutathione reductase (GR) activity was determined by measuring the decrease in absorbance in a reaction mixture of 750 µL of potassium phosphate buffer (pH 7.2, 0.1 M) and 50 µL of enzyme source and incubated at 37 °C for 10 min and later 50 µL of 2 mM NADPH (in 1 % NaHCO3), and 50 µL of oxidized glutathione was added, and the absorbance at 340 nm was monitored using spectrophotometer [28]. Glutathione-S-transferase (GST) activity was determined following the formation of conjugate of GSH and CDNB at 340 nm in a reaction mixture containing 50 µL GSH (20 mM), 50 µL CDNB (20 mM), sample (50 µL) and 880 µL phosphate buffer (0.1 mM, pH 6.5) containing 1 mM EDTA. The increase in absorbance was monitored for 5 min using spectrophotometer [29].
Lutein profiling
Lutein was extracted from serum, kidney and heart homogenates according to Lakshminarayana et al. [14]. Extraction mixture contained serum (0.5 mL), DCM:MeOH (3 mL, v/v, 2:1) with 2 mM α-tocopherol and hexane (1.5 mL). Lutein was released to upper hexane/DCM phase upon centrifugation at 3000 rpm at 4 °C for 5 min. The extraction procedure was repeated three times. Extracts were pooled, evaporated to dryness using nitrogen, dissolved in mobile phase and analyzed by HPLC with the method as mentioned elsewhere. Kidney and heart homogenates (10 %, w/v with normal saline) were saponified separately with 2 mL of KOH at 60 °C for 45 min and vortexed every 15 min during saponification with an addition of 2 mL of ice-cold deionized water before lutein extraction.
Statistical analysis
The data are presented as mean values ± SD/SEM. Statistical significance between the groups was interpreted by one-way ANOVA followed by Tukey’s test using GraphPad Prism (v. 5.00). Significance level was set at p < 0.05.
Results
Lutein (95 ± 2 %) purified from marigold petals had a spectrum with λ max of 444 nm, comparable with standard lutein. Isolated lutein was used for mixed micelles preparation for animal intubation (39 nmol/rat/day in 200 µL) studies.
Fasting blood glucose, feeding efficiency, organ weight and urine output
Streptozotocin-injected rats (after 2 weeks) had fasting blood glucose (FBG) level of 13.9 ± 0.8 mmol/L which was significantly higher than the rats in group I (6.2 ± 0.2 mmol/L). Induction of diabetes resulted in decrease (10 %) in body weight gain, whereas in case of rats in group I, an increase in body weight gain (15 %) was evident. The change in body weight found to be corresponded inversely with the food and water intake of rats in group I (16 ± 1.1 g/day and 20 ± 3 mL/24 h) and group II (26 ± 2.4 g/day and 120 ± 25.3 mL/24 h).
Lutein intubation for 8 weeks to rats in group III did not alter the FBG level (12.8 ± 1.6 mmol/L), but rats in group II had significantly increased FBG (22.0 ± 0.8 mmol/L), whereas its level in rats of group I remained low at 5.0 ± 0.2 mmol/L. Decrease in FBG contradicting with the weight gain (12 %) and food and water intake of rats in group III, whereas the group II had no change in weight gain, but there was a change in food and water intake (Fig. 1). Over the experimental span, the body weight increased in group I (35 %) and group III (12 %), but did not change in group II. Further, there was detectable lutein level in serum (69 ± 18 nmol/dL), kidney (14.75 nmol/dL) and heart (1.35 nmol/g) respectively in group III rats, whereas no lutein was detected in group I and group II.
Daily food intake (left) and water intake (right) during the time course (0–8 weeks) of lutein treatment between groups. Values are mean ± SD. aValues differ significantly (p < 0.05) from group I and bvalues differ significantly (p < 0.05) from group II. Group I = normal rats, group II = diabetic control rats and group III = diabetic rats fed with lutein
Biweekly urine glucose was measured to assess the severity of diabetes upon STZ injection. Throughout the experimental span, urine glucose was significantly higher (p < 0.05) in group II (4.4 ± 0.8–7.2 ± 0.4 g/24 h) compared to group I (0.1 ± 0.0–0.2 ± 0.0 g/24 h). Intubation of lutein to group III resulted in lower urine glucose level (day 56, 55 %) than group II (Fig. 2), indicating an inverse correlation between circulating lutein and urine glucose.
Effect of lutein on biweekly urine glucose levels. Values are mean ± SD (n = 11/group). aValues differ significantly (p < 0.05) from group I and bvalues differ significantly (p < 0.05) from group II. Group I = normal rats, group II = diabetic control rats and group III = diabetic rats fed with lutein
Results on the oral glucose tolerance test also showed significant decrease in blood glucose level at 30 min (27 %) in rats of group III when compared to rats in group II, but at other time intervals, no significant change was observed. Rats in group II and group III both had significantly higher (p < 0.05) glucose level at all observed intervals of time when compared to rats in group I (Fig. 3). Rats in group III had highest blood glucose level (3.5-fold) at 60 min than rats in group I (131 ± 0.98 mg/dL), whereas rats in group II had highest blood glucose level (four fold compared to rats in group I, 129 ± 0.23 mg/dL) at 30 min. Results imply better glucose tolerance in rats of group III compared to rats in group II.
Effect of lutein on oral glucose tolerance (after oral glucose load of 10 g/kg body weight) on 48th day of treatment. Values are mean ± SD (n = 11/group). aValues differ significantly (p < 0.05) from group I and bvalues differ significantly (p < 0.05) from group II. Group I = normal rats, group II = diabetic control rats and group III = diabetic rats fed with lutein
Aldose reductase and sorbitol dehydrogenase activity
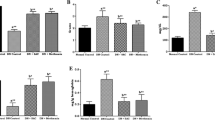
The activities of AR and SDH were investigated in heart and kidney and are illustrated in Fig. 4. Kidney and heart of rats in group II showed an increase (p < 0.05) in activity of AR (1.7- and 0.8-fold) and SDH (0.4- and 0.9-fold), respectively, when compared to rats in group I. However, activity of AR (0.3- and 0.2-fold) and SDH (0.2- and 0.3-fold) was decreased in both kidney and heart of rats in group III compared to rats in group II.
Oral lutein administration prevented activities of aldose reductase and sorbitol dehydrogenase in kidney (a, b) and heart (c, d) of experimental diabetic rats. Values are mean ± SD (n = 11/group). aValues differ significantly (p < 0.05) from group I and bvalues differ significantly (p < 0.05) from group II. Group I = normal rats, group II = diabetic control rats and group III = diabetic rats fed with lutein
MDA level and antioxidant status
Induction of diabetes resulted in elevated levels of MDA in serum, kidney and heart (3.1-, 1.6- and 1.5-fold), respectively, in rats of group II when compared to rats in group I, whereas its level was decreased significantly (0.3-, 0.7- and 0.7-fold) in rats of group III when compared to rats in group II is comparable with rats of group I. Similarly, rats in group II had decreased level of GSH in serum and kidney (0.4- and 0.6-fold) compared to rats in group I. GSH levels in rats of group III was elevated significantly (1.8- and 1.9-fold) toward its levels in rats of group I when compared to rats in group II. However, no change was evident in glutathione levels in heart (Fig. 5) between rats of all groups. Results indicate that lutein increases the glutathione level and prevents lipid peroxidation in kidney, whereas it may directly scavenge lipid peroxides without affecting glutathione level in heart under diabetic condition.
Effect of lutein on lipid peroxides (MDA, left) level and glutathione (right) level in serum (a), kidney (b) and heart (c) of diabetic rats. Values are mean ± SD (n = 11/group). aValues differ significantly (p < 0.05) from group I and bvalues differ significantly (p < 0.05) from group II. Group I = normal rats, group II = diabetic control rats and group III = diabetic rats fed with lutein
Effects of lutein intubation on the activities of radical scavenging enzymes such as SOD, CAT, GPx, GST and GR in diabetic rats were investigated (Table 1). In serum and kidney, there was an increased activity of SOD (35 and 22 %), CAT (130 and 42 %), GR (112 and 19 %) and GST (40 and 24 %), whereas decreased activity of GPx (31 and 65 %) was observed in rats of group II, when compared to rats in group I, but heart exhibited a different trend of antioxidant status with decreased activity of SOD (28 %),CAT (26 %), GPx (45 %), GR (40 %) and GST (30 %), respectively, in rats of group II when compared to rats in group I. Amelioration of altered antioxidant enzymes was evident in rats of group III when compared to rats in group II and was comparable with that of rats in group I.
Histopathogical characteristics of heart and kidney
STZ-induced hyperglycemic rats were found to develop pathological changes in kidney and heart (Fig. 6). Results show that STZ-induced hyperglycemia caused more interstitial space and increased number of intercalated disk structures in heart sections of group II. Kidney sections reveal more of macrophage infiltration into Bowman capsule along with capsule thickening in rats of group II when compared to rats in group I. Lutein treatment for 8 weeks showed improved glomerular capillary size in kidney and reduced interstitial space in heart. An increase in the weight of kidney (12 %) and decrease in the heart weight (25 %) was evident in diabetic rats compared to control rats. Treatment of lutein to diabetic rats resulted in no significant change in kidney or heart weight when compared to rats in group II (Table 2).
Lutein prevented pathophysiology of heart (above) and kidney (below) (H&E, ×200 magnification) of streptozotocin-induced diabetic rats. Arrow symbol indicates intercalated space in heart sections and dotted arrow indicates thickened Bowman’s capsule due to macrophage infiltration in kidney. Group 1 = normal rats, group II = diabetic control rats, group III = lutein-fed diabetic rats
Discussion
Streptozotocin-induced diabetic rat is an acceptable model for endogenous chronic oxidative stress studies [30]. Kidney and heart are considered to be one among the main targets for examinations of alterations associated with chronic hyperglycemia since they are markedly sensitive to cellular damage and insult [31]. Cellular damage reported to occur due to impaired antioxidant mechanism which also shown to have role in the development of insulin resistance [32]. Reducing oxidative stress with feeding antioxidant shown to reverse insulin resistance by inhibiting redox sensitive phosphorylation and downregulation of insulin receptor substrate-1 [33]. The CARDIA investigation revealed that higher serum carotenoid levels were correlated with reduced complications of diabetes in insulin resistance in non-smokers [34]. In this study, we have examined the effect of lutein intubation on key enzymes of polyol pathway and oxidative stress markers in heart and kidney of STZ-induced diabetic rats. Results showed that intubation of antioxidant lutein to diabetic rats for 8 weeks reduced FBG. This may be due to increased lutein absorption. Increased lutein bioavailability depends on the potential incorporation of absorbable mixed micelles in enterocytes [35]. Baskaran et al. [13] and Lakshminarayana et al. [14] have shown oleic acid micelles significantly improve the plasma response of lutein. The bioavailability of lutein observed in this study may be due to the intubated micellar lutein. Bioavailable antioxidants improve glucose regulation [34].
Diabetic rats were characterized by decreased body weight, enlarged kidney and reduced heart size as compared to normal control rats. These features are typical signs of diabetic complications [36]. Lutein is shown to have no harmful effect on kidney and heart, but with slight increase in body weight (12 %) though no significant (Table 2) between day 1 and day 56 of treatment. Oral administration of lutein had a better glucose tolerance and is also evidenced with lower urine glucose levels. Possibly lutein’s antiglycemic effect uniting with calorie restriction may extend life span in diabetic patients. In this regards, further studies are needed. Further, as in the case of lutein as observed in the present study, curcumin has been reported to ameliorate hyperglycemic complications [37].
Diabetic rats exhibited hypertrophied glomerulus, thickening of Bowman’s capsule and inner wrinkled lining in kidney morphology, whereas heart exhibited increased intercalated space between intercalated disk [38, 39]. Heart sections of lutein-fed diabetic rats revealed reduced intercalated space and also decrease in number of intercalated disk. Kidney sections of lutein-fed diabetic animals had decreased Bowman’s capsule thickening compared to diabetic rats. It is evident from the results, an ameliorating effect of lutein on structural abnormalities associated with diabetes as reported by Muruganandan et al. [40].
Chronic hyperglycemia induces non-enzymatic modification of simple sugars on other proteins leading to the formation of AGEs. AGEs accumulation in the tissue leads to the generation of ROS, a possible mechanism causing oxidative stress [41]. Glucose flux from glycolysis to polyol pathway is the main culprit in increased oxidative stress (Fig. 7) [2]. Therefore, effect of feeding lutein on aldose reductase and sorbitol dehydrogenase activities in kidney and heart was quantified. Diabetic rats (group II) had significantly higher AR and SDH activities in both kidney and heart compared to normal control rats (Fig. 3). Increased activities of AR depleted NADPH and may be resulted in decreased GSH. Intubation with lutein to diabetic rats resulted normalization of AR and SDH toward group I (normal control). It appears that biologically available lutein exhibits antihyperglycemic effect as evidenced by decreased FBG and also decreased food and water intake in the group II (diabetic control) rats. Decreased FBG may have accounted for lower activity of AR and SDH in both liver and kidney of lutein-fed group. As in the case of lutein, curcumin was found to decrease AR activity without any effect on SDH and FBG in diabetic rats [37]. In that way, lutein may be a superior antioxidant molecule with antihyperglycemic potential. Bioavailability of lutein decreased FBG and might have accounted for the decreased AR, SDH and blood glucose in the lutein-fed diabetic rats. Further, the superior antihyperglycemic potential of lutein compared with curcumin may be due to the structural and functional difference between these molecules.
Inferring the MDA and GSH levels and elucidating the changes in the activities of antioxidant enzymes are good markers of oxidative stress implication [4]. Increased MDA level and decreased GSH level in group II compared with group I suggest that there is an increased oxidative stress in kidney and heart of diabetic rats which may be due to elevated glucose levels (Fig. 4). Antioxidants reported to modulate the oxidative stress. Alpha-tocopherol increased mRNA level of gamma-glutamylcysteine synthetase, rate limiting enzyme in glutathione biosynthesis [42]. As in the case of α-tocopherol, lutein being an antioxidant, its activity might have increased the glutathione level. However, in-depth studies are necessary to conclude this mechanism. Moreover, decreasing the activities of AR and SDH might have also contributed in part for the decrease in GSH level and ultimately MDA level. Lutein treatment prevented the alterations similar with that of turmeric and curcumin in diabetic cataract [37]. The exact action of lutein on these enzyme activities needs further investigations at molecular level.
In the present study, diabetic rats also exhibited decreased activities of GPX in serum, kidney and heart. Possible reason for the reduced GPX activity may be due to the depletion of reduced GSH, whereas SOD, CAT, GR and GST activities were increased in serum and kidney, but heart displayed decreased activities of all these enzymes in diabetic condition. Upon intubation of lutein, the activity of these enzymes was modulated toward the normal control rats (Table 1). Possible reason for this effect may be the increased GSH level in lutein-treated diabetic rats as evidenced with α-tocopherol in diabetic lens [42].
Because of the protective action of lutein against STZ-induced heart and kidney damage, it is possible to conclude that glucose tolerance could be one of the reasons for the decrease in oxidative stress markers studied in the lutein-treated diabetic animals. However, the possibility of antioxidant activity of lutein could not be ruled out for the potent protection offered in lutein-administered diabetic animals.
Conclusion
Due to potent antioxidant nature and bioavailability of lutein when given with mixed micelles, lutein ameliorates glucose intolerance by decreasing the activities of AR and SDH. Decreased polyol pathway replenishes GSH:GSSG pool and ameliorates the altered antioxidant defense system in heart and kidney of diabetic rats. Lutein or any other antioxidant is not sufficient enough to completely prevent diabetic-mediated pathophysiological complications, but they seem to be an appropriate coadjuvant treatment for the impairments associated with chronic hyperglycemia mediated oxidative stress.
References
Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J (2000) Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 26(3):163–176
Bantle JP (2009) Dietary fructose and metabolic syndrome and diabetes. J Nutr 139:1263S–1268S. doi:10.3945/jn.108.098020
Ceriello A (2000) Oxidative stress and glycemic regulation. Metabolism 49:27–29. doi:10.1016/S0026-0495(00)80082-7
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57:715S–724S
Johansen JS, Harris AK, Rychly DJ, Ergul A (2005) Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 4:5. doi:10.1186/1475-2840-4-5
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9. doi:10.2337/diabetes.48.1.1
Ying W (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10:179–206. doi:10.1089/ars.2007.1672
Chew BP, Brown CM, Park JS, Mixter PF (2003) Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res 23:3333–3339
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23:171–201. doi:10.1146/annurev.nutr.23.011702.073307
Nidhi B, Sharavana G, Ramaprasad TR, Vallikannan B (2015) Lutein derived fragments exhibit higher antioxidant and anti-inflammatory properties than lutein in lipopolysaccharide induced inflammation in rats. Food Funct. doi:10.1039/C4FO00606B
Hu B-J, Hu Y-N, Lin S, Ma W-J, Li X-R (2011) Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol 4:303–306. doi:10.3980/j.issn.2222-3959.2011.03.19
Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K et al (2010) Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 53:971–979. doi:10.1007/s00125-009-1655-6
Baskaran V, Sugawara T, Nagao A (2003) Phospholipids affect the intestinal absorption of carotenoids in mice. Lipids 38:705–711. doi:10.1007/s11745-003-1118-5
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V (2006) Enhanced lutein bioavailability by lyso-phosphatidylcholine in rats. Mol Cell Biochem 281:103–110. doi:10.1007/s11010-006-1337-3
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V (2007) Lutein and zeaxanthin in leafy greens and their bioavailability: olive oil influences the absorption of dietary lutein and its accumulation in adult rats. J Agric Food Chem 55:6395–6400. doi:10.1021/jf070482z
Lakshminarayana R, Raju M, Krishnakantha TP, Baskaran V (2005) Determination of major carotenoids in a few Indian leafy vegetables by high-performance liquid chromatography. J Agric Food Chem 53:2838–2842. doi:10.1021/jf0481711
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Huggett ASG, Nixon DA (1957) Use of glucose oxidase, peroxidase and o-dianisidine in determination of blood and urinary glucose. Lancet 270(6991):368–370. doi:10.1016/S0140-6736(57)92595-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. doi:10.1016/0003-2697(79)90738-3
Owens CW, Belcher RV (1965) A colorimetric micro-method for the determination of glutathione. Biochem J 94:705–711. doi:10.1042/bj0940705
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226. doi:10.1016/0003-2697(76)90326-2
Driver AS, Kodavanti PRS, Mundy WR (2000) Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181. doi:10.1016/S0892-0362(99)00069-0
Kim HY, Oh JH (1999) Screening of Korean forest plants for rat lens aldose reductase inhibition. Biosci Biotechnol Biochem 63:184–188. doi:10.1271/bbb.63.184
Gerlach U, Hiby W (1974) Sorbitol dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3. Academic Press, New York, pp 569–573
Flohe L, Otting F (1984) Superoxide dismutase assays. Methods Enzymol 105:93–104
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 175:408–413. doi:10.1016/0003-2697(88)90564-7
Warholm M, Guthenberg C, von Bahr C, Mannervik B (1985) Glutathione transferases from human liver. Methods Enzymol 113:499–504
Low PA, Nickander KK, Tritschler HJ (1997) The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes 46:S38–S42. doi:10.2337/diab.46.2.S38
Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847. doi:10.1161/01.CIR.97.18.1837
Vincent HK, Bourguignon CM, Weltman AL, Vincent KR, Barrett E, Innes KE et al (2009) Effects of antioxidant supplementation on insulin sensitivity, endothelial adhesion molecules, and oxidative stress in normal-weight and overweight young adults. Metabolism 58:254–262. doi:10.1016/j.metabol.2008.09.022
Evans JL, Maddux BA, Goldfine ID (2005) The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 7:1040–1052. doi:10.1089/ars.2005.7.1040
Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee D-H (2007) Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the coronary artery risk development in young adults (CARDIA)/young adult longitudinal trends in antioxidants (YALTA). Clin Chem 53:447–455. doi:10.1373/clinchem.2006.074930
Faulks RM, Southon S (2005) Challenges to understanding and measuring carotenoid bioavailability. Biochim Biophys Acta 1740:95–100. doi:10.1016/j.bbadis.2004.11.012
Mogensen CE (1987) Early diabetic renal involvement and nephropathy. The diabetes annual, vol 3, pp 306–324
Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB (2005) Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Investig Ophthalmol Vis Sci 46:2092. doi:10.1167/iovs.04-1304
Romero MJ, Yao L, Sridhar S, Bhatta A, Dou H, Ramesh G et al (2013) L-citrulline protects from kidney damage in type 1 diabetic mice. Front Immunol 4:480. doi:10.3389/fimmu.2013.00480
Kumar V, Ahmed D, Gupta PS, Anwar F, Mujeeb M (2013) Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of Melastoma malabathricum Linn. leaves in streptozotocin induced diabetic rats. BMC Complement Altern Med 13:222. doi:10.1186/1472-6882-13-222
Muruganandan S, Gupta S, Kataria M, Lal J, Gupta P (2002) Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology 176:165–173. doi:10.1016/S0300-483X(02)00069-0
Mohamed AK, Bierhaus A, Schiekofer S, Tritschler H, Ziegler R, Nawroth PP (1999) The role of oxidative stress and NF-κB activation in late diabetic complications. BioFactors 10:157–167. doi:10.1002/biof.5520100211
Costagliola C, Iuliano G, Menzione M, Rinaldi E, Vito P, Auricchio G (1986) Effect of vitamin E on glutathione content in red blood cells, aqueous humor and lens of humans and other species. Exp Eye Res 43:905–914. doi:10.1016/0014-4835(86)90069-2
Acknowledgments
The authors are grateful to Dr. Mahenderkar, ex-chief Editor, Journal of Food Science and Technology, for editing the manuscript with respect to English language. This work was financially supported by the Major Laboratory Project, CSIR-Central Food Technological Research Institute, Mysore, India. Gurunathan Sharavana acknowledges the award of Senior Research Fellowship by the Department of Biotechnology, Government of India, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharavana, G., Joseph, G.S. & Baskaran, V. Lutein attenuates oxidative stress markers and ameliorates glucose homeostasis through polyol pathway in heart and kidney of STZ-induced hyperglycemic rat model. Eur J Nutr 56, 2475–2485 (2017). https://doi.org/10.1007/s00394-016-1283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1283-0