Abstract
Aims
To compare the effects of replacing regular dietary oils intake with sesame (SO), canola (CO), and sesame–canola (SCO) oils (a novel blend), on cardiometabolic markers in adults with type 2 diabetes mellitus (T2DM), in a triple-blind, three-way, randomized, crossover clinical trial.
Methods
Participants were assigned to receive SO, CO, and SCO in three 9-week phases (4 weeks apart). Cardiometabolic makers (serum lipids, Apolipoprotein, cardiovascular risk scores, kidney markers, and blood pressure) were considered at the beginning and the end of intervention phases.
Results
Ninety-two, ninety-five, and ninety-five participants completed the SO, SCO, and CO periods, respectively. After CO consumption, serum Apo A-1 concentrations were significantly higher compared with the SCO period in the whole population (p < 0.05). A considerable reduction in visceral adiposity index values was seen in the CO compared with the SO period in males (p < 0.05). Serum high-density lipoprotein concentration was also significantly higher after the SO intake compared with SCO in females (p < 0.05). The between-period analysis showed a substantial reduction in diastolic blood pressure in the SCO period compared with the CO and SO periods and lower systolic blood pressure after SCO versus CO intake in males (p < 0.05).
Conclusions
Canola oil might protect CVD through improving Apo A-1 levels in patients with T2DM (particularly in females) and visceral adiposity index in male patients. However, the blend oil might beneficially affect blood pressure in men. Future sex-specific studies might warrant the current findings.
Registry of clinical trials
This trial was registered in the Iranian Registry of Clinical Trials (IRCT, registration ID: IRCT2016091312571N6).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past decades, the prevalence of type 2 diabetes mellitus (T2DM) has been increasing [1]. According to a report by the International Diabetes Federation (IDF), in 2015, 415 million people were affected and 642 million individuals will be diagnosed with T2DM in 2040 [2]. T2DM is a strong independent risk factor for cardiovascular diseases (CVDs) such as myocardial infarction (MI), stroke, and peripheral vascular diseases [3], which considered as the major cause of death among individuals with T2DM (47.2%) [4, 5].
Dietary fats might have a strong association with CVDs [6, 7]. More attention to the quality of dietary fats rather than their quantity led to a change in dietary recommendations to substitute saturated and trans-fatty acids with unsaturated fats [8, 9]; and to increase the intake of polyunsaturated (PUFAs) [10] and monounsaturated (MUFAs) fatty acids [11]. MUFAs are suggested to have beneficial effects on cardiovascular risk factors [12,13,14], although, a systemic review and meta-analysis on 83 randomized clinical trials reported that omega-3, omega-6, or total PUFA has little or no effect on prevention and treatment of T2DM [15].
Canola oil (CO) is rich in alpha-linolenic acid (ALA) [16], as well as MUFAs [17]. Randomized clinical trials (RCTs) have shown the effects of CO on different cardiovascular risk factors. A systematic review and meta-analysis of our group on RCTs regarding the effects of CO on cardiovascular risk factors indicated that CO consumption decreases total cholesterol (TC) (− 0.27 mmol/l), low-density lipoprotein cholesterol (LDL-C) (− 0.23 mmol/l), LDL-C to high-density lipoprotein cholesterol ratio (LDL:HDL ratio) (− 0.21), TC:HDL ratio (− 0.13), Apolipoprotein B (Apo B) (− 0.03 g/l), and Apo B:Apo A-1 ratio (− 0.02) compared to other edible oils (P < 0.05), while no significant effects were observed on triglycerides (TG), Apo A, lipoprotein (a) (Lp (a)), systolic and diastolic blood pressure (SBP and DBP) [18]. The findings of another meta-analysis concerning the effects of CO intake on body weight and body composition measurements showed no significant effects on body mass index (BMI) and body composition measurements, and just a slight decrease was observed in body weight [19].
The main functional ingredients of sesame oil (SO) are polyunsaturated fatty acids, MUFAs, vitamin E [20], and lignans, including sesamin, episesamin, and sesamolin [21]. In addition, sesame seeds has been also consumed as a therapeutic agent in Asia for decades [22]. A limited number of studies have investigated the effect of sesame oil on cardiometabolic health. Findings of two systematic reviews and meta-analyses were indicative of no significant effect of sesame seed and its products on circulating HDL-C, TC, and LDL-C; while, sesame fractions led to a substantial reduction in serum TG levels [23] and blood pressure [24]. According to these reviews, only a few number of studies examined the effect of sesame oil on the lipid profile and blood pressure. All of them were graded as high risk based on Cochrane collaboration’s risk of bias assessment tool with an intervention duration up to 6 weeks [23, 24].
To the best of our knowledge, no study has been designed to compare the impacts of these oils on cardiometabolic markers. Therefore, in the current study, we have presented the effects of replacing household ordinary consumed oils, with SO, CO, and sesame–canola oil (a novel blend of sesame and canola oil (SCO), consist of 60% canola oil and 40% sesame oil) on cardiometabolic markers, including lipid profile, Apolipoproteins, CVD risk scores, blood pressure, visceral adiposity index (VAI), and kidney markers in patients with T2DM. Additionally, it is worth to mention that findings of other investigations of our team concerning the effects of these oils on glycemic control and liver function enzymes in adult patients with T2DM revealed no significant difference between the treatment oils [25]. Moreover, based on our analysis, these oils might not differently affect body fat and composition in these patients [26] as well as adults without any chronic diseases [27].
Materials and methods
Study design
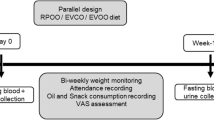
The present study is derived from a parent triple-blind, randomized, crossover clinical trial designed to investigate the effects of replacing regular consumed oils with SO, SCO, and CO on blood glucose, cardiometabolic markers, and body weight and composition in patients with T2DM and their spouses. The methodology of the parent study was completely described elsewhere [28]. In brief, all participants received intervention oils in three 9-week intervention phases. There were 3 clinical visits in each phase (at the start, in the middle, and at the end). Blood samples were taken after an overnight fasting (10–12 h) at the beginning and the end of each phase. Anthropometric indices (body weight, waist and hip circumferences, and body composition), the medication changes, and dietary and physical activity records were measured or collected in all clinical visits. The ethical approval of the current RCT was granted by the ethics committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Reference number: IR.SSU.REC.1395.25) on 29th May 2016. In addition, this RCT was registered in the Iranian Registry of Clinical Trials (IRCT) on 14th November 2016 (registration ID: IRCT2016091312571N6, URL: https://en.irct.ir/trial/12622) and written informed consents were obtained from all individuals participated in this study. As the number of participants completed the study were different for other outcome variables (such as anthropometric measurements), only the cardiometabolic markers including serum lipid profile, Apolipoproteins, CVD risk scores, and blood pressure in patients with T2DM are reported in the current study.
Inclusion and exclusion criteria
Adults (18–60 years old) with the following criteria were included: (1) with 6-month to 10-year history of T2DM; (2) taking the oral anti-glycemic agents (not insulin therapy or any other injectable glycemic control medication); (3) consumed stable doses of lipid-lowering medications (at least for 3 months prior to starting the study); (4) without history of any other diseases such as CVDs, coronary artery bypass grafting (CABG), kidney or liver diseases, and cancers. Individuals who (1) changed their dietary habits (going on any diet), (2) went on insulin therapy, (3) experienced pregnancy or chronic diseases, including CVDs or cancers during the study, and (4) did not intend to continue the project were excluded.
Intervention
After a 1 month of run-in-period, participants were randomly assigned to 6 rolling methods to consume SO, SCO, and CO for 9-week intervention phases. The intervention periods were separated by 4-week intervals as washout periods (sunflower oil was provided for the run-in and washout periods). Details of the order of intervention oils intakes as well as the intervention program are fully illustrated in the study protocol paper [28]. The intervention oils were provided for the household use of participants as much as they requested. Neither participants nor personnel and statisticians were aware of the type of intervention oils up to the end of the study. The strategy used for randomization, randomization concealment and blinding is fully described in the protocol [28]. The refining and advanced purification process as well as odor removal and color elimination processes, that were used during the producing procedure, provided us with intervention oils which were similar in their odor and color. The fatty acid profile of the intervention oils was assessed using gas chromatography with flame ionizer detector [(GC-FID) (Youngling, model: YL6500 GC)] [28]. The mean percentage of the main fatty acids content of intervention oils are provided in Supplementary Table 1.
Cardiometabolic assessments
Blood samples were taken after an overnight fasting (10–12 h) at the beginning and the end of all phases. TG, TC, HDL-C, LDL-C, Apo A-1, Apo B, Lp(a), urea, and creatinine were analyzed from serum samples by an auto-analyzer (Alpha-classic, Iran, model: AT + +) using standard Pars Azmun kits. Intra- and inter-assay coefficients of variances for blood markers are provided in Supplementary Table 2. Blood pressure was measured after 5-min rest when participants were in the sitting mode, for the right arm with at least 1-min interval, using a sphygmomanometer (Riester, Germany, model: Diplomat-presameter). Anthropometric measurements were assessed using standard methods at the beginning, in the middle, and at the end of each phase. All anthropometric and blood pressure assessments were done three times at each visit and the mean was recorded [28]. Cardiovascular risk scores were calculated using age, gender, SBP, TC, and HDL-C by Framingham equations [29, 30]. Visceral adiposity index (VAI) was estimated as an independent risk factor related to cardiovascular events by the separate formulas for males and females using WC, BMI, TG, and HDL-C [31]. The equation suggested by the chronic kidney disease epidemiology collaboration (CKD EPI) was used to calculate the estimated glomerular filtration rate (eGFR) [32].
Dietary and physical activity assessments
Although the participants were asked to keep their dietary intake and physical activity stable, their dietary intake and physical activity were also controlled. To assess the stability of the dietary intake, 3-day weighed food records (three consecutive days, 2 weekdays and 1 weekend day) were completed by all participants and were collected at the start, in the middle, and at the end of each intervention phase. In addition, a digital kitchen scale (model: Electronic kitchen scale, SF-400) was provided for participants to record 3-day cooking forms and record the ingredients of the cooked foods with their weights. The daily intake of all consumed food and beverages were computed and converted to gram/day by household measures and food weights provided by individuals. Daily energy and nutrients intakes were calculated using Nutritionist IV software (version 3.5.2, Axxya Systems, Redmond, Washington, USA) modified for Iranian foods. Physical activity was also assessed by 3-day records (2 weekdays and 1 weekend day) filled at the beginning, in the middle, and the end of each phase. Its data were converted to metabolic equivalent-min/day (Met-min/day) using the updated version of the compendium of physical activities [33]. All dietary and physical activity records were checked at return by a trained nutritionist.
Sample size and power calculation
The following formula [n = [(z 1 − α/2 + z1 − β)2 × s 2]/2Δ2] [34] was used to calculate sample size considering serum glucose as the primary outcome [35]. Type 1 and 2 errors were set at 5% and 10%, respectively, and a minimum number of 34 participants was determined. As we aimed to have enough power to conduct sex-stratified analyses, we decided to enter 50 males and 50 females. The present study targeted to report the effect of intervention oils on secondary outcomes including cardiometabolic and kidney markers. We calculated the power of the current study for each outcome using G*Power software (version 3.1.9.7, Germany). Our calculations revealed that the study had more than 80% power for comparison of change values between at least two different oils in all participants as well as males and females.
Statistical analysis
The normal distribution of quantified variables was checked using Kolmogorov–Smirnov test. As this was a crossover clinical trial, the carry-over effect was considered for all outcomes using linear mixed effect model. If a carry-over effect was detected for an outcome variable, the comparison of change values between intervention oils was done for the first phase of the study using general linear model, univariate analysis. Baseline and after-intervention period measurements were compared using the general linear model, repeated measures procedure. If no carry-over effect was observed, the after-intervention and change values between the intervention phases were compared using linear mixed model with considering rolling method, as between subjects’ factor in the crude model and multivariable adjusted model (adjustments for age, sex, baseline BMI, the calculated intervention oils consumed per subject, changes in physical activity level, the energy intake in each intervention period, and baseline values). All these procedures were also performed based on gender. Finally, all analyses were performed in participants who did not change their glucose-lowering and lipid-modifying medications/supplements during the whole study period (sensitivity analysis). The results are expressed as means ± standard errors (SEs). All analyses were conducted using IBM SPSS (version 20; IBM Corporation, USA). p-values equal to or less than 0.05 were considered as statistically significant.
Results
Participants
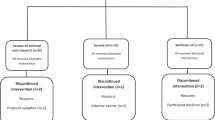
One hundred and two individuals with T2DM were included in the present investigation to randomly consume the three intervention oils. During the follow-up duration, six participants were excluded due to the following reasons: three did not intend to continue the project, one migrated to another city, one was diagnosed with CVD, and the medication therapy of another patient was changed to insulin therapy. In addition, in the SO intervention period, three participants did not intend to give pre- or post-intervention blood samples. One participant was also excluded from the statistical analysis because of no compliance based on dietary food records data, although completed all study phases. Finally, 92, 95, and 95 patients had complete data for SO, CO, and SCO intervention periods, respectively. The study flow diagram is described in Fig. 1. The mean age of individuals who participated in the study was 49.17 ± 0.70 years, and 48.42% of the study population were males. The baseline characteristics of the study participants are summarized in Supplementary Table 3.
Dietary and physical activity assessment
Table 1 represents participants’ energy as well as nutrients intake, and the physical activity level based on each intervention phase. No significant difference was observed between intervention phases regarding calorie, macronutrients, saturated fatty acids intake and physical activity level. Considerable between-duration differences in MUFAs and PUFAs intake were observed (P < 0.05). Sex-stratified dietary intakes and physical activity in different intervention periods are provided in Supplementary Table 4.
The effects of intervention oils on cardiometabolic markers in the whole population
The crude and multivariable-adjusted mean changes in serum lipid profile markers, lipoproteins, CVD risk scores, VAI, blood pressure, and kidney markers for the 3 intervention oils are provided in Table 2. It should be noted that the carry-over effect was reported only for eGFR levels. Therefore, the comparison of dietary oils effects on eGFR levels was conducted for the first phase.
After adjustment for possible confounders, the consumption of CO caused a significant increase in Apo A-1 concentration (mean change and SE: 5.22 ± 2.37 mg/dl, p = 0.04). In addition, the post-treatment serum Apo A-1 levels were significantly higher in the CO consumption period compared with the SCO period (p = 0.029).
SO intake also caused a significant increase in DBP (mean change and SE: 0.27 ± 0.12, p = 0.021) as well as a considerable increase in serum creatinine levels (mean change and SE: 0.006 ± 0.02, p = 0.026) and eGFR (mean change and SE: 10.24 ± 3.19, p = 0.014) (Table 2). However, no significant differences were observed in blood pressure and kidney markers between the intervention periods (p > 0.05). As it is shown in Table 2, no significant within- or between-period effects were observed for the other markers.
The effects of intervention oils on cardiometabolic markers in male and female participants
The effects of treatment oils on lipid and Apolipoproteins’ profile in male and female participants with T2DM are shown in Tables 3 and 4. The post-treatment serum HDL-C levels were significantly higher in the SO period compared with the SCO period in females (p = 0.006). Furthermore, SCO consumption resulted in a considerable decrease in Apo A-1 concentration in women (p = 0.043); and the mean change in serum APO A-1 levels was significantly different between the CO and SCO periods in the multivariable-adjusted model (mean change and SE: 4.60 ± 3.15 vs. − 5.78 ± 2.93; p = 0.044) in females (Table 4). Neither within- nor between-period analysis revealed any significant effects on other lipid and lipoprotein markers in both sexes.
None of the intervention oils could significantly affect CVD risk scores in both sexes. No between-period difference was revealed. SO and CO consumption differently affected the VAI in males (mean change and SE: 0.66 ± 0.33 in SO period vs. − 43 ± 0.35 in CO period, p = 0.029).
The comparison of the post-intervention levels showed a considerably lower SBP levels in the SCO period compared with the CO period in men (p = 0.042). CO and SO consumption both resulted in a significant increase in DBP in men (mean change and SE: 0.52 ± 0.17, p = 0.003, and 0.49 ± 0.17, p = 0.018, Table 3); however, SCO had a significantly decreasing effect on DBP in this group (mean change and SE: − 0.37 ± 0.16, p = 0.034). In addition, analyses indicated that SCO intake significantly reduced DBP compared to CO and SO in males (p < 0.05, Table 3). Consuming SCO could considerably increase DBP in females (mean change and SE: 0.37 ± 0.18, p = 0.044, Table 4); however, the intervention oils were not significantly different regarding their effect on blood pressure in this gender (Table 4).
SO consumption could significantly increase eGFR levels in females (mean change and SE: 9.12 ± 4.53, p = 0.048), while there were no significant changes in this index in males (p > 0.05). In addition, the intervention oils were not significantly different in their effects on kidney markers.
Sensitivity analysis
The analysis revealed that 12 participants had changed doses or types of their hypoglycemic or lipid-lowering medication during the study period. We conducted the analyses on lipid profile, CVD risk scores, and VAI by excluding participants experiencing the medication changes. The sensitivity analysis revealed that the increase in serum Apo A-1 concentration after CO consumption remained significant and after intervention values were still higher for CO period compared with SCO period for all participants; however, the difference in change values between CO and SCO periods was disappeared (p > 0.05). In addition, the decrease in VAI values was still significantly higher in CO compared to SO period, in males (p < 0.05). Furthermore, Serum HDL values were still significantly higher after the SO compared with SCO intake period (p < 0.05). All other significant effects were disappeared in the sensitivity analysis for lipid profile, Apolipoproteins, and cardiovascular disease risk scores (Supplementary Tables 5–7).
Discussion
This study was designed to examine the effects of replacing typical household oil intake with SO, CO, and SCO on cardiometabolic markers. The findings demonstrate that post-treatment levels of serum Apo A-1 were significantly higher in CO period compared with the SCO period in the whole population; however, by sensitivity analysis the difference was disappeared. Furthermore, a considerable decrease in VAI values was seen in CO compared with SO intake period in males. Serum HDL-C values were also significantly higher after the SO period compared with SCO in females. These effects remained significant after performing sensitivity analysis. In women, according to the between-oil comparisons, CO consumption resulted in an increase in serum Apo A-1 levels which was significantly different from SCO period. This effect was vanished after the sensitivity analysis. SBP was significantly lower after SCO intake compared with CO in males. The between-period analysis also showed a substantial reduction in DBP in the SCO period compared with the CO and SO periods in males.
Monounsaturated fatty acids, PUFAs, dietary fibers, and polyphenols are substantial dietary components associated with cardiovascular risk markers [36]. Canola oil is low in saturated fat (7%) and has considerable amounts of PUFAs (21% linoleic acid and 11% ALA), MUFAs (61% oleic acid), and phytosterols [37] and these characteristics have made it a good option for protecting humans against cardiovascular diseases. On the other hand, SO is a rich source of PUFAs (43%), MUFAs (40%), vitamin E, and some lignans (sesamin, sesamolin, and sesaminol) [20]. It has been suggested that the incorporation of conventional or high-oleic CO to a diet in the replacement of saturated fats is an effective approach to improve lipid profile [38]. In addition, a study on animals reported that SO might increase the expression of some genes which are involved in lipid metabolisms [39]. An investigation by our team concerning the interaction effects of rs670 variant of APOA-1 gene with cardiometabolic markers after consuming SO, CO, and SCO on patients with T2DM found that the inter-individual variations of lipid profile markers in response to dietary interventions might be mediated by rs670 variant of APOA-1 gene [40]. It was indicated that serum levels of HDL-C and TG:HDL ratio were increased and decreased following CO intake in A-allele carriers rather than non-A-allele carriers, respectively [40]. In addition, a considerable genotype-dietary oil interactions were observed for LDL:HDL, TC:HDL and TG:HDL ratios in another study concerning the interaction between cholesteryl ester transfer protein (CETP) TaqIB gene polymorphism and SO, CO, and SCO consumption on metabolic response in patients with T2DM [41].
Our findings showed similar effects of CO, SCO, and SO on lipid profile and cardiovascular risk scores in patients with T2DM except for HDL-C and Apo A-1. In women, HDL-C concentration tended to be significantly decreased by SCO compared with SO. Furthermore, in the whole population and in females, consuming CO led to a significant increase in Apo A-1 compared with SCO. In support of our results, several studies have shown the similar effects of CO compared with other edible oils on some lipid profile markers [42, 43]. In addition, a long-term intervention trial in adults with hypercholesterolemia reported a significant effect of CO on LDL-C, although no substantial differences were observed between CO and control groups regarding the effect on TC, LDL-C, HDL-C, and TG [44]. In contrast, Bowen et al. reported that CO-based diets could decrease TC, LDL-C, Apo B; however, the concentration of Apo A-1, HDL-C, and TG were not affected [38]. CO significantly reduced VAI, an index calculated using serum TG, HDL-C, BMI, and waist circumference, when compared with SO in men. The increase in VAI is associated with cardiometabolic risk [31], showing that CO intake might have more favorable effects on this index rather than SO, which can be related to TG lowering and HDL-C increasing effect of this oil. The conflicting results between men and women might be related to some polymorphisms which exert opposite effects on several lipid profile markers, specifically HDL-C and Apo A-1 following interventions in different genders [45], showing the interaction of several polymorphisms and edible oils on lipid profile in males or females. Moreover, it is proposed that sex hormones might affect insulin sensitivity [46] which in turn affects lipid profile responsiveness to dietary interventions [47].
The results of the current study showed that the SCO consumption yielded to a significant decrease in DBP when compared with other oils in males. Substantial lower SBP levels were also observed after SCO consumption compared with CO in men which might show the synergistic effect of the combination of SO and CO on blood pressure in males. SCO benefits from both SO and CO healthy components. Depending on the available nutrients such as lignans and vitamin E, SO might exert its antihypertensive effects via some mechanisms like modulating the expression of some genes associated with the renin–angiotensin–aldosterone system (RAS) [48,49,50,51,52]. It is noteworthy that RAS has been considered as the major mediator of hypertension in males, whereas some other mediators except RAS are more important in women [53], which might interpret our different findings in males and females. The previous investigations have led to inconsistent results regarding the effect of SO on blood pressure. It has been suggested that consuming 35 g/day of SO in individuals with hypertension led to endothelial function and blood pressure improvement [54]. In contrast with the above studies, the blood pressure was not affected significantly by SO consumption in the present study. In line with our results, a randomized crossover clinical trial showed that consuming 25 g/day of sesame (50 mg/day sesamin) for 5 weeks did not significantly affect blood pressure [55]. Furthermore, another study showed that a daily intake of 4.5 g/day SO has no considerable effect on blood pressure [56]. In addition to polyphenols, polyunsaturated fatty acids might reduce blood pressure. It has been shown that a high-SFA diet led to an increase in blood pressure compared with high-PUFAs diet [57]. A meta-analysis revealed that n-3 PUFAs intake may reduce SBP and DBP by 2.3 and 1.5 mmHg, respectively [58]. Alpha-linolenic acid, as one of the most abundant n-3 PUFAs in CO, may decrease blood pressure due to its capability to be converted to the most significant n-3 long-chain fatty acids, namely EPA and DHA [59]. It is proposed that consuming 0.7 g/day of DHA might reduce blood pressure by 3.3 mmHg [60]. Randomized clinical trials were inconclusive regarding the effect of CO on blood pressure. In a 6-month parallel study done by Baxheinrich et al. which compared the effects of a CO and an olive oil-based diet, differing in ALA content, the decline in DBP was significantly more in the CO group compared with the olive oil group [61]. The improvement of endothelium function by n-3 PUFAs was observed in young adults with high metabolic risk [62]. On the other hand, the results of a meta-analysis suggested no antihypertensive effects for ALA [63]. Moreover, a trial showed that consuming 40 g/day CO could not significantly change the blood pressure in women with osteoporosis [64] which was consistent with our results.
One of the most common microvascular complications of type 1 or 2 diabetes is nephropathy. It could be occurred in less than half (20–40%) of the individuals with T2DM [65]. eGFR could be measured to assess the renal function in patients. It has been proposed that the decrease of eGFR is associated with increased risk of coronary heart diseases, heart failure, and mortality [66]. A novel finding of the current study was the increase of eGFR in the SO consumption phase in the whole population and females; however, no significant differences were observed between the intervention oils. The possible mechanisms accounting for the effects of SO on kidney health can be speculated by polyphenols content. According to the pathogenic role of oxidative stress in renal diseases [67], consuming antioxidant-rich foods might lead to alleviate or lessen the severe consequences of oxidative stress on organs [68]. Several animal studies assessed the effects of sesamin or SO on kidney health. One of them found that sesamin might protect the body against age-related kidney dysfunction regarding its anti-inflammatory and anti-oxidative properties [69]. Furthermore, SO may attenuate renal injuries in rats as well by inhibiting renal oxidation [70]. In this case, several studies on rats demonstrated that SO might inhibit nitric oxide production and is a hydroxyl radical scavenger [71, 72], causing renal protective effects [70, 73]. Hydroxyl radicals are defined as the mediator of lipid peroxidation [72] producing by nitric oxide and superoxide onion reaction [74].
To our knowledge, this study is the first study to compare the effect of canola, sesame, and the blend of canola and sesame oils on cardiometabolic health in patients with T2DM. Even though, our study was designed to compare two edible oils and a blended one (as a novel oil product) which all of them can be considered as healthy oils. This was a randomized, triple-blind, and crossover clinical trial that might lead to a lower risk of bias and greater statistical power than parallel studies [75]. In addition, acting participants as their own control is the most valuable characteristic of such a study design, decreasing confounding variables and increasing precision [76]. In addition, due to the nature of crossover design, a lower number of participants are needed. In the present study, most of the subjects completely participated all phases of the study, leading to low attrition. Furthermore, 9-week intervention periods can be considered as a long duration among most of the crossover studies previously conducted in this field [38, 77, 78].
Since we aimed at examining the effect of replacing the household oil intake with the intervention oils in the ordinary consumption amounts (similar to the real life), it was not possible to measure the exact amounts of consumed oils by the participants, which is one of the limitations of our study. Measuring the serum fatty acids composition would be a much more appropriate way to assess the participants’ adherence, but we were not able to do these measurements due to the financial limitations. Hence, we tried to assess the consumption amounts using the weight of the given and returned oil containers and the 3-day weighted food records. In addition, to remove the contamination and to have a higher quality oil, purification, deodorization, eliminating the taste and odor of the oil were conducted and the process might affect the physicochemical and micronutrients content of the intervention oils. However, it should be noted, that all the intervention oils were undergone the same processing methods. Although this was a crossover study, women included in this study were in the menstruating age and the intervention duration were 9 weeks; therefore, the menstruation and hormonal changes would take place in different time points of the intervention periods and this might confound the effects in women. It needs to be stated the majority of significant findings were disappeared after performing the sensitivity analysis based on the type or the dose of their lipid/glucose-lowering medications, thus, our results should be interpreted with caution.
Conclusion
The present study revealed a significant higher Apo A-1 levels after CO compared with SCO period in patients with T2DM. Furthermore, this dietary oil significantly reduced VAI values compared to SO in males. After-intervention, serum HDL-C values were also significantly higher in females after SO compared to SCO consumption. The post-intervention levels of SBP were significantly lower after SCO compared with CO consumption in males. In addition, SCO significantly reduced DBP compared to the other intervention oils in males. To clarify the reasons for gender-specific responses, gene-oil interaction studies are merited.
Abbreviations
- Apo A-1:
-
Apolipoprotein A-1
- Apo B:
-
Apolipoprotein B
- BMI:
-
Body mass index
- CO:
-
Canola oil
- CVDs:
-
Cardiovascular diseases
- DBP:
-
Diastolic blood pressure
- DHA:
-
Docosahexaenoic acid
- eGFR:
-
Estimated glomerular filtration rate
- EPA:
-
Eicosapentaenoic acid
- FBS:
-
Fasting blood sugar
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- LP (a):
-
Lipoprotein (a)
- MI:
-
Myocardial Infarction
- MUFAs:
-
Monounsaturated fatty acids
- PUFAs:
-
Polyunsaturated fatty acids
- SBP:
-
Systolic blood pressure
- SCO:
-
Sesame–canola oil
- SFAs:
-
Saturated fatty acids
- SO:
-
Sesame oil
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- VAI:
-
Visceral adiposity index
References
Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94(3):311–321
Atlas D (2015) International diabetes federation. IDF Diabetes Atlas, 7th edn Brussels, Belgium: International Diabetes Federation
Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE (2005) Identifying individuals at high risk for diabetes: the atherosclerosis risk in communities study. Diabetes Care 28(8):2013–2018
Morrish N, Wang S-L, Stevens L, Fuller J, Keen H, Group WMS (2001) Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44(2):S14
Acs A, Ludwig C, Bereza B, Einarson T, Panton U (2017) Prevalence of cardiovascular disease in type 2 diabetes: a global systematic review. Value Health 20(9):A475
Weisburger JH (1997) Dietary fat and risk of chronic disease: mechanistic insights from experimental studies. J Am Diet Assoc 97(7 Suppl):S16-23. https://doi.org/10.1016/s0002-8223(97)00725-6
Guasch-Ferre M, Becerra-Tomas N, Ruiz-Canela M, Corella D, Schroder H, Estruch R, Ros E, Aros F, Gomez-Gracia E, Fiol M, Serra-Majem L, Lapetra J, Basora J, Martin-Calvo N, Portoles O, Fito M, Hu FB, Forga L, Salas-Salvado J (2017) Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the prevencion con dieta mediterranea (PREDIMED) study. Am J Clin Nutr 105(3):723–735. https://doi.org/10.3945/ajcn.116.142034
Dworatzek PD, Arcudi K, Gougeon R, Husein N, Sievenpiper JL, Williams SL (2013) Nutrition therapy. Can J Diabet 37:S45–S55
Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, De Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J (2015) Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 132(8):691–718
Eyre H, Kahn R, Robertson RM, Committee AAACW, Members AAACWC, Clark NG, Doyle C, Hong Y, Gansler T, Glynn T (2004) Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 109(25):3244–3255
Franz MJ, Bantle JM, Beebe CA, Brunzell JD, Chiasson J-L, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian A (2002) American Diabetes Association Position Statement: Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. J Am Diet Assoc 102(1):109–118
Kris-Etherton PM, Pearson TA, Wan Y, Hargrove RL, Moriarty K, Fishell V, Etherton TD (1999) High–monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am J Clin Nutr 70(6):1009–1015. https://doi.org/10.1093/ajcn/70.6.1009
Qian F, Korat AA, Malik V, Hu FB (2016) Metabolic effects of monounsaturated fatty acid–enriched diets compared with carbohydrate or polyunsaturated fatty acid–enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 39(8):1448–1457. https://doi.org/10.2337/dc16-0513
Jeppesen C, Schiller K, Schulze MB (2013) Omega-3 and omega-6 fatty acids and type 2 diabetes. Curr DiabRep 13(2):279–288
Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L (2019) Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. https://doi.org/10.1136/bmj.l4697
Brenna JT, Salem N Jr, Sinclair AJ, Cunnane SC (2009) α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids 80(2–3):85–91
Johnson GH, Keast DR, Kris-Etherton PM (2007) Dietary modeling shows that the substitution of canola oil for fats commonly used in the United States would increase compliance with dietary recommendations for fatty acids. J Am Diet Assoc 107(10):1726–1734
Amiri M, Raeisi-Dehkordi H, Sarrafzadegan N, Forbes SC, Salehi-Abargouei A (2020) The effects of Canola oil on cardiovascular risk factors: a systematic review and meta-analysis with dose-response analysis of controlled clinical trials. Nutr Metab Cardiovasc Disease
Raeisi-Dehkordi H, Amiri M, Humphries KH, Salehi-Abargouei A (2019) The effect of canola oil on body weight and composition: a systematic review and meta-analysis of randomized controlled clinical trials. Adv Nutr 10(3):419–432. https://doi.org/10.1093/advances/nmy108
Sankar D, Rao MR, Sambandam G, Pugalendi K (2006) A pilot study of open label sesame oil in hypertensive diabetics. J Med Food 9(3):408–412
Kita S, Matsumura Y, Morimoto S, Akimoto K, Furuya M, Oka N, Tanaka T (1995) Antihypertensive effect of sesamin. II. Protection against two-kidney, one-clip renal hypertension and cardiovascular hypertrophy. Biol Pharm Bull 18(9):1283–1285
Namiki M (2007) Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr 47(7):651–673
Khalesi S, Paukste E, Nikbakht E, Khosravi-Boroujeni H (2016) Sesame fractions and lipid profiles: a systematic review and meta-analysis of controlled trials. Br J Nutr 115(5):764–773. https://doi.org/10.1017/s0007114515005012
Khosravi-Boroujeni H, Nikbakht E, Natanelov E, Khalesi S (2017) Can sesame consumption improve blood pressure? A systematic review and meta-analysis of controlled trials. J Sci Food Agric 97(10):3087–3094. https://doi.org/10.1002/jsfa.8361
Raeisi-Dehkordi H, Amiri M, Zimorovat A, Moghtaderi F, Zarei S, Forbes SC, Salehi-Abargouei A (2020) Canola oil compared with sesame and sesame-canola oil on glycaemic control and liver function in patients with type 2 diabetes: a three-way randomized triple-blind cross-over trial. Diabetes Metab Res Rev:e3399
Raeisi-Dehkordi H, Amiri M, Moghtaderi F, Zimorovat A, Rahmanian M, Mozaffari-Khosravi H, Salehi-Abargouei A (2021) Effects of sesame, canola and sesame-canola oils on body weight and composition in adults with type 2 diabetes mellitus: a randomized, triple-blind, cross-over clinical trial. J Sci Food Agric. https://doi.org/10.1002/jsfa.11265
Moghtaderi F, Amiri M, Zimorovat A, Raeisi-Dehkordi H, Rahmanian M, Hosseinzadeh M, Fallahzadeh H, Salehi-Abargouei A (2020) The effect of canola, sesame and sesame-canola oils on body fat and composition in adults: a triple-blind, three-way randomised cross-over clinical trial. Int J Food Sci Nutr:1–10
Amiri M, Ghaneian MT, Zare-Sakhvidi MJ, Rahmanian M, Nadjarzadeh A, Moghtaderi F, Raeisi-Dehkordi H, Zimorovat A, Jafari F, Reza JZ (2019) The effect of canola oil compared with sesame and sesame-canola oil on cardio-metabolic biomarkers in patients with type 2 diabetes: Design and research protocol of a randomized, triple-blind, three-way, crossover clinical trial. ARYA Atherosclerosis 15 (4)
Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97(18):1837–1847
Anderson KM, Odell PM, Wilson PWF, Kannel WB (1991) Cardiovascular disease risk profiles. Am Heart J 121(1):293–298
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A (2010) Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 33(4):920–922. https://doi.org/10.2337/dc09-1825
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32 (9; SUPP/1):S498-S504
Chow S-C, Wang H, Shao J (2007) Sample size calculations in clinical research. CRC Press
Jenkins DJ, Kendall CW, Vuksan V, Faulkner D, Augustin LS, Mitchell S, Ireland C, Srichaikul K, Mirrahimi A, Chiavaroli L (2014) Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care 37(7):1806–1814
Chen PR, Chien KL, Su TC, Chang CJ, Liu T-L, Cheng H, Tsai C (2005) Dietary sesame reduces serum cholesterol and enhances antioxidant capacity in hypercholesterolemia. Nutr Res 25(6):559–567. https://doi.org/10.1016/j.nutres.2005.05.007
Gunstone F (2011) Vegetable oils in food technology: composition, properties and uses. Wiley
Bowen KJ, Kris-Etherton PM, West SG, Fleming JA, Connelly PW, Lamarche B, Couture P, Jenkins DJ, Taylor CG, Zahradka P (2019) Diets enriched with conventional or high-oleic acid canola oils lower atherogenic lipids and lipoproteins compared to a diet with a western fatty acid profile in adults with central adiposity. J Nutr 149(3):471–478. https://doi.org/10.1093/jn/nxy307
Narasimhulu CA, Selvarajan K, Litvinov D, Parthasarathy S (2015) Anti-atherosclerotic and anti-inflammatory actions of sesame oil. J Med Food 18(1):11–20
Ramezani-Jolfaie N, Aghaei S, Yazd EF, Moradi A, Mozaffari-Khosravi H, Zimorovat A, Raeisi-Dehkordi H, Moghtaderi F, Amiri M, Yasini Ardakani SA, Salehi-Abargouei A (2020) Association of rs670 variant of APOA-1 gene with cardiometabolic markers after consuming sesame, canola and sesame-canola oils in adults with and without type 2 diabetes mellitus. Clin Nutr ESPEN 38:129–137
Ramezani-Jolfaie N, Aghaei S, Farashahi Yazd E, Moradi A, Mozaffari-Khosravi H, Amiri M, Raeisi-Dehkordi H, Moghtaderi F, Zimorovat A, Yasini Ardakani SA, Salehi-Abargouei A (2020) The combined effects of cholesteryl ester transfer protein (CETP) TaqIB gene polymorphism and canola, sesame and sesame-canola oils consumption on metabolic response in patients with diabetes and healthy people. J Cardiovasc Thorac Res 12(3):185–194
Nielsen NS, Pedersen A, Sandström B, Marckmann P, Høy C-E (2002) Different effects of diets rich in olive oil, rapeseed oil and sunflower-seed oil on postprandial lipid and lipoprotein concentrations and on lipoprotein oxidation susceptibility. Br J Nutr 87(5):489–499
Gillingham LG, Gustafson JA, Han S-Y, Jassal DS, Jones PJ (2011) High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br J Nutr 105(3):417–427
Sarkkinena ES, Uusitupaa MI, Pietinen P, Aro A, Ahola I, Penttilä I, Kervinen K, Kesäniemi YA (1994) Long-term effects of three fat-modified diets in hypercholesterolemic subjects. Atherosclerosis 105(1):9–23
von Eckardstein A, Funke H, Chirazi A, Chen-Haudenschild C, Schulte H, Schönfeld R, Köhler E, Schwarz S, Steinmetz A, Assmann G (1994) Sex-specific effects of the glutamine/histidine polymorphism in apo A-IV on HDL metabolism. Arteriosc Thromb Journal Vascu Biol 14(7):1114–1120
Bruns CM, Kemnitz JW (2004) Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J 45(2):160–169. https://doi.org/10.1093/ilar.45.2.160
Petersen KS, Bowen KJ, Tindall AM, Sullivan VK, Johnston EA, Fleming JA, Kris-Etherton PM (2020) The effect of inflammation and insulin resistance on lipid and lipoprotein responsiveness to dietary intervention. Curr Dev Nutr. https://doi.org/10.1093/cdn/nzaa160
Matsumura Y, Kita S, Tanida Y, Taguchi Y, Morimoto S, Akimoto K, Tanaka T (1998) Antihypertensive effect of sesamin. III. Protection against development and maintenance of hypertension in stroke-prone spontaneously hypertensive rats. Biol Pharm Bull 21(5):469–473
Martín-Peláez S, Castañer O, Konstantinidou V, Subirana I, Muñoz-Aguayo D, Blanchart G, Gaixas S, de la Torre R, Farré M, Sáez GT (2017) Effect of olive oil phenolic compounds on the expression of blood pressure-related genes in healthy individuals. Eur J Nutr 56(2):663–670
Matsumura Y, Kita S, Morimoto S, Akimoto K, Furuya M, Oka N, Tanaka T (1995) Antihypertensive effect of sesamin. I. Protection against deoxycorticosterone acetate-salt-induced hypertension and cardiovascular hypertrophy. Biol Pharm Bull 18(7):1016–1019
Namiki M (1995) The chemistry and physiological functions of sesame. Food Rev Intl 11(2):281–329
Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N (2002) Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res 72(5):309–314
Reckelhoff JF (2018) Sex differences in regulation of blood pressure. In: Sex-Specific Analysis of Cardiovascular Function. Springer, pp 139–151
Karatzi K, Stamatelopoulos K, Lykka M, Mantzouratou P, Skalidi S, Zakopoulos N, Papamichael C, Sidossis LS (2013) Sesame oil consumption exerts a beneficial effect on endothelial function in hypertensive men. Eur J Prev Cardiol 20(2):202–208
Wu JH, Hodgson JM, Puddey IB, Belski R, Burke V, Croft KD (2009) Sesame supplementation does not improve cardiovascular disease risk markers in overweight men and women. Nutr Metab Cardiovasc Dis 19(11):774–780
Khajehdehi P (2000) Lipid-lowering effect of polyunsaturated fatty acids in hemodialysis patients. J Ren Nutr 10(4):191–195
Langley-Evans SC, Clamp AG, Grimble RF, Jackson AA (1996) Influence of dietary fats upon systolic blood pressure in the rat. Int J Food Sci Nutr 47(5):417–425
Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ (2002) Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. LWW
Dittrich M, Jahreis G, Bothor K, Drechsel C, Kiehntopf M, Blüher M, Dawczynski C (2015) Benefits of foods supplemented with vegetable oils rich in α-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: a double-blind, randomized, controlled trail. Eur J Nutr 54(6):881–893
Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA (2007) Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J Nutr 137(4):973–978
Baxheinrich A, Stratmann B, Lee-Barkey YH, Tschoepe D, Wahrburg U (2012) Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of alpha-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br J Nutr 108(4):682–691. https://doi.org/10.1017/s0007114512002875
Leeson C, Mann A, Kattenhorn M, Deanfield J, Lucas A, Muller D (2002) Relationship between circulating n-3 fatty acid concentrations and endothelial function in early adulthood. Eur Heart J 23(3):216–222
Wendland E, Farmer A, Glasziou P, Neil A (2006) Effect of α linolenic acid on cardiovascular risk markers: a systematic review. Heart 92(2):166–169
Azemati M, Shakerhosseini R, Hekmatdos A, Alavi-Majd H, Hedayati M, Houshiarrad A, Hosseini M, Taherian ME, Noroozi MF, Rashidi M, Amraie A (2012) Comparison of the effects of canola oil versus sunflower oil on the biochemical markers of bone metabolism in osteoporosis. J Res Med Sci 17(12):1137–1143
Bailey CJ, Day C (2012) Diabetes therapies in renal impairment. Br J Diab Vasc Dis 12(4):167–171
Barzilay JI, Davis BR, Pressel SL, Ghosh A, Rahman M, Einhorn PT, Cushman WC, Whelton PK, Wright JT Jr (2018) The effects of eGFR change on CVD, renal, and mortality outcomes in a hypertensive cohort treated with 3 different antihypertensive medications. Am J Hypertens 31(5):609–614
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4(8):118
Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC (2012) Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 17(4):311–321
Shimoyoshi S, Takamoto D, Masutomi H, Kishimoto Y, Amano A, Ono Y, Shibata H, Ishigami A (2017) Sesamin and sesamin combined with alpha-tocopherol improve age-related kidney dysfunction. Innovation Aging 1 (suppl_1):458–458
Hsu D-Z, Li Y-H, Chu P-Y, Periasamy S, Liu M-Y (2011) Sesame oil prevents acute kidney injury induced by the synergistic action of aminoglycoside and iodinated contrast in rats. Antimicrob Agents Chemother 55(6):2532–2536
Hsu D-Z, Liu M-Y (2004) Effects of sesame oil on oxidative stress after the onset of sepsis in rats. Shock 22(6):582–585
Hsu D-Z, Liu M-Y (2004) Sesame oil protects against lipopolysaccharide-stimulated oxidative stress in rats. Crit Care Med 32(1):227–231
Hsu D-Z, Su S-B, Chien S-P, Chiang P-J, Li Y-H, Lo Y-J, Liu M-Y (2005) Effect of sesame oil on oxidative-stress-associated renal injury in endotoxemic rats: involvement of nitric oxide and proinflammatory cytokines. Shock 24(3):276–280
Takeyama N, Shoji Y, Ohashi K, Tanaka T (1996) Role of reactive oxygen intermediates in lipopolysaccharide-mediated hepatic injury in the rat. J Surg Res 60(1):258–262
Mackay DS, Jew S, Jones PJ (2017) Best practices for design and implementation of human clinical trials studying dietary oils. Prog Lipid Res 65:1–11. https://doi.org/10.1016/j.plipres.2016.10.003
Wang D, Bakhai A (2006) Clinical trials: a practical guide to design, analysis, and reporting. Remedica
Iggman D, Gustafsson IB, Berglund L, Vessby B, Marckmann P, Risérus U (2011) Replacing dairy fat with rapeseed oil causes rapid improvement of hyperlipidaemia: a randomized controlled study. J Intern Med 270(4):356–364. https://doi.org/10.1111/j.1365-2796.2011.02383.x
Jones PJH, Senanayake VK, Pu S, Jenkins DJA, Connelly PW, Lamarche B, Couture P, Charest A, Baril-Gravel L, West SG, Liu X, Fleming JA, McCrea CE, Kris-Etherton PM (2014) Dha-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr 100(1):88–97. https://doi.org/10.3945/ajcn.113.081133
Acknowledgements
We appreciate the participants for their voluntary involvement in the project. In addition, we are thankful from Shahid Sadoughi University of Medical Sciences and Neshatavar food industry company (Datis Corporation) for their joint funding of this study. Additionally, scientific support and close cooperation of research council of Nutrition and Food Security Research Center and diabetes research center of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, are greatly appreciated.
Funding
The current study was funded by Shahid Sadoughi University of Medical Sciences (http://www.ssu.ac.ir) and Neshatavar food industry (Datis Corporation; http://www.neshatavar.com/?l=EN). Datis Corporation also provided all the treatment oils consumed during the study including canola, sesame, sesame–canola and sunflower oils. Datis Corporation had no role in design and conduct of this manuscript; collection, management, analysis, and interpretation of the data; and preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
ASA and MA conceived and designed the study. MA and FM recruited the participants and followed them. MA and FM conducted the data collection. MA, HRD, FM, AZ and MM performed the data entry. ASA conducted the statistical analyses. MA wrote the first draft of the manuscript. ASA is the guarantor of the submitted work and takes the full responsibility for the work as a whole. All authors approved the final draft of the manuscript and agreed to be accountable for all aspects of the work, ensuring its integrity and accuracy.
Corresponding author
Ethics declarations
Conflict of interest
The study was jointly funded by Shahid Sadoughi University of Medical sciences and Datis Corporation. The investigators declared that they did not have a direct financial relationship with Datis Corporation and Shahid Sadoughi University of Medical Sciences received the funds and delivered them to the investigators. Datis Corporation did not take any part in the conception, design, the execution of the study protocol, and the reporting of the study results. The corporation did not have any other relationship with the investigators. The authors declare that they have no other potential personal or financial conflicts of interest. The principal investigator (ASA) declares that he has full access to the data and samples provided by this project.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amiri, M., Raeisi-Dehkordi, H., Moghtaderi, F. et al. The effects of sesame, canola, and sesame–canola oils on cardiometabolic markers in patients with type 2 diabetes: a triple-blind three-way randomized crossover clinical trial. Eur J Nutr 61, 3499–3516 (2022). https://doi.org/10.1007/s00394-022-02898-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02898-9