Abstract
Purpose
Pomegranate fruit, Punica granatum L. (Punicaceae), and its constituents have been shown to inhibit inflammation. In this study, we aimed to assess the effects of freeze-dried pomegranate (PWE) on PGE2 production in IL-1β-stimulated SK-N-SH cells.
Methods
An enzyme immunoassay (EIA) was used to measure prostaglandin E2 (PGE2) production from supernatants of IL-1β-stimulated SK-N-SH cells. Expression of COX-2, phospho-IκB, and phospho-IKK proteins was evaluated, while NF-κB reporter gene assay was carried out in TNFα-stimulated HEK293 cells to determine the effect of PWE on NF-κB transactivation. Levels of BACE-1 and Aβ in SK-N-SH cells stimulated with IL-1β were measured with an in cell ELISA.
Results
PWE (25–200 μg/ml) dose dependently reduced COX-2-dependent PGE2 production in SK-N-SH cells stimulated with IL-1β. Phosphorylation of IκB and IKK was significantly (p < 0.001) inhibited by PWE (50–200 μg/ml). Our studies also show that PWE (50–200 μg/ml) significantly (p < 0.01) inhibited NF-κB transactivation in TNFα-stimulated HEK293 cells. Furthermore, PWE inhibited BACE-1 and Aβ expression in SK-N-SH cells treated with IL-1β.
Conclusions
Taken together, our study demonstrates that pomegranate inhibits inflammation, as well as amyloidogenesis in IL-1β-stimulated SK-N-SH cells. We propose that pomegranate is a potential nutritional strategy in slowing the progression of neurodegenerative disorders such as Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is still the most common cause of dementia accounting for 50–75 % of all cases [1], especially in the elderly [2]. As the population of the European continent continues to age, it is predicted that AD will continue to be a major public health problem. Consequently, there is need to identify and develop therapeutic strategies aimed at delaying progression of AD.
Neurodegeneration in AD is linked to the accumulation of senile plaques, which consist of small peptides, known as amyloid-β (Aβ), and intracellular neurofibrillary tangles, consisting of aggregates of hyperphosphorylated tau protein [3]. Neuroinflammation is a process that principally involves activation of astrocytes and microglia by inflammatory mediators in AD [4, 5]. However, in spite of the widely reported roles of microglia and astrocytes in neuroinflammation, it has been suggested that PGE2 produced in neurons may contribute to the self-propagating processes involved in AD. For instance, Hoshino et al. [6] showed that PGE2 stimulates the production of Aβ in cultured human neuroblastoma (SH-SY-5Y) cells. Also, reports have demonstrated elevated levels of PGE2 and COX-2 in the brains of AD patients [7, 8]. Inhibition of PGE2 production and COX-2 expression therefore provides a critical target for reducing the contributions of neurons to the self-perpetuating cycle of neuroinflammation.
The production of COX-2 and other inflammatory factors is regulated by the transcription factor, nuclear factor-kappa B (NF-κB), which has been shown to be widely expressed in the brain. Evidences have been put forward that NF-κB signalling pathways may be activated in AD brains [9]. These have been supported by reports, demonstrating that Aβ peptides could activate NF-κB in neurons [10]. NFκB pathway therefore provides an important target in the understanding of mechanisms involved in modulating inflammation in the neurons.
Accumulation of extracellular Aβ plaques in neurons is one of the important pathological hallmarks in AD. Also, the beta-site amyloid precursor protein (APP) cleaving enzyme1 (BACE-1) plays a key role in the processing of Aβ and its aggregation through catalysing APP [11]. Various studies have demonstrated that the transcription of BACE-1 is controlled by NF-κB and thus Aβ production in neurons [12–15]. In this regard, targeting BACE-1 and Aβ production could be a potential strategy in slowing down the progression of AD.
Pomegranate fruit (Punica granatum L.) is widely consumed for its broad spectrum of nutritional and health benefits. Pomegranate contains polyphenols and tannins, which have been shown to be responsible for most of its nutritional benefits. Extracts and bioactive constituents of pomegranate fruit have been shown to suppress inflammation. Components such as punicalagin and punicalin have been shown to reduce nitric oxide and PGE2 production in intestinal cells [16, 17]. In vitro and in vivo studies showed that pomegranate produced significant reduction in egg albumin-induced hind paw inflammation following intraperitoneal and intracerebroventricular administrations in rats, reduction in carrageenan-induced paw oedema, and NO production and iNOS expression in RAW 264.7 cells [18, 19]. Recently, we showed that one of the bioactive components of pomegranate, punicalagin, inhibited neuroinflammation in LPS-activated microglia [20]. In spite of accumulating evidence showing that inflammation in neurons contributes to the pathology in AD, it is not currently known whether pomegranate or its constituents produce any direct effect on these cells. In this study, we have evaluated the activity of freeze-dried pomegranate juice on PGE2 production in IL-1β-stimulated SK-N-SH cells. In the light of the importance of neuroinflammation to amyloidogenesis, we also investigated whether pomegranate could inhibit BACE-1 and Aβ protein expression in IL-1β-activated neuronal cells.
Materials and methods
Materials
Pomegranate juice (POM Wonderful LLC, Los Angeles, CA) was freeze-dried to a solid sample (PWE), which was then reconstituted in sterile water and stored at −20 °C. Pomegranate juice used in this study was made from fruit skins, which has been standardised to ellagitannins, as punicalagins (80–85 %) and free ellagic acid (1.3 %) as determined by high-performance liquid chromatography [21].
Cell culture
The human neuroblastoma (SK-N-SH) cells were obtained from the HPA Culture Collection (Salisbury, UK) and were grown in MEM Eagle’s medium (Life Technologies, UK). Medium was supplemented with 10 % foetal bovine serum (Sigma, UK), 2 mM l-glutamine, 1 mM sodium pyruvate, 40 units/ml penicillin/streptomycin (Sigma, UK). Confluent monolayers were passaged routinely by trypsinisation. Cultures were grown at 37 °C in 5 % CO2 until 80 % confluence, and the medium was to serum-free MEM the day before treatment.
HEK293 cells were obtained from the HPA Culture Collection (Salisbury, UK) and were grown in MEM Eagle’s medium (Life Technologies, UK). Medium was supplemented with 10 % foetal bovine serum (Sigma, UK), 2 mM l-glutamine, 1 mM sodium pyruvate, 40 units/ml penicillin/streptomycin (Sigma, UK). Confluent monolayers were passaged routinely by trypsinisation. Cultures were grown at 37 °C in 5 % CO2 until 80 % confluence.
PGE2 measurement
Quantification of PGE2 accumulation was carried out in SK-N-SH cells by seeding in 96-well plates (2 × 105/well), cultured for 48 h, and incubated with or without IL-1β (10 U/ml) in the absence or presence of PWE (25–200 μg/ml) for 24 h. PGE2 concentration was assessed in cell supernatants with a commercially available kit (Arbor Assays, Ann Arbor, MI, USA), followed by measurement at 450 nm with a microplate reader. Experiments were performed at least three times and in triplicate.
Sandwich ELISA for COX-2, phospho-IκBα, and phospho-IKKα
Protein expressions of COX-2, phospho-IκBα, and phospho-IKKα were determined using an ELISA for human COX-2, phospho-IκBα, and phospho-IKKα. Cultured SK-N-SH cells were stimulated with IL-1β (10 U/ml) in the presence or absence of PWE (25–200 μg/ml) for 24 h (COX-2), or 5 min (phospho-IκBα and phospho-IKKα). At the end of the experiments, cells were washed with phosphate-buffered saline (PBS) and lysed with 400 μl cell lysis buffer [20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1 % Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin] and incubated on ice for 5 min. The cells were then scraped and centrifuged at 13,500 rpm. Cell lysates were collected and measured for levels of COX-2, phospho-IκBα, and phospho-IKKα, using a PathScan® sandwich ELISA kit (Cell Signalling Technology), according to the manufacturer’s instructions.
Transient transfection and luciferase reporter gene assay
In order to determine the effect of PWE on the transactivation of NF-κB, a luciferase reporter gene assay was carried out. HEK293 cells were seeded out at a concentration of 4 × 105 cells/ml. Twenty-four hours later, cells were transfected with a Cignal® NF-κB Reporter (luc) (SABiosciences), using TransIT®-LT1 transfection reagent (Mirus Bio LLC) and incubated for a further 16 h at 37 °C in 5 % CO2. Twenty-four hours later, transfected HEK293 cells were stimulated with TNFα (1 ng/ml) in the presence or absence of PWE (25–200 μg/ml) for 6 h. NF-κB-mediated gene expression was measured with ONE-Glo luciferase assay kit (Promega, Southampton, UK) according to the manufacturer’s instructions.
In Cell ELISA for BACE1 and Aβ
In Cell ELISA is used for quantitative protein analysis directly in adherent cell cultures and was used to measure BACE-1 and Aβ protein expression following stimulation of SK-N-SH cells with IL-1β, as described earlier [22]. The protocol was based on the MaxDiscovery In Cell ELISA kit (Bio Scientific, Texas). SK-N-SH cells were seeded out in a 96-well plate (2.5 × 105 cells/ml). At 80 % confluence, cells were pre-treated with PWE (25–200 μg/ml) 30 min before stimulation with IL-1β (10 U/ml) for 24 h. At the end of stimulation, cells were washed with 100 μl PBS, fixed, and permeabilised. Primary antibodies (rabbit anti-BACE-1 or rabbit anti-Aβ) were diluted 1:100 and added to each sample well and incubated at room temperature for 1 h. This was followed by incubation with HRP-conjugated anti-rabbit IgG antibody at room temperature for 1 h. TMB solution was added, followed by stop solution and the plate read at 450 nm using a Tecan F50 microplate reader. GAPDH was used as internal control.
Determination of cell viability
Viability of SK-N-SH cells treated with IL-1β (10 U/ml) in the presence or absence of PWE (25–200 μg/ml) was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in 96-well plates (2 × 105 cells/ml) and incubated for 48 h. Thereafter, cells were pre-treated with PWE (25–200 μg/ml) prior to stimulation with IL-1β (10 U/ml). Twenty-four hours after stimulation, culture medium was replaced with MTT solution (5 mg/ml) and incubated for 4 h at 37 °C in 5 % CO2. Thereafter, 150 µl of MTT solution was replaced with DMSO and mixed thoroughly on a plate shaker and read at 540 nm.
Cell viability was also measured using the lactate dehydrogenase (LDH) assay [23]. LDH is a cytosolic enzyme that is an indicator of cellular toxicity. When the plasma membrane is damaged, LDH is released into cell culture media. Cells were seeded in 96-well plates (2 × 105 cells/ml) and incubated for 48 h. Thereafter, cells were pre-treated with PWE (25–200 μg/ml) prior to stimulation with IL-1β (10 U/ml). Cells were then lysed and supernatants collected for LDH assay. LDH levels in supernatants were determined using the CytoTox 96® non-radioactive cytotoxicity assay kit (Promega, Southampton).
Statistical analysis
Values of all experiments were represented as mean ± SEM of at least three experiments. Values were compared using t test (two groups) or one-way ANOVA with post hoc Student–Newman–Keuls test (multiple comparisons). Levels of significance were set at *p < 0.05, **p < 0.01, ***p < 0.001.
Results
PWE reduced PGE2 production by inhibiting cyclooxygenase-2 (COX-2) protein expression in IL-1β-activated SK-N-SH cells
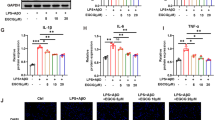
In the presence of IL-1β (10 U/ml), there was a marked increase (***p < 0.01) in PGE2 production in supernatants of SK-N-SH cells, when compared to unstimulated cells. However, treatment with PWE (25–200 μg/ml) for 30 min prior to stimulation with IL-1β resulted in significant reduction (***p < 0.001) in PGE2 production, in comparison with IL-1β control (Fig. 1). PGE2 is synthesised through the enzymatic activity of COX-2. Consequently, we sought to determine whether the effect of PWE on PGE2 was mediated through inhibition of the activities of this enzyme. Interestingly, experiments showed that PWE (25–200 μg/ml) produced significant reduction (***p < 0.001) in COX-2 protein levels in IL-1β-stimulated SK-N-SH cells (Fig. 2).
PWE reduced PGE2 production in IL-1β-stimulated SK-N-SH cells. Cells were stimulated with IL-1β (10 U/ml) in the presence or absence of PWE (25–200 μg/ml) pre-incubated for 30 min. After 24 h, supernatants were collected for PGE2 measurement. All values are expressed as mean ± SEM for three independent experiments. Data were analysed using one-way ANOVA for multiple comparisons with post hoc Student–Newman–Keuls test, *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with IL-1β control
PWE inhibited COX-2 protein expression in IL-1β-stimulated SK-N-SH cells. Cells were stimulated with IL-1β (10 U/ml) in the presence or absence of PWE (25–200 μg/ml) pre-incubated for 30 min. After 24 h, COX-2 protein expression was determined using PathScan® sandwich ELISA. All values are expressed as mean ± SEM for at least three independent experiments. Data were analysed using one-way ANOVA for multiple comparisons with post hoc Student–Newman–Keuls test, *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with IL-1β control
PWE inhibited NF-κB-dependent reporter gene expression in TNFα-activated HEK293 cells
In order to determine the effect of PWE on the transactivation of NF-κB, a luciferase reporter gene assay was carried out. We observed that stimulation of transfected cells with TNFα (1 ng/ml) resulted in activation of the NF-κB-driven luciferase expression (Fig. 3). Pre-incubation with PWE (25 μg/ml) did not affect luciferase expression. However, pre-treatment with 50, 100, and 200 μg/ml of PWE resulted in significant (p < 0.01) and concentration-dependent inhibition of NF-κB-driven luciferase expression, demonstrating that PWE suppresses NF-κB-dependent gene expression in general.
TNFα-induced NF-κB-dependent gene expression in HEK293 cells was inhibited by PWE. Transfected cells were incubated with different concentrations of PWE followed by stimulation with TNFα (1 ng/ml) for an additional 6 h. Luminescence was them measured. All values are expressed as mean ± SEM for three independent experiments performed in triplicate. Data were analysed using one-way ANOVA for multiple comparisons with post hoc Student–Newman–Keuls test, *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with TNFα control
PWE inhibited IL-1β-dependent IκB and IKK phosphorylation in SK-N-SH cells
Based on our observation that PWE inhibited NF-κB-mediated gene expression in general, we sought to investigate its effect on IKK and IκB phosphorylation following stimulation with IL-1β (10 U/ml). Using a sandwich ELISA kit, we observed that IL-1β treatment resulted in phosphorylation of IKK and IκB in IL-1β-treated cells, compared with unstimulated cells. These were significantly inhibited by pre-treatment with 50, 100, and 200 μg/ml of PWE (Figs. 4, 5).
PWE inhibited IL-1β-induced IκB phosphorylation in SK-N-SH cells. Cells were stimulated with IL-1β (10 U/ml) in the presence or absence of PWE (25–200 μg/ml) pre-treated for 30 min. After 5 min, p-IκBα protein expression was determined using PathScan® sandwich ELISA. All values are expressed as mean ± SEM for three independent experiments. Optical densities were measured at 450 nm with a microplate reader. Data were analysed using one-way ANOVA for multiple comparisons with post hoc Student–Newman–Keuls test, *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with IL-1β control
PWE inhibited IL-1β-induced IKK phosphorylation in SK-N-SH cells. Cells were stimulated with IL-1β (10 U/ml) in the presence or absence of PWE (25–200 μg/ml) for 5 min. At the end of incubation period, p-IKKα protein expression was determined using PathScan® sandwich ELISA. All values are expressed as mean ± SEM for three independent experiments. Optical densities were measured at 450 nm with a microplate reader. Data were analysed using one-way ANOVA for multiple comparisons with post hoc Student–Newman–Keuls test, *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with IL-1β control
Pre-treatment of SK-N-SH cells with PWE resulted in inhibition of BACE-1 and Aβ proteins
Exposure of the cells to IL-1β resulted in a marked increase in both BACE-1 and Aβ proteins (Figs. 6, 7). However, pre-treatment with PWE (50,100, and 200 μg/ml) significantly reduced the levels of BACE-1 and Aβ proteins.
PWE inhibited IL-1β-induced BACE-1 in SK-N-SH cells. Cells were pre-treated with PWE (25–200 μg/ml) 30 min before stimulation with IL-1β (10 U/ml) for 24 h. At the end of stimulation, levels of BACE-1 were determined using MaxDiscovery In Cell ELISA kit. All values are expressed as mean ± SEM for three independent experiments. Optical densities were measured at 450 nm with a microplate reader. Data were analysed using one-way ANOVA for multiple comparisons with post hoc Student–Newman–Keuls test, *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with IL-1β control. GAPDH was used as internal control
PWE attenuated IL-1β-induced Aβ production in SK-N-SH cells. Cells were pre-treated with PWE (25–200 μg/ml) 30 min before stimulation with IL-1β (10 U/ml) for 24 h. At the end of stimulation, Aβ production was determined using MaxDiscovery In Cell ELISA kit. All values are expressed as mean ± SEM for three independent experiments. Optical densities were measured at 450 nm with a microplate reader. Data were analysed using one-way ANOVA for multiple comparisons with post hoc Student–Newman–Keuls test, *p < 0.05, **p < 0.01, ***p < 0.001 in comparison with IL-1β control. GAPDH was used as internal control
PWE did not affect the viability of SK-N-SH cells
In order to show that PWE did not affect viability of SK-N-SH cells at concentrations used in this experiment, an MTT assay was performed. Results showed that treatment with PWE (25–200 μg/ml) did not have significant effect on the viability of the cells (Fig. 8a). LDH assay also showed that concentrations of PWE used for pharmacological investigations did not affect viability (Fig. 8b). These results suggest that the observed effects of PWE were not due to cytotoxicity as a result of decreased live cells.
Pre-treatment with PWE (25–200 μg/ml) did not affect the viability of SK-N-SH cells stimulated with IL-1β (10 U/ml). Cells were per-incubated for 30 min with PWE (25–200 μg/ml) in the presence or absence of IL-1β for 24 h. At the end of the incubation period, MTT and LDH assays were carried out on cells. All values are expressed as mean ± SEM for three independent experiments
Discussion
Studies on the role of neuroinflammation in AD have focused mainly on the activity of the microglia. However, studies have shown that inflammation in neurons also contributes to the self-perpetuating processes leading to neuronal loss. For example, studies have shown that PGE2 is able to stimulate Aβ production in SH-SY5Y cells [6]. Furthermore, IL-18 has been shown to increase BACE-1 expression in differentiated SH-SY5Y cells [24]. We therefore investigated whether pomegranate could affect IL-1β-induced PGE2 production, as well as BACE-1 and Aβ production in SK-N-SH cells.
Studies have shown that elevated levels of COX-2 and its metabolic product PGE2 were observed in AD brains, while COX-2 inhibitors markedly reduce the risk of AD [25]. Recent in vivo studies also show that long-term treatment of APP transgenic mice with NSAIDs significantly diminished inflammatory factors and its dependant Aβ deposition [26]. Our results show that pomegranate significantly inhibited COX-2-mediated PGE2 production in IL-1β-stimulated SK-N-SH cells, suggesting that pomegranate could reduce the toxic effects of PGE2 overproduction in neurons.
NF-κB plays a crucial role in regulating the transcription of a wide variety of genes during neuroinflammation and neurodegeneration. In the cytoplasm, NF-κB is coupled with IκB, an inhibitory protein which stays inactive. On activation, IκB undergoes phosphorylation by IKK, resulting in the liberation of NF-κB. This free NF-κB translocates into the nucleus and binds to the promoter region of respective genes such as COX-2. Furthermore, NF-κB activation has been shown to control the transcription of the BACE-1 and APP genes in neurons [13]. To investigate the effect of pomegranate on NF-κB-mediated gene expression in general, a reporter gene assay was carried out. Results show that pomegranate significantly inhibited NF-κB-driven luciferase expression in TNFα-stimulated HEK293 cells, suggesting that this compound is able to attenuate NF-κB-mediated gene expression. To gain a better understanding on the modulatory action of pomegranate on NF-κB signalling pathway, we studied its activity on upstream protein targets. Results show that pomegranate blocked phosphorylation of IκB and IKK in SK-N-SH neuronal cells stimulated with IL-1β; this outcome might suggest that pomegranate acts through interference with NF-κB pathway in neurons. These results were consistent with the outcome of studies conducted by Romier-Crouzet et al. [27] in human intestinal cells. They reported that polyphenolic aqueous extract of pomegranate significantly suppressed NF-κB-mediated NO, PGE2, IL-8 production in IL-1β-activated Caco-2 cells. Our results also reflect the results of studies conducted by Ahmed et al. [28] where it was shown that pomegranate inhibited NF-κB in IL-1β-activated human chondrocytes.
In AD brains, Aβ is generated through proteolysis of APP by β-secretase enzymes. Several studies have identified BACE-1 as an important β-secretase enzyme, which effectively cleaves membrane-bound APP [29]. In vivo studies in transgenic mice have also revealed that BACE-1 is highly involved in Aβ plaque formation, and employing BACE-1 blockers has completely reversed Aβ production [30]. As BACE-1 transcription has been shown to be controlled by NF-κB [13], and since we have shown that pomegranate inhibits NF-κB signalling in SK-N-SH cells, we investigated whether pomegranate would block BACE-1 protein in IL-1β-stimulated SK-N-SH cells. Expectedly, IL-1β induced marked increase in BACE-1 expression in these cells, and this increase was significantly blocked with pomegranate pre-treatment. Interestingly, pomegranate also inhibited Aβ protein induced by IL-1β, suggesting that its effect is probably mediated through the observed interference with BACE-1 enzymatic activity.
This in vitro evidence of the potential nutritional benefits of pomegranate in AD does not prove bioavailability or in vivo biological activity of pomegranate polyphenols following oral intake in humans. However, bioavailability studies have shown that bioactive polyphenols in pomegranate are absorbed from the gastrointestinal tract. Lei et al. [31] reported that punicalagin and ellagic acid reached a plasma concentration of 30 μg/ml and 213 ng/ml, respectively, following oral administration in rats. A study in rabbits reported that pomegranate constituents become bioavailable 2 h after oral ingestion of concentrated pomegranate extract, ellagic acid reaching a plasma value of 247 ng/ml [32]. In a human study, Seeram et al. administered 180 ml of pomegranate juice containing 25 mg ellagic acid and 318 mg ellagitannins to a human subject. Results of this study showed that ellagic acid was detected in human plasma at a concentration of 31.9 ng/ml 1 h after ingestion [33]. Furthermore, ellagitannins in pomegranate have been shown to be metabolised by gut bacteria into urolithins that readily enter systemic circulation. These metabolites appeared in human systemic circulation within a few hours of consumption of pomegranate products, reaching maximum concentrations between 24 and 48 h [34].
To provide benefits in CNS diseases such as AD, pomegranate polyphenols must permeate the blood–brain barrier (BBB). It is not yet clear whether biologically active levels of these compounds could be detected in the CNS following oral administration. However, a study by Farbood et al. [35] showed that oral administration of 100 mg/kg ellagic acid for 7 days prevented cognitive and long-term potentiation deficits in rats. The outcome of this study suggests that ellagic acid permeated the BBB to act in the CNS.
Our study did not establish if the concentrations of pomegranate used in the experiments contain quantities of ellagic acid and ellagitannins which reflect levels which have been detected in plasma. However, we can conclude that bioactive polyphenols in pomegranate could be absorbed from the gastrointestinal tract.
In conclusion, we have provided further data, showing that pomegranate inhibits induced inflammation in SK-N-SH cells. It appears that the effects of pomegranate on inflammatory processes in SK-N-SH cells result in a reduction in BACE-1 and the neurotoxic Aβ. We propose that pomegranate is a potential nutritional strategy in slowing the progression of neuroinflammatory diseases such as AD, possibly through its anti-inflammatory effect. Further pharmacokinetic studies in animals and humans are needed to confirm whether pomegranate polyphenols permeate the BBB and reach biologically active levels in the brain.
References
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368:387–403
Castellani RK, Rolston RK, Smith MA (2010) Alzheimer disease. Dis Mon 56:484–546
Selkoe DJ (2001) Alzheimer’s disease. Genes, proteins, and therapy. Physiol Rev 81:741–766
Messmer K, Reynolds GP (2005) An in vitro model of inflammatory neurodegeneration and its neuroprotection. Neurosci Lett 388:39–44
Chen CH, Zhou W, Liu S, Deng Y, Cai F, Tone M, Tone Y, Tong Y, Song W (2012) Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int J Neuropsychopharmacol 15:77–90
HoshinoT NT, Homan T, Tanaka K, Sugimoto Y, Araki W, Narita M, Narumiya S, Suzuki T, Mizushima T (2007) Involvement of prostaglandin E2 in production of amyloid-peptides both in vitro and in vivo. J Biol Chem 282:32676–32688
Yasojima K, Schwab C, McGeer EG, McGeer PL (1999) Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res 830:226–236
Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ 2nd, Morrow JD (1999) Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology 53:1495–1498
Paris D, Patel N, Quadros A, Linan M, Bakshi P, Ait-Ghezala G, Mullan M (2007) Inhibition of Abeta production by NF-kappaB inhibitors. Neurosci Lett 415:11–16
Bales KR, Du Y, Dodel RC, Yan GM, Hamilton-Byrd E, Paul SM (1998) The NF–kappaB/Rel family of proteins mediates Abeta-induced neurotoxicity and glial activation. Brain Res Mol Brain Res 57:63–72
Marwarha G, Raza S, Meiers C, Ghribi O (2014) Leptin attenuates BACE1 expression and amyloid-β genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta 1842:1587–1595
Bourne KZ, Ferrari DC, Lange-Dohna C, Rossner S, Wood TG, Perez-Polo JR (2007) Differential regulation of BACE1 promoter activity by NFB in neurons and glia upon exposure to Ab peptides. J Neurosci Res 85:1194–1204
Buggia-Prevot V, Sevalle J, Rossner S, Checler F (2008) NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42. J Biol Chem 283:10037–10047
Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C, Catalano MG, Smith MA, Perry G, Danni O, Boccuzzi G, Tabaton M (2012) AGEs/RAGE complex upregulates BACE1 via NF-κB pathway activation. Neurobiol Aging 33:196-e13–196-e27
Camandola S, Poli G, Mattson MP (2000) The lipid peroxidation product 4-hydroxy-2,3-nonenal inhibits constitutive and inducible activity of nuclear factor kappa B in neurons. Brain Res Mol Brain Res 85:53–60
Lee SI, Kim BS, Kim KS, Lee S, Shin KS, Lim JS (2008) Immunosuppressive activity of punicalagin via inhibition of NFAT activation. Biochem Biophys Res Commun 371:799–803
Romier B, Van De Walle J, During A, Larondelle Y, Schneider YJ (2008) Modulation of signalling nuclear factor-kappaB activation pathway by polyphenols in human intestinal Caco-2 cells. Br J Nutr 100:542–551
Oucharif A, Khalki H, Chaib S, Mountassir M, Aboufatima R, Farouk L, Benharraf A, Chait A (2012) Comparative study of the anti-inflammatory and antinociceptive effects of two varieties of Punica granatum. Pharm Biol 50:429–438
Lee CJ, Chen LG, Liang WL, Wang CC (2010) Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem 118:315–322
Olajide OA, Kumar A, Velagapudi R, Okorji U, Fiebich BL (2014) Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol Nutr Food Res 58:1843–1851
Seeram NP, Lee R, Hardy ML, Heber D (2005) Large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep Purif Technol 41:49–55
Olajide OA, Velagapudi R, Okorji U, Sarker S, Fiebich BL (2014) Picralima nitida seeds suppress PGE2 production by interfering with multiple signalling pathways in IL-1β-stimulated SK-N-SH neuronal cells. J Ethnopharmacol 152:377–383
Decker T, Lohmann-Matthes ML (1988) A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 115:61–69
Sutinen EM, Pirttilä T, Anderson G, Salminen A, Ojala JO (2012) Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J Neuroinflamm 9:199
Pasinetti GM, Aisen PS (1998) Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience 87:319–324
Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM (2000) Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci 20:5709–5714
Romier-Crouzet B, Van De Walle J, During A, Joly A, Rousseau C, Henry O, Larondelle Y, Schneider YJ (2009) Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem Toxicol 47:1221–1230
Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM (2005) Punica granatum L. extract inhibits IL–1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappa B in human chondrocytes in vitro. J Nutr 135:2096–2102
Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V (1999) Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402:537–540
Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R (2001) Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci 4:231–232
Lei F, Xing DM, Xiang L, Zhao YN, Wang W, Zhang LJ, Du LJ (2003) Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J Chromatogr B Analyt Technol Biomed Life Sci 796:189–194
Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM (2008) Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL–1beta-induced PGE2 production in human chondrocytes in vitro. J Inflamm (Lond) 5:9
Seeram NP, Lee R, Heber D (2004) Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta 348:63–68
Bialonska D, Kasimsetty SG, Khan SI, Ferreira D (2009) Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J Agric Food Chem 57:10181–10186
Farbood Y, Sarkaki A, Dianat M, Khodadadi A, Haddad MK, Mashhadizadeh S (2015) Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci 124:120–127
Acknowledgments
This study was carried out in part with funding by the Alexander von Humboldt Foundation to Dr. Olumayokun Olajide. We wish to thank Mr. Oluwatodimu Sam-Dahunsi for assisting with freeze-drying of pomegranate juice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Velagapudi, R., Baco, G., Khela, S. et al. Pomegranate inhibits neuroinflammation and amyloidogenesis in IL-1β-stimulated SK-N-SH cells. Eur J Nutr 55, 1653–1660 (2016). https://doi.org/10.1007/s00394-015-0984-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0984-0