Abstract
Neuroinflammation is a key factor in cognitive dysfunction and neurodegenerative diseases such as Alzheimer’s disease (AD), so inhibiting neuroinflammation is considered as a potential treatment for AD. Epigallocatechin-3-gallate (EGCG), a polyhydroxyphenol of green tea, has been found to exhibit anti-oxidative, anti-inflammatory and neuroprotective effects. The aim of this study was to investigate the inhibitory effect of EGCG on inflammation and its mechanism. In this study, BV2 cells were simultaneously exposed to lipopolysaccharides (LPS) and the amyloid-β oligomer (AβO) to induce inflammatory microenvironments. Inflammatory cytokines and NLRP3 inflammasome-related molecules were detected by RT-PCR and Western Blot. The results show that EGCG inhibits LPS/AβO-induced inflammation in BV2 cells through regulating IL-1β, IL-6, and TNF-α. Meanwhile, EGCG reduces the activation of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome and levels of intracellular ROS in BV2 cells treated with LPS/AβO by affecting the mitochondrial membrane potential (MMP). Further research found that EGCG inhibited MMP through regulating thioredoxin-interacting protein (TXNIP) in LPS/AβO-induced neuroinflammation. In conclusion, EGCG may alleviate LPS/AβO-induced microglial neuroinflammation by suppressing the ROS/ TXNIP/ NLRP3 pathway. It may provide a potential mechanism underlying the anti-inflammatory properties of EGCG for alleviating AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a kind of primary degenerative encephalopathy, that occurs in the elderly and presenile characterized by persistent cognitive dysfunction and behavioral impairment. Neuropathological changes of AD include β-amyloid protein (Aβ) deposition, abnormal neurofibrillary tangles (NFTs) and loss of neurons in the brain(Edwards 2019). It was reported that chronic deposition of Aβ stimulates the persistent activation of microglial cells and increases IL-1β levels, which initiates chronic inflammation, eventually causes neuronal death, and develops into cognitive impairment in AD(Leng and Edison 2021). Besides, nucleotide oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation has been implicated in microglial inflammation and AD(Chen et al. 2021; Zhang et al. 2020a, b). Therefore, prevention of inflammasome activation may be a potential intervention in the treatment of AD(Luo et al. 2022).
Recent studies suggested that ROS production is induced by many NLRP3 inflammasome stimulators, and elevated ROS is essential for inflammasome activation (Dominic et al. 2022). ROS are shown to be a critical factor triggering NLRP3 inflammasome formation and activation in response to many exogenous stimuli (Billingham et al. 2022). The primary factor contributing to the elevation of reactive oxygen species (ROS) levels is mitochondrial dysfunction (Angelova and Abramov 2018). Furthermore, thioredoxin-interacting protein (TXNIP), the endogenous inhibitor of the ROS scavenging protein thioredoxin (TRX), is a bridge between oxidative stress and the NLRP3 inflammasome. In addition, TXNIP is vital for the activation of the NLRP3 inflammasome (Du et al. 2020; Mohamed et al. 2021).
Epigallocatechin-3-gallate (EGCG) is a polyhydroxyphenol of green tea (Camellia sinensis) (Kochman et al. 2020). It was found that EGCG markedly decreased reactive oxygen species (ROS), inflammation factors, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (Zhang et al. 2019). Due to the antioxidant effects of EGCG, the neuroprotective effects of EGCG against Aβ-induced neuronal loss and tau toxicity in AD models were also reported in several studies (Zhang et al. 2020a, b). Therefore, EGCG can be selected for the potential targets in alleviating AD (Zhang et al. 2020a, b; Valverde-Salazar et al. 2023; Zhong et al. 2019). EGCG, as a common polyphenolic natural product, has been widely studied in neuroinflammation, with anti-neuroinflammatory and anti-Aβ aggregation effects. However, no relevant research has been carried out on the ROS/TXNIP/NLRP3 pathway.
In the present study, we found that EGCG inhibited inflammation produced by microglial BV2 stimulated by LPS and AβO, which may explain the beneficial health effects of EGCG on AβO-mediated AD toxicity. The anti-inflammatory mechanism of EGCG in inhibiting NLRP3 inflammasome activation through mitochondrial ROS/TXNIP/NLRP3 axis was investigated by Western Blot, RT-PCR, immunohistochemistry, intracellular ROS detection, mitochondrial membrane potential fluorescence staining and cell transfection.
Materials and methods
Chemicals and Reagents
EasySee Western Blot Kit was obtained from TransGen Biotech (Beijing, China). Lipopolysaccharide (LPS, Sigma-Aldrich, USA) was dissolved in sterile water and prepared into 2 mg/mL mother liquor, stored in -20℃ refrigerator. PH is the most important factor affecting the stability of EGCG (Solarbio, China). EGCG is extremely unstable in neutral and alkaline aqueous solutions, so we dissolved EGCG in DMSO (DMSO:92 mg/mL 200.71 mM) to form mother liquor to adjust PH < 7 Store in the refrigerator at -20 ℃ away from light MCC950 (Med Chem Express, USA) was dissolved in DMSO to form 20 mΜ reserve solution and stored in -80℃ refrigerator. Mitoquinone (Mitoq, Med Chem Express, USA) was dissolved in DMSO to form 40 mΜ reserve solution and stored at -80 ℃ refrigerator. The NLRP3 activator adenosine triphosphate (ATP, Med Chem Express, USA); the ROS scavenger N-acetylcysteine (NAC, Beyotime Biotechnology, China) and the ROS activator Tert-butyl hydrogen peroxide (TBHP, Thermo Fisher, USA) reagents are all ready for use. All other reagents were at the highest analytical levels.
Culture and Treatment of BV2 Cells
The BV2 cells (Beijing, China) were cultured in incomplete high-glucose DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in an incubator at 37 ℃ with 5% CO2.
BV2 cells will be implanted on the 12-well plate 12 h before the experiment to ensure that the cell density and number meet the experimental requirements. In the EGCG group, BV2 cells were exposed to EGCG (5 µM, 10 µM, and 20 µM) solution or MCC950 (10 µM) or NAC (20 mM) or Mitoquinone (Mitoq, 0.2 µM) for 1 h. Then the cells were treated with LPS (1 µg/mL) for 1 h and Aβ1−42 oligomer (AβO) (10 µg/mL) for 6 h (Zhong et al. 2019). Untreated cells were used as control. Besides, BV2 cells were treated with the NLRP3 activator ATP (5 mM) for the final 30 min or the ROS activator TBHP (100 µM) for the final 3 h.
Aβ1−42 Oligomer (AβO) Preparation and Identification
AβO was prepared as previously described (Jian et al. 2019). In short, The HFIP (1,1,1,3,3,3-hexafluoride-o-2-propanol) (Sigma-Aldrich, USA) is first cooled by placing it on ice in a fume hood. Then, 1 mg of Aβ 1−42 peptide (GL Biochem, China) was dissolved to 1 mM in 222 µL cold HFIP. At room temperature (RT), the solution was incubated for 1 h, and then the solution was incubated for 10 min on ice. After incubation, the solution was dried overnight at RT in a fume hood. Then, the peptide film was dissolved in 44.34 µL dimethyl sulfoxide (DMSO) and ultrasound oscillated for 10 min. The solution is quickly distributed in aliquots of 5.43 µL in a sterile centrifuge tube and stored at -80℃. The day before each experiment, the tube was added with an F12 medium to make it a 100 µM concentration solution. Then, the solution was incubated overnight at 4℃. The Aβ oligomers appeared in the supernatant after being centrifuged at 14,000×g for 10 min at 4 ℃. To confirm the existence of Aβ oligomers, we used anti-Aβ1–42 antibody (Proteintech, China) to analyze by Western Blot and Immunocytochemistry (ICC). The day before each experiment begins, the Aβ1–42 oligomer peptide was dissolved in an F12 medium and incubated overnight in 4 ℃ refrigerators.
Cell Counting Kit-8 (CCK8) Assay
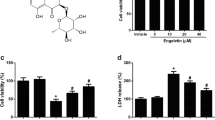
BV2 cells (1 × 104 per well) were seeded in a 96-well culture plate in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin and cultured for 12 h. Then, cells were incubated without or with EGCG (5, 10 and 20 µM) conditions for 24 h. Finally, 10 µL of CCK-8 reagent (Beyotime Biotechnology, China) with 90 µL Serum-free medium was added to each well at 37 ℃ for 90 min, and the OD value was measured at 450 nm with a microplate reader. Cell survival was normalized to 100% in the untreated group.
Western Blot Analysis
Proteins of BV2 cells were performed as described previously (Long et al. 2020). Antibodies used in this study were anti-IL-1β, anti-TNF-α, anti-Iba-1, anti-NLRP3, and anti-Caspase-1 (1:1000, Abcam, China); anti-ASC (1:1000, Cell Signaling Technology, MA); anti-IL-6 (1:1000, Bioss, China); anti-GAPDH (1:1000, Boster Biological Technology, China); anti-Aβ1−42 (1:1000, Proteintech, China). The blots were visualized using an enhanced chemiluminescence system (Bio-Rad, CA) and the bands were quantified using Image J software. Then all band’s intensity were normalized against GAPDH.
Real-time PCR Analysis
The transcriptional levels of the objective genes were measured through Real-time PCR protocols. First, the total RNAs were extracted from the BV2 cells using the PureLink RNA mini kit (Thermo, China). Then, the reverse transcription reaction was performed according to the manufacturer’s instructions (TransGen, China), resulting in the first strand of total complementary DNA (cDNA). Real-time PCR was performed on cDNA templates using the CFX96 Real-time PCR system (Bio-Rad, USA). The primers used in this study are listed in Table 1. The results were normalized against GAPDH as an internal ctrl.
Immunofluorescence Assay
For immunofluorescence assay, BV2 cells grown on coverslips were plated in a 24-well plate coated with 400 µL Poly- D -lysine/lamine (PLL), After incubating with the indicated agent, the cells were rinsed in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde in PBS for 20 min, followed by permeabilization with 0.25% Triton X-100 in PBS for 8 min at room temperature, and cells were washed three times before being blocked by 5% FBS in PBS. The fixed cells were incubated with anti-Aβ1−42 (1:100, Proteintech, China) or anti-Iba-1 (1:100, Abcam, China) at 4 ℃ overnight, followed by incubation of Alexa Fluor-conjugated 488 (FITC) and 568 (CY3) secondary antibodies for 1 h at RT. Subsequently, coverslips were mounted onto slides in Prolong Gold mounting media with DAPI (Invitrogen, China). Fluorescent imaging was performed using a fluorescence microscope according to the manufacturer’s instructions.
Detection of Intracellular ROS
The intracellular production of ROS in BV2 cells was measured by DCFH-DA (Solarbio, China). Briefly, BV2 cells were cultured to 70% confluency and then treated with or without EGCG (10 µM), NAC (20 mM), TBHP (100 µM), LPS/AβO-induced for 6 h. After treatment, the cells were washed twice with PBS and incubated with DCFH-DA in the dark at 37 ℃ for 0.5 h. ROS production was visualized by a fluorescence microscope at a fluorescent signal (488 nm excitation and 525 nm emission).
Detection of Intracellular Mitochondria Membrane Potential (MMP)
JC-1 is an ideal fluorescent probe for the detection of mitochondrial membrane potential (MMP). When MMP is increased, JC-1 aggregates in the mitochondrial matrix to form polymers and produce red fluorescence, while when MMP is decreased, JC-1 cannot aggregate in the mitochondrial matrix, but only to form monomers and emit green fluorescence. Therefore, the change of MMP can be represented by the ratio of red fluorescence to green fluorescence. Briefly, cells were cultured in a 12-well plate and subjected to different experimental treatments. Then, cells were treated with a JC-1 solution for 20 min of incubation at 37℃. Finally, labeled-cells were washed twice with buffer solution. MMP was measured by a fluorescence microscope.
BV2 Cells Transfection
Small interfering RNA (siRNA) of TXNIP and Negative control, as well as the TXNIP overexpressed plasmid vector pEX-3 and the empty vector of ctrl plasmid pEX-3 were synthesized by Shanghai GenePharma (China). Transfection was performed by Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. After 6 h transfection, the culture medium was replaced with fresh medium and incubated for an additional 12 h. The silencing efficiency was confirmed by Real-time PCR after transfection. A sequence of TXNIP siRNA and Negative ctrl used in this study were listed in Table 2.
Statistical Analysis
All data were analyzed by Ordinary one-way ANOVA. Statistical analyses and graphical representations were carried out using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA). Data are expressed as means ± standard error of the mean (SEM). An alpha level of 0.05 was used for all statistical tests. *p < 0.05, **p < 0.01, ***p < 0.001.
Results
EGCG Inhibits LPS/AβO-induced Activation and Inflammation in BV2 Cells
First, we identified that EGCG was not cytotoxic to BV2 cells at the concentration of (5 µM, 10 µM, 20 µM) through CCK8 assay in our study (Fig. S1A). Secondly, BV2 cells were treated with LPS (1 µg/mL for 1 h) and AβO (10 µM for 6 h) to induce inflammatory response (Zhong et al. 2019). And mRNA expression of inflammatory factors (IL-1β, IL-6, TNF-α) were detected using RT-PCR and (Fig. 1A-C). Besides, the protein levels of Aβ42, Iba-1, IL-1β, IL-6, TNF-α were detected (Fig. 1D-I). It showed that the microglial activation marker of Iba-1, as well as pro-inflammatory factors of IL-1β, IL-6, TNF-α were significantly elevated in LPS/AβO-induced BV2 cells, while EGCG reversed this elevation, suggesting that EGCG has an inhibitory effect on LPS/AβO-induced inflammation in BV2 cells. Then Iba-1, the microglial activation marker, was evaluated by using immunofluorescence staining. Immunofluorescence results showed that EGCG reduces Aβ deposition in BV2 cells (Fig. 1J).
EGCG Inhibits LPS/AβO-induced activation and inflammation in BV2 cells (A-C) The mRNA levels of IL-1β, IL-6, and TNF-α were analyzed by RT-PCR(n = 3). BV2 cells were treated with EGCG (0 µm, 5 µM, 10 µM and 20 µM), followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (D-I) The protein expressions of Aβ42, Iba‐1, IL‐1β, IL‐6, and TNF-α were analyzed by Western Blot (n = 3). BV2 cells were treated with EGCG (0 µM, 5 µM,10 µM and 20µM), followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h.(E-I) The relative protein level of Aβ42, Iba‐1, IL‐1β, IL‐6, TNF-α, and GAPDH as the loading ctrl(n = 3). (J) Immunofluorescence staining was used to detect the effect of Iba-1 on BV2 cell activation and AβO deposition. *p < 0.05, **p < 0.01, ***p < 0.001
EGCG Inhibits LPS/AβO-induced Inflammation by Suppressing NLRP3 Inflammasome in BV2 Cells
We sought to determine whether NLRP3 inflammasome activation is involved in the inhibition of EGCG on LPS/AβO-induced inflammation in BV2 cells. The protein levels of NLRP3 inflammasome-associated molecules, including NLRP3, ASC, Caspase-1, as well mRNA of NLRP3 were measured. The results investigated LPS/AβO induced increases in NLRP3, ASC, Caspase-1, while EGCG inhibited the increase (Fig. 2A-E), suggesting that EGCG inhibited LPS/AβO-induced NLRP3 inflammasome activation in BV2 cells.
EGCG inhibits LPS/AβO-induced inflammation by suppressing NLRP3 inflammasome in BV2 cells. (A) The mRNA level of NLRP3 was analyzed by RT-PCR(n = 3). BV2 cells were treated with EGCG (0 µM, 5 µM, 10 µM and 20 µM), followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h.(B)The expressions of NLRP3, ASC, and Caspase-1 were analyzed by Western Blot(n = 3). BV2 cells were treated with EGCG (0 µM, 5 µM, 10 µM and 20 µM), followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h.(C-E) The relative protein levels of NLRP3, ASC, Caspase-1, and GAPDH as the loading ctrl(n = 3). (F) The mRNA level of IL-1β was analyzed by RT-PCR(n = 3). BV2 cells were treated without or with EGCG (10 µM), inflammasome inhibitor (MCC950, 10 µM), or the NLRP3 activator (ATP 5 µM) for 1 h, followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h(n = 3).(G) The expression of IL-1β was analyzed by Western Blot(n = 3). (H) The relative protein levels of IL-1β and GAPDH as the loading ctrl(n = 3). *p < 0.05, **p < 0.01, ***p < 0.001
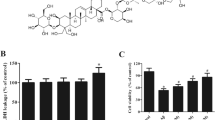
To further confirm the role of NLRP3 inflammasome activation in inhibition of EGCG on LPS/AβO-induced inflammation, the NLRP3 inflammasome inhibitor MCC950, and the NLRP3 activator ATP were used in the study. Here, 10 µM of EGCG was used, for it showed the best inhibition effect in the above experiment, and IL-1β expression was measured, because IL-1β initiates the inflammation response and affects the release of other inflammatory cytokines such as TNF-α, IL-6 (Shimodaira et al. 2018). The result showed that the IL-1β expression in LPS/AβO-induced BV2 was downregulated by MCC950 (Fig.S1B, C and Fig. 2F-H). EGCG inhibited IL-1β expression, while the NLRP3 activator ATP partially reversed the inhibitory effect of EGCG on IL-1β expression.
EGCG Inhibits the Activation of NLRP3 Inflammasome by Reducing Oxidative Stress in LPS/AβO Induced BV2 Cells
ROS is reported to be essential for the activation of NLRP3 inflammasomes (Long et al. 2020). Therefore, we intended to determine whether the suppressing of EGCG on NLRP3 Inflammasome is related to the inhibiting of oxidative stress. Firstly, the intracellular ROS levels in BV2 cells were detected by fluorescent probe staining. LPS/AβO induced an increase of ROS in BV2 cells while EGCG inhibited ROS in BV2 cells (Fig. 3A). Furthermore, ROS inhibitor (NAC, 20 mM) inhibited intracellular ROS levels and ROS activator (TBHP, 100 µM) reversed the inhibition of EGCG on ROS levels in BV2 cells (Fig. S1D, E and Fig. 3B). Besides, the IL-1β and NLRP3 inflammasome related proteins of NLRP3, ASC, Caspase-1 were detected by Western Blot. The results found that EGCG inhibited the expression of IL-1β, NLRP3, ASC, and Caspase-1, and this effect was partially reversed by TBHP (Fig. 3C-G). These findings suggest that EGCG may inhibit NLRP3 inflammasome activation by reducing oxidative stress in LPS/AβO induced BV2 cells.
EGCG inhibits NLRP3 inflammasome by reducing oxidative stress in LPS/AβO induced BV2 cells. (A) Intracellular ROS levels in BV2 cells were measured with fluorescent probe staining. BV2 cells were treated with EGCG (0 µM, 5 µM, 10 µM and 20 µM), followed by stimulation with LPS (µg/mL) for 1 h and AβO (10 µM) for 6 h. (B) Intracellular ROS levels in BV2 cells were measured with fluorescent probe staining. BV2 cells were treated without or with EGCG (10 µM), ROS inhibitor (NAC, 20 mM), or ROS activator (TBHP, 100 µM) for 1 h, followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (C) The expressions of IL-1β, NLRP3, ASC and Caspase-1 were analyzed by Western Blot(n = 3). BV2 cells were treated without or with EGCG (10 µM), ROS inhibitor (NAC, 20mM), or ROS activator (TBHP, 100 µM) for 1 h, followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (D-G) The relative protein levels of IL-1β, NLRP3, ASC, Caspase-1 and GAPDH as the loading ctrl(n = 3). *p < 0.05, **p < 0.01, ***p < 0.001
EGCG Inhibits the Production of ROS due to Mitochondrial Dysfunction
We further explored whether EGCG inhibits the production of ROS by mitochondrial dysfunction in LPS/AβO induced BV2 cells. EGCG inhibited intracellular ROS levels, and Mitoquinone (Mitoq), a specific inhibitor of mitochondrial ROS, presented similar effects (Fig.S1F, G).
Then, we examined the change of mitochondrial membrane potential (MMP) by using the JC-1 fluorescence probe. MMP in normal cells shows red fluorescent of JC-1 dimers while loss of MMP caused the increase of green fluorescent of JC-1 monomers. As shown in Fig. 4A, green fluorescence increased in the LPS/AβO group, suggesting that LPS/AβO treatment triggered mitochondrial damage. Mitoq and EGCG reduced mitochondrial damage induced by LPS/AβO, and TBHP reversed the protective effect of EGCG (Fig.S1H). Similarly, Mitoq or EGCG inhibited the expression of NLRP3 inflammasome-related molecules of NLRP3, ASC, Caspase-1 induced by LPS/AβO and TBHP partially blocked this effect of EGCG (Fig. 4B-F). According to the above results, we revealed that EGCG inhibits the production of ROS due to mitochondrial dysfunction.
EGCG inhibits the production of ROS due to mitochondrial dysfunction. (A) The level of mitochondrial membrane potential (MMP) in BV2 cells was detected by fluorescent probe staining. BV2 cells were treated without or with EGCG (10 µM), and the specific inhibitor of mitochondrial ROS (Mitoq, 0.2 µM), or ROS activator (TBHP, 100 µM) for 1 h, followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (B) The expressions of IL-1β, NLRP3, ASC, and Caspase-1 were analyzed by Western Blot(n = 3). BV2 cells were treated without or with EGCG (10 µM), and the specific inhibitor of mitochondrial ROS (Mitoq, 0.2 µM), or ROS activator (TBHP, 100 µM) for 1 h, followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (C-F) The relative protein levels of IL-1β, NLRP3, ASC, Caspase-1 and GAPDH as the loading ctrl(n = 3). *p < 0.05, **p < 0.01, ***p < 0.001
TXNIP is Involved in Mediating the Inhibitory Effect of EGCG on the Activation of the NLRP3 Inflammasome in BV2 Cells
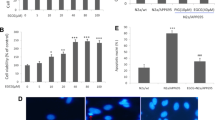
TXNIP is a key signal linking oxidative stress and NLRP3 inflammasome activation (Li et al. 2019). Studies have confirmed that TXNIP, NLRP3 and IL-1β overexpression in the brain of patients with AD (Li et al. 2019). So, we explored the role of TXNIP in oxidative stress and NLRP3 inflammasome activation in BV2 cells induced jointly by LPS/AβO. In Fig. 5A and B, the results of Western Blot showed that EGCG suppressed the protein expression of TXNIP in LPS/AβO-induced BV2 cells. In Fig. 5C, mRNA levels of TXNIP in BV2 cells showed similar trends, among them, the EGCG concentration of 10 µM had the best inhibitory effect. Therefore, TXNIP knockdown (TXNIP siRNA) and overexpression (TXNIP pEX-3) were constructed. The protein levels of TXNIP, NLRP3, ASC, Caspase-1 and IL-1β were increased by LPS/AβO treatment and decreased by TXNIP knockdown. Besides, Western Blot showed that the protein levels of IL-1β, NLRP3 and TXNIP were increased in the LPS/AβO group, and decreased in the TXNIP knockout group. Similarly, EGCG inhibited its expression, while TXNIP overexpression partially reversed the inhibition of EGCG (Fig. 5D-I).
TXNIP is Involved in Mediating the Inhibitory Effect of EGCG on the Activation of the NLRP3 Inflammasome in BV2 Cells (A) Western Blot analysis showed protein expression of TXNIP(n = 3). BV2 cells were treated with EGCG (0 µM, 5 µM, 10 µM, 20 µM) followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (B) The relative protein levels of TXNIP as the loading ctrl(n = 3). BV2 cells were treated with EGCG (0 µM, 5 µM, 10 µM, 20 µM) followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (C) The mRNA level of TXNIP was analyzed by RT-PCR(n = 3). BV2 cells were treated with EGCG (0 µM, 5 µM, 10 µM, 20µM), followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (D) The expressions of IL-1β, NLRP3, ASC, Caspase-1 and TXNIP were analyzed by Western Blot(n = 3). TXNIP knockdown (TXNIP siRNA) and overexpression (TXNIP pEX-3) were constructed. BV2 cells were treated without or with EGCG (10 µM), followed by stimulation with LPS (1 µg/mL) for 1 h and AβO (10 µM) for 6 h. (E-I) The relative protein levels of IL-1β, NLRP3, ASC, Caspase-1, TXNIP and GAPDH as the loading ctrl(n = 3). *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
Neuroinflammation is an important factor in the pathogenesis of AD and plays an important role in the occurrence and development of AD (Li et al. 2019). Accumulation of misfolded proteins is a hallmark of AD, and dysregulation of the clearance of misfolded proteins by microglia leads to neuroinflammation under pathological conditions. The causes of neuroinflammation in the brain are complex, and some studies suggest that the accumulation of amyloid behind the production of inflammatory factors in the brain. LPS stimulation alone can only cause an increase in the levels of proinflammatory cytokines in the cells, which cannot fully mimic the external environment of brain glial cells. Therefore, we used LPS/AβO model according to previous studies.
NLRP3 inflammasome is an intracellular multiprotein complex composed of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and cysteine protease Caspase-1, and contributes to inflammatory responses in various diseases, including cardiovascular diseases (Takahashi 2022). The formation of NLRP3 inflammasome causes auto-activation of Caspase-1 which leads to the processing of pro-IL-1β and pro-IL-18 to their forms, resulting in inflammation (Huang et al. 2021). Cross-regulation between NLRP3 inflammasome-driven IL-1β and IL-18 contributes to the pathophysiology of LPS-induced inflammation (Fujimura et al. 2023). Therefore, in this thesis, we chose inflammasomes as the indicator of neuroinflammation, investigated the inhibitory effect of EGCG on inflammasomes, and revealed the specific regulatory mechanism.
In previous studies, EGCG was found to inhibit systemic inflammation-induced neuroinflammation and amyloidosis, as well as memory impairment, and to improve dendritic integrity (Lee et al. 2013) and synaptic protein expression levels in the brain of APP/PS1 mice (Bao et al. 2020). In this study, the anti-inflammatory mechanism of EGCG was investigated in vitro. Consistent with the previous study, EGCG inhibited the activation of inflammasome. On this basis, this study attempts to explain the anti-inflammatory effect of EGCG from a new perspective, from EGCG to restore mitochondrial dysfunction, reduce intracellular ROS production, inhibit TXNIP, and reduce the expression of TXNIP. The link between oxidative stress and neuroinflammatory activation via the ROS/TXNIP/NLRP3 pathway provides a new possible target for EGCG treatment of neuroinflammation-related diseases.
This study has demonstrated the possible mechanism of EGCG in alleviating neuroinflammation in vitro. However, the pharmacokinetics of EGCG in vivo and how EGCG interact with other molecules in the brain still need to be explored in vivo. Interestingly, we found that EGCG inhibited inflammasome activation through ROS/TXNIP/NLRP3 pathway in vitro. Although the level of inflammation was increased in EGCG treatment group, the level of inflammatory molecules was still lower than that induced by LPS + AβO. This suggests that EGCG may not only inhibit the activation of inflammasome through this pathway, but also provide more ideas for our later research.
Data Availability
The data that support the findings of this study are available from the corresponding author, [T.C], upon reasonable request.
Code Availability
All software used in the course of this study is officially licensed software.
References
Angelova PR, Abramov AY (2018) Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS LETT 592(5):692–702
Bao J, Liu W, Zhou HY, Gui YR, Yang YH, Wu MJ, Xiao YF, Shang JT, Long GF, Shu XJ (2020) Epigallocatechin-3-gallate alleviates cognitive deficits in APP/PS1 mice. CURR MED SCI 40(1):18–27
Billingham LK, Stoolman JS, Vasan K, Rodriguez AE, Poor TA, Szibor M, Jacobs HT, Reczek CR, Rashidi A, Zhang P, Miska J, Chandel NS (2022) Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. NAT IMMUNOL 23(5):692–704
Chen J, Sun J, Hu Y, Wan X, Wang Y, Gao M, Liang J, Liu T, Sun X (2021) MicroRNA-191-5p ameliorates amyloid-β (1–40) -mediated retinal pigment epithelium cell injury by suppressing the NLRP3 inflammasome pathway. FASEB J 35(4):e21184
Dominic A, Le NT, Takahashi M (2022) Loop between NLRP3 inflammasome and reactive oxygen species. ANTIOXID REDOX SIGN 36(10–12):784–796
Du J, Wang Y, Tu Y, Guo Y, Sun X, Xu X, Liu X, Wang L, Qin X, Zhu M, Song E (2020) A prodrug of epigallocatechin-3-gallate alleviates high glucose-induced pro-angiogenic factor production by inhibiting the ROS/TXNIP/NLRP3 inflammasome axis in retinal Müller cells. EXP EYE RES 196:108065
Edwards FA (2019) A unifying hypothesis for Alzheimer’s Disease: from plaques to Neurodegeneration. TRENDS NEUROSCI 42(5):310–322
Fujimura K, Karasawa T, Komada T, Yamada N, Mizushina Y, Baatarjav C, Matsumura T, Otsu K, Takeda N, Mizukami H, Kario K, Takahashi M (2023) NLRP3 inflammasome-driven IL-1β and IL-18 contribute to lipopolysaccharide-induced septic cardiomyopathy. J MOL CELL CARDIOL 180:58–68
Huang Y, Xu W, Zhou R (2021) NLRP3 inflammasome activation and cell death. CELL MOL IMMUNOL 18(9):2114–2127
Jian M, Kwan JS, Bunting M, Ng RC, Chan KH (2019) Adiponectin suppresses amyloid-β oligomer (AβO)-induced inflammatory response of microglia via AdipoR1-AMPK-NF-κB signaling pathway. J NEUROINFLAMM 16(1):110
Kochman J, Jakubczyk K, Antoniewicz J, Mruk H, Janda K (2020) Health benefits and Chemical composition of Matcha Green Tea: a review. Molecules 26(1):85
Lee YJ, Choi DY, Yun YP, Han SB, Oh KW, Hong JT (2013) Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J NUTR BIOCHEM 24(1):298–310
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? NAT REV NEUROL 17(3):157–172
Li L, Ismael S, Nasoohi S, Sakata K, Liao FF, McDonald MP, Ishrat T (2019) Thioredoxin-interacting protein (TXNIP) Associated NLRP3 Inflammasome activation in human Alzheimer’s Disease Brain. J ALZHEIMERS DIS 68(1):255–265
Long Y, Liu X, Tan XZ, Jiang CX, Chen SW, Liang GN, He XM, Wu J, Chen T, Xu Y (2020) ROS-induced NLRP3 inflammasome priming and activation mediate PCB 118- induced pyroptosis in endothelial cells. ECOTOX ENVIRON SAFE 189:109937
Luo T, Zhou X, Qin M, Lin Y, Lin J, Chen G, Liu A, Ouyang D, Chen D, Pan H (2022) Corilagin Restrains NLRP3 Inflammasome Activation and Pyroptosis through the ROS/TXNIP/NLRP3 Pathway to Prevent Inflammation. OXID MED CELL LONGEV. 2022: 1652244
Mohamed IN, Li L, Ismael S, Ishrat T, El-Remessy AB (2021) Thioredoxin interacting protein, a key molecular switch between oxidative stress and sterile inflammation in cellular response. WORLD J DIABETES 12(12):1979–1999
Shimodaira T, Matsuda K, Uchibori T, Sugano M, Uehara T, Honda T (2018) Upregulation of osteopontin expression via the interaction of macrophages and fibroblasts under IL-1b stimulation. Cytokine 110:63–69
Takahashi M (2022) NLRP3 inflammasome as a key driver of vascular disease. CARDIOVASC RES 118(2):372–385
Valverde-Salazar V, Ruiz-Gabarre D, García-Escudero V (2023) Alzheimer’s Disease and Green Tea: Epigallocatechin-3-Gallate as a modulator of inflammation and oxidative stress. ANTIOXIDANTS-BASEL 12(7):1460
Zhang Q, Wu Y, Guan Y, Ling F, Li Y, Niu Y (2019) Epigallocatechin gallate prevents senescence by alleviating oxidative stress and inflammation in WI-38 human embryonic fibroblasts. RSC ADV 9(46):26787–26798
Zhang Y, Dong Z, Song W (2020a) NLRP3 inflammasome as a novel therapeutic target for Alzheimer’s disease. SIGNAL TRANSDUCT TAR 5(1):37
Zhang S, Zhu Q, Chen JY, OuYang D, Lu JH (2020b) The pharmacological activity of epigallocatechin-3-gallate (EGCG) on Alzheimer’s disease animal model: a systematic review. Phytomedicine 79:153316
Zhong X, Liu M, Yao W, Du K, He M, Jin X, Jiao L, Ma G, Wei B, Wei M (2019) Epigallocatechin-3-Gallate attenuates microglial inflammation and neurotoxicity by suppressing the activation of Canonical and Noncanonical Inflammasome via TLR4/NF-κB pathway. MOL NUTR FOOD RES 63(21):e1801230
Funding
This work was supported by the National Science Foundation of China (Nos. 82274124); Natural Science Research Project of Anhui Educational Committee (KJ2021A0234); Anhui provincial College Students’ Innovation and Entrepreneurship Training Program (S202310366094).
Author information
Authors and Affiliations
Contributions
Yan-yan Xiao: Conducted the research, wrote an original draft, prepared Figs. 1, 2, 3, 4 and 5. Cheng-lin Yang: Formal analysis, Writing-review & editing,prepared Supplementary Fig. 1. Na-na Si: prepared Figs. 1, 2, 3, 4 and 5. Tao Chu: Formal analysis. Xin-tong Yuan: Formal analysis. Jia-hui Yu: Data curation. Xiang-tao Chen: Conducted the research, wrote an original draft, formulation or evolution of overarching research goals and aims. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent for publication
All authors agree to publish the paper.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Yang, C., Si, N. et al. Epigallocatechin-3-gallate Inhibits LPS/AβO-induced Neuroinflammation in BV2 Cells through Regulating the ROS/TXNIP/NLRP3 Pathway. J Neuroimmune Pharmacol 19, 31 (2024). https://doi.org/10.1007/s11481-024-10131-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11481-024-10131-z