Abstract
Purpose
This study aimed to determine the effects of fish oil-derived n-3 PUFA on glycemic control and lipid profiles in type 2 diabetic patients with abdominal obesity.
Methods
In a randomized, double-blind, placebo-controlled trial, 100 type 2 diabetic patients with abdominal obesity were randomized into two groups including 4 g/day of fish oil (2.4 g n-3 PUFA) or placebo (corn oil) for 6 months. Serum fatty acid, body composition, as well as markers of glucose regulation and lipid parameters were measured before and after intervention.
Results
Thirty-five men and 64 women aged 65.4 ± 5.3 years completed the intervention. Although body composition was unchanged, serum EPA and DHA were higher in the fish oil group than those in the placebo group (P < 0.001 and P < 0.001, respectively). Serum triglyceride (TG) decreased (P = 0.007), whereas high-density lipoprotein cholesterol (HDL-C) increased (P = 0.006) in the fish oil group compared with the placebo group after 6 months. Serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), the ratio of LDL-C to HDL-C, and glycemic control (measured by serum glucose, glycated hemoglobin, insulin, and homeostasis model assessment–insulin resistance) were not significantly different between the two groups after 6 months.
Conclusions
This study showed that 6 months of fish oil supplement had no statistically significant effects on glycemic control, but improved TG and HDL-C in type 2 diabetic patients with abdominal obesity.
Trial registration
Chictr.org ChiCTR-TRC-14005084.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is currently one of the most important chronic noncommunicable diseases and has become an enormous worldwide medical challenge; thus, it has turned into a major public health issue. Staggeringly, the number of adults with diabetes in the world increased from 108 million in 1980 to 422 million in 2014 [1]. The estimated prevalence of diabetes in a representative sample of Chinese adults was 11.6%, making China the country with the highest prevalence of diabetes in Asia and the largest absolute disease burden for diabetes in the world [2]. Obesity, especially abdominal obesity, is a major contributor to insulin resistance and type 2 diabetes [3]. Most people with type 2 diabetes are obese, and obesity itself can directly cause some degree of insulin resistance. Interestingly, Greenland Eskimos who consumed large amounts of fish have a remarkably low incidence of diabetes even when being obese [4]. This potential beneficial effect has been ascribed to the high n-3 polyunsaturated fatty acids (PUFA) content of fish oil: eicosapentaenoic acid (EPA, 20:5 n-3) and docosahexaenoic acid (DHA, 22:6 n-3) [5], although one recent study has reported that it relates to genetic and physiological adaptations to a diet rich in PUFA [6].
Subsequent prospective studies on the impact of fish intake and risk of type 2 diabetes have produced inconsistent outcomes, with the risk of type 2 diabetes increased in some [7–9], but decreased in others [10–12]. The recent systematic review and meta-analysis provided evidence that marine n-3 PUFA had protective effects on type 2 diabetes in Asian populations, but was positively associated with risk of type 2 diabetes in Western populations [13]. In addition, the majority of randomized clinical trials support the triglyceride (TG)-lowering effect of fish oil supplementation in type 2 diabetic patients [14], but the effects on glycemic control are still inconclusive: Fasting blood glucose was either deteriorated [15–18] or unchanged [19–24]. It is important to note, however, that these studies are limited by small sample size and short trial duration. Moreover, there are few randomized controlled trials in Asian type 2 diabetic patients.
To our knowledge, the effects of fish oil-derived n-3 PUFA on glucose and lipid metabolism have not yet been studied in type 2 diabetic patients with abdominal obesity. Therefore, we performed a randomized, double-blind, placebo-controlled trial to evaluate the effects of 6-month supplementation of fish oil on glycemic control and lipid profiles in Chinese type 2 diabetic patients with abdominal obesity.
Methods
Study population
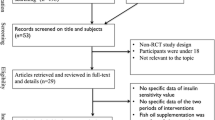
We recruited participants from the diabetes clinic at the Guanlin hospital in Yixing City, China, and performed the trial from September 2014 to March 2015. All participants had known type 2 diabetes as defined by World Health Organization (WHO) criteria (fasting glucose ≥7.0 mmol/L or 2-h postprandial glucose ≥11.1 mmol/L) and abdominal obesity as measured by Working Group on Obesity of China (WGOC) criteria (waist circumference ≥85 cm for men and ≥80 cm for women) [25]. Exclusion criteria included using n-3 PUFA supplements or lipid-lowering treatment, allergy to fish oil, gastrointestinal disorders, pregnancy or lactation, malignant tumor, hyperthyroidism, or other serious diseases known to influence glucose or lipid metabolism. The study protocol was approved by the ethic committee of Zhongda Hospital Affiliated Southeast University, and written informed consent was obtained from each participant prior to enrollment. This trial has been registered in the Chinese clinical trial registry at http://www.chictr.org.cn with the following registration number: ChiCTR-TRC-14005084.
Study design and intervention
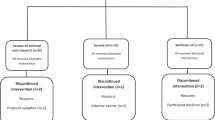
We conducted a randomized, double-blind, placebo-controlled trial of parallel design to evaluate the effects of fish oil-derived n-3 PUFA supplementation on blood glucose control and lipid levels. One hundred subjects were randomly assigned to four 1-g capsules/d fish oil (containing 1.34 g EPA and 1.07 g DHA) or a placebo control (four identical-looking capsules/d that contained corn oil) for 6 months. The fatty acid composition of the two types of oil is given in Table 1. Compliance was monitored by monthly check-ins and returning empty bottles during the study period. Participants were asked to swallow the whole capsules before their main meals to avoid unmasking and maintain stable medications, diet pattern, and physical activity throughout the supplement period. The sample size was calculated after fixing the probability of type 1 error at 0.05 and that of type 2 error at 0.10 for changes in fasting glucose (mean difference = 1.0 mmol/L, SD = 1.5 mmol/L). The randomization was developed using a computer-generated random numbers. Both participants and investigators were blinded for treatment allocation until the completion of the final data analysis.
Measurements
Waistline, hipline, height, systolic and diastolic blood pressure were measured according to standardized protocols. Serum fatty acid composition and body composition were determined before and after 6 months of fish oil supplementation for each subject. For serum fatty acid analysis, a total of 1 mL fasting serum samples were mixed with 100 μL BHT methanol (100 μg/mL) and 2 mL 20% sulfuric acid methanol solution and then incubated with 80 °C for 10 min. After cooling to room temperature, the 2 mL hexane and saturated sodium chloride were added to the solution, respectively. The solution was mixed thoroughly and stood for about 30 min before centrifuging at 3000 r/min for 15 min. The 1 mL supernatant was dried under nitrogen and then dissolved in 100 μL hexane for analysis. Concentrations of fatty acids in serum and capsules were detected by gas chromatography (Agilent, 6890 N, USA). Body composition was assessed by bioelectrical impedance analysis (Tanita, BC-420, Japan).
Measurements in the hospital were performed at baseline, after 3 and 6 months of intervention. In all visits, fasting venous blood sample was obtained from each study subject to measure serum glucose, glycated hemoglobin (HbA1c), insulin, TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels.
Assessments of serum fasting glucose and lipids parameters (including TG, TC, HDL-C, and LDL-C) were performed by automatic biochemical analyzer (Beckman, DxC800, USA). HbA1c concentration was determined by chromatography (Bio-Rad, D10, USA) and insulin by chemiluminescence (Roche, FG_cobase 8000, Switzerland). The insulin resistance was estimated using homeostasis model assessment–insulin resistance (HOMA-IR) formula (Fasting insulin × Fasting glucose/22.5).
Statistical analysis
Measurement data are presented as mean ± SD for normally distributed data sets or median (with interquartile range) for skewed data sets. Skewed data were logarithmically transformed prior to analysis and back transformed for presentation in tables. Assumptions of normality were checked by using the Kolmogorov–Smirnov test. Categorical data are presented as frequencies and percentages. Student’s t test (for normally distributed variables), Mann–Whitney U test (for skewed variables), or Chi square test was used to compare groups at baseline as appropriate. The end values of the outcomes were compared by using two factors repeated-measures ANOVA. A model of analysis of covariance (ANCOVA) with the baseline value as a covariate was used to evaluate the effects of fish oil supplementation on serum fatty acid content and body composition, because these data were sampled only at two time points (at baseline and at the end of the intervention). Calculations were performed in PASW statistics 18.0 (SPSS Inc, Chicago, IL, USA). A value of P < 0.05 was considered as statistically significant.
Results
Baseline characteristics
Of the 100 subjects randomized in the trial, 99 subjects completed the final follow-up after 6-month supplementation and one subject assigned to the fish oil group left the study due to his own plan of moving out. Data from this subject were excluded from all results. No side effects were reported.
The baseline characteristics of the study population are shown in Table 2. Their overall mean age was 65.4 ± 5.3 years, and the majority (64.6%) of the subjects were women. Subjects randomly assigned to receive fish oil or placebo were similar in demographics.
Serum n-3 PUFA composition
Subjects receiving fish oil had a median (25th, 75th percentile) EPA and DHA of 0.55% (0.16, 1.35) and 2.15% (0.65, 3.61) at baseline, which was increased to 2.15% (1.21, 2.73) and 5.28% (3.42, 6.79) after 6 months of treatment, respectively (Fig. 1). Relative to the placebo group, fish oil significantly increased EPA and DHA (P < 0.001 and P < 0.001, respectively) after the 6-month follow-up.
Median concentrations of a EPA and b DHA in serum before and after 6 months of intervention. EPA eicosapentaenoic acid, DHA docosahexaenoic acid. a,bSignificant difference between 0 month and 6 months within group (P < 0.001), calculated by paired t test. x,ySignificant difference between groups on 6 months (P < 0.001), calculated by ANCOVA with the baseline value as the covariate
Body composition
Table 3 shows that neither fish oil nor placebo significantly affected body composition during the intervention.
Glycemic control
Fasting serum glucose, HbA1c, and insulin were not significantly influenced by fish oil treatment when compared with placebo (Table 4). Similarly, the insulin resistance determined by HOMA remained unchanged.
Lipid profiles
The TG concentration was significantly lower (−21.25% compared with 2.89% in the placebo group after 6 months; P = 0.007), with the largest reduction occurring in the first 3 months, followed by a progressive reduction later on (Table 4). However, the HDL-C concentration was significantly higher (P = 0.006) after 6 months of intervention, rose from 1.36 ± 0.35 on baseline to 1.56 ± 0.39 mmol/L on 6 months. Serum TC, LDL-C, and the ratio of LDL-C to HDL-C were not significantly affected by fish oil treatment when compared with placebo.
Discussion
In this randomized, double-blind, placebo-controlled trial, fish oil-derived n-3 PUFA supplementation at a dose of 2.4 g/day for 6 months significantly decreased TG and increased HDL-C in type 2 diabetic patients with abdominal obesity. Rather, we found no changes in TC, LDL-C, ratio of LDL-C to HDL-C, body composition, and glycemic control (serum glucose, HbA1c, insulin, and HOMA-IR) due to the consumption of fish oil. Significant alterations were noted in serum EPA and DHA, suggesting that subjects complied with fish oil supplementation.
In our study, serum glucose control was neither deteriorated nor ameliorated by moderate doses of fish oil supplementation. In contrast, researchers discovered the deleterious effects of fish oil on glucose control between the 1980s and the 1990s [15–17]. Popp-Snijders [26] noticed these adverse effects are predominantly occurred when the dosage is high, subjects are obese, and no oral diabetic medication is prescribed. Given the limitation of small sample size and short duration, the results should be interpreted with some caution. Further studies of doses of 2–3 g/day of n-3 PUFA showed no such deleterious impact [27–30], even if the subjects are obese [20, 22, 31] or Asians [32–34]. The lack of positive results might be explained by the long duration of insulin resistance in type 2 diabetic patients in addition to relatively short-term experimental periods. But results of a recent study have shown that treatment with a daily dose of 3.9 g n-3 PUFA for 9 months did not alter glycemic control in subjects with impaired glucose regulation [35]. Consequently, the question of the potential role of the fish oil supplementation remains open.
The decrease in TG, without changes in TC and LDL-C, is in accordance with most previous studies in type 2 diabetic patients. The TG-lowering effect may be related to decreased hepatic TG synthesis and increased fatty acid oxidation [36]. In addition, decreased very low density lipoprotein (VLDL) secretion [37] and augmented hepatic glucose output [38] have also played a pivotal role in this process. In animal studies, researchers have observed that these favorable changes may be attributed to the increased adiponectin and decreased TNF-α and resistin levels in plasma [39]. Furthermore, we previously found the low n-3 meal can strengthen the postprandial response of lipid between hypertriglyceridemia group and healthy control [40]. We also recently reported low n-6/n-3 PUFA ratio had a beneficial effect on cardiovascular risk factors by enhancing favorable lipid profiles in rats fed a high-fat diet [41].
While fish oils lower TG almost uniformly, doubts have been raised about the optimum EPA/DHA ratio. The most recent meta-analyses of randomized clinical trials reported that a greater decreasing tendency was observed in HbA1c, insulin, TG, and TC within high-ratio (EPA/DHA > 1.5) subgroup when compared to either low-ratio (EPA/DHA < 1.4) or intermediate (1.4 ≤ EPA/DHA ≤ 1.5) subgroups [14]. An appropriate cutoff for this ratio still needs to be elucidated, which is of great help for the development of functional oil from fish.
In contrast to the effect on TG level, HDL-C level tends to remain unchanged in type 2 diabetic patients treated with n-3 PUFA [15–17, 19, 21, 22, 34, 38]. It is worth noting, however, we observed that fish oil supplementation induced increases in HDL-C, which was in agreement with the results of a few previous studies [42–44]. It is well known that HDL-C is considered a protective factor against coronary heart disease. In individuals with abdominal obesity and insulin resistance, small HDL particle size represents another feature of the dyslipidemic profile that is common in this population [45]. Fish oil raised HDL-2a cholesterol and HDL-2b cholesterol concentrations as compared with corn oil, although there were no significant effects of fish oil on HDL-C [46]. Additionally, purified EPA and DHA administration increased HDL2 cholesterol, but was without effect on HDL-C. EPA also significantly decreased HDL3 cholesterol in moderately obese type 2 diabetic patients with treated hypertension [38]. Despite this, EPA supplementation increased HDL in patients with diabetes and comorbid major depressive depression [47]. Unfortunately, the present study did not evaluate changes in the HDL subfractions.
It is intriguing that the fasting glucose, TC, and LDL-C were increased in both intervention and control groups, with no significant between-group difference nevertheless. This phenomenon was probably not caused by changes in lifestyle, because weight and body composition were stable during the study period. One possible factor could be the long duration of insulin resistance in type 2 diabetic patients in addition to relatively older ages. This deterioration could be more active in individuals with abdominal obesity [48]. Another possible explanation might be that lipid-lowering treatment was prohibited throughout the study. However, it cannot be excluded that other undetermined mechanisms may also be involved in the amplification of fasting glucose, TC, and LDL-C, and additional studies are needed for a deeper understanding of its causes.
In summary, this study illustrated that 6 months of fish oil supplement had no statistically significant effects on glycemic control, but improved TG and HDL-C in type 2 diabetic patients with abdominal obesity. Further large, long-term, randomized, controlled trials and greater inclusion of biomarkers will be necessary to determine the specific effects of fish oil on glucose and lipid metabolism in diabetes.
References
NCD-RisC (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387:1513–1530. doi:10.1016/s0140-6736(16)00618-8
Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G (2013) Prevalence and control of diabetes in Chinese adults. JAMA 310:948–959. doi:10.1001/jama.2013.168118
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846. doi:10.1038/nature05482
Mouratoff GJ, Carroll NV, Scott EM (1967) Diabetes mellitus in Eskimos. JAMA 199:107–112
Bang HO, Dyerberg J, Sinclair HM (1980) The composition of the Eskimo food in north western Greenland. Am J Clin Nutr 33:2657–2661
Fumagalli M, Moltke I, Grarup N, Racimo F, Bjerregaard P, Jorgensen ME, Korneliussen TS, Gerbault P, Skotte L, Linneberg A, Christensen C, Brandslund I, Jorgensen T, Huerta-Sanchez E, Schmidt EB, Pedersen O, Hansen T, Albrechtsen A, Nielsen R (2015) Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 349:1343–1347. doi:10.1126/science.aab2319
Djousse L, Gaziano JM, Buring JE, Lee IM (2011) Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr 93:143–150. doi:10.3945/ajcn.110.005603
Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB (2009) Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 90:613–620. doi:10.3945/ajcn.2008.27424
van Woudenbergh GJ, van Ballegooijen AJ, Kuijsten A, Sijbrands EJ, van Rooij FJ, Geleijnse JM, Hofman A, Witteman JC, Feskens EJ (2009) Eating fish and risk of type 2 diabetes: a population-based, prospective follow-up study. Diabetes Care 32:2021–2026. doi:10.2337/dc09-1042
Brostow DP, Odegaard AO, Koh WP, Duval S, Gross MD, Yuan JM, Pereira MA (2011) Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr 94:520–526. doi:10.3945/ajcn.110.009357
Nanri A, Mizoue T, Noda M, Takahashi Y, Matsushita Y, Poudel-Tandukar K, Kato M, Oba S, Inoue M, Tsugane S, Japan Public Health Center-based Prospective Study G (2011) Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 94:884–891. doi:10.3945/ajcn.111.012252
Villegas R, Xiang YB, Elasy T, Li HL, Yang G, Cai H, Ye F, Gao YT, Shyr Y, Zheng W, Shu XO (2011) Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr 94:543–551. doi:10.3945/ajcn.111.013193
Zheng JS, Huang T, Yang J, Fu YQ, Li D (2012) Marine N-3 polyunsaturated fatty acids are inversely associated with risk of type 2 diabetes in Asians: a systematic review and meta-analysis. PLoS ONE 7:e44525. doi:10.1371/journal.pone.0044525
Chen C, Yu X, Shao S (2015) Effects of omega-3 fatty acid supplementation on glucose control and lipid levels in type 2 diabetes: a meta-analysis. PLoS ONE 10:e0139565. doi:10.1371/journal.pone.0139565
Glauber H, Wallace P, Griver K, Brechtel G (1988) Adverse metabolic effect of omega-3 fatty acids in non-insulin-dependent diabetes mellitus. Ann Intern Med 108:663–668
Schectman G, Kaul S, Kissebah AH (1988) Effect of fish oil concentrate on lipoprotein composition in NIDDM. Diabetes 37:1567–1573
Borkman M, Chisholm DJ, Furler SM, Storlien LH, Kraegen EW, Simons LA, Chesterman CN (1989) Effects of fish oil supplementation on glucose and lipid metabolism in NIDDM. Diabetes 38:1314–1319
Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V (2006) Effects of n-3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 84:540–550
Annuzzi G, Rivellese A, Capaldo B, Di Marino L, Iovine C, Marotta G, Riccardi G (1991) A controlled study on the effects of n-3 fatty acids on lipid and glucose metabolism in non-insulin-dependent diabetic patients. Atherosclerosis 87:65–73
Pelikanova T, Kohout M, Valek J, Kazdova L, Base J (1993) Metabolic effects of omega-3 fatty acids in type 2 (non-insulin-dependent) diabetic patients. Ann N Y Acad Sci 683:272–278
Morgan WA, Raskin P, Rosenstock J (1995) A comparison of fish oil or corn oil supplements in hyperlipidemic subjects with NIDDM. Diabetes Care 18:83–86
McManus RM, Jumpson J, Finegood DT, Clandinin MT, Ryan EA (1996) A comparison of the effects of n-3 fatty acids from linseed oil and fish oil in well-controlled type II diabetes. Diabetes Care 19:463–467
Luo J, Rizkalla SW, Vidal H, Oppert JM, Colas C, Boussairi A, Guerre-Millo M, Chapuis AS, Chevalier A, Durand G, Slama G (1998) Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care 21:717–724
Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, Guerre-Millo M, Rizkalla SW (2007) Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 86:1670–1679
WGOC (2004) Guidelines for the prevention and control of overweight and obesity among Chinese adults (Excerpt). Acta Nutrimenta Sinica 26:1–4. doi:10.3321/j.issn:0512-7955.2004.01.001
Popp-Snijders C, Bilo HJ, Heine RJ (1990) Fish oil and glycemic control: importance of dose. Diabetes Care 13:80–81
McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR (1993) Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 36:33–38
Neil HA, Ceglarek U, Thiery J, Paul S, Farmer A, Holman RR (2010) Impact of atorvastatin and omega-3 ethyl esters 90 on plasma plant sterol concentrations and cholesterol synthesis in type 2 diabetes: a randomised placebo controlled factorial trial. Atherosclerosis 213:512–517. doi:10.1016/j.atherosclerosis.2010.09.013
Sirtori CR, Paoletti R, Mancini M, Crepaldi G, Manzato E, Rivellese A, Pamparana F, Stragliotto E (1997) N-3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. Am J Clin Nutr 65:1874–1881
Vessby B, Boberg M (1990) Dietary supplementation with n-3 fatty acids may impair glucose homeostasis in patients with non-insulin-dependent diabetes mellitus. J Intern Med 228:165–171
Puhakainen I, Ahola I, Yki-Jarvinen H (1995) Dietary supplementation with n-3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 61:121–126
Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S (2008) Effects of omega-3 fatty acid supplements on serum lipids, apolipoproteins and malondialdehyde in type 2 diabetes patients. East Mediterr Health J 14:305–313
Pooya S, Jalali MD, Jazayery AD, Saedisomeolia A, Eshraghian MR, Toorang F (2010) The efficacy of omega-3 fatty acid supplementation on plasma homocysteine and malondialdehyde levels of type 2 diabetic patients. Nutr Metab Cardiovasc Dis 20:326–331. doi:10.1016/j.numecd.2009.04.002
Wong CY, Yiu KH, Li SW, Lee S, Tam S, Lau CP, Tse HF (2010) Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with Type 2 diabetes mellitus. Diabet Med 27:54–60. doi:10.1111/j.1464-5491.2009.02869.x
Clark LF, Thivierge MC, Kidd CA, McGeoch SC, Abraham P, Pearson DW, Horgan GW, Holtrop G, Thies F, Lobley GE (2016) Fish oil supplemented for 9 months does not improve glycaemic control or insulin sensitivity in subjects with impaired glucose regulation: a parallel randomised controlled trial. Br J Nutr 115:75–86. doi:10.1017/s0007114515004274
Marsh JB (2003) Lipoprotein metabolism in obesity and diabetes: insights from stable isotope kinetic studies in humans. Nutr Rev 61:363–375
Nestel PJ (1990) Effects of N-3 fatty acids on lipid metabolism. Annu Rev Nutr 10:149–167. doi:10.1146/annurev.nu.10.070190.001053
Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ (2002) Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 76:1007–1015
Yang ZH, Miyahara H, Takemura S, Hatanaka A (2011) Dietary saury oil reduces hyperglycemia and hyperlipidemia in diabetic KKAy mice and in diet-induced obese C57BL/6J mice by altering gene expression. Lipids 46:425–434. doi:10.1007/s11745-011-3553-1
Song Z, Yang L, Shu G, Lu H, Sun G (2013) Effects of the n-6/n-3 polyunsaturated fatty acids ratio on postprandial metabolism in hypertriacylglycerolemia patients. Lipids Health Dis 12:181. doi:10.1186/1476-511X-12-181
Yang LG, Song ZX, Yin H, Wang YY, Shu GF, Lu HX, Wang SK, Sun GJ (2016) Low n-6/n-3 PUFA ratio improves lipid metabolism, inflammation, oxidative stress and endothelial function in rats using plant oils as n-3 fatty acid source. Lipids 51:49–59. doi:10.1007/s11745-015-4091-z
Jain S, Gaiha M, Bhattacharjee J, Anuradha S (2002) Effects of low-dose omega-3 fatty acid substitution in type-2 diabetes mellitus with special reference to oxidative stress—a prospective preliminary study. J Assoc Physicians India 50:1028–1033
Kesavulu MM, Kameswararao B, Apparao C, Kumar EG, Harinarayan CV (2002) Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab 28:20–26
Pedersen H, Petersen M, Major-Pedersen A, Jensen T, Nielsen NS, Lauridsen ST, Marckmann P (2003) Influence of fish oil supplementation on in vivo and in vitro oxidation resistance of low-density lipoprotein in type 2 diabetes. Eur J Clin Nutr 57:713–720. doi:10.1038/sj.ejcn.1601602
Pascot A, Lemieux I, Prud’homme D, Tremblay A, Nadeau A, Couillard C, Bergeron J, Lamarche B, Despres JP (2001) Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res 42:2007–2014
Petersen M, Pedersen H, Major-Pedersen A, Jensen T, Marckmann P (2002) Effect of fish oil versus corn oil supplementation on LDL and HDL subclasses in type 2 diabetic patients. Diabetes Care 25:1704–1708
Mocking RJ, Assies J, Bot M, Jansen EH, Schene AH, Pouwer F (2012) Biological effects of add-on eicosapentaenoic acid supplementation in diabetes mellitus and co-morbid depression: a randomized controlled trial. PLoS ONE 7:e49431. doi:10.1371/journal.pone.0049431
Haffner SM (2006) Abdominal obesity, insulin resistance, and cardiovascular risk in pre-diabetes and type 2 diabetes. Eur. Heart J. Suppl. 8:B20–B25
Acknowledgements
Both fish oil and corn oil capsules were manufactured by By-Health Co. (Guangdong, China) according to our requirements. We are indebted to all the people who kindly participated in this study. This study was supported by the Grant from the National Natural Science Foundation of China (No. 81573144), the nutrition research foundation from the Chinese Nutrition Society (No. 201326), the Fundamental Research Funds for the Central Universities, and the Graduate Research and Innovation Projects of Colleges in Jiangsu Province (No. KYLX15_0175).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, F., Wang, Y., Zhu, Y. et al. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: a randomized, double-blind, placebo-controlled trial. Eur J Nutr 56, 2415–2422 (2017). https://doi.org/10.1007/s00394-016-1352-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1352-4