Abstract
Background
Type 2 diabetes (T2D), one of the major common human health problems, is growing at an alarming rate around the globe. Alpha-glucosidase and dipeptidyl peptidase IV (DPP-IV) enzymes play a significant role in development of T2D. Hence, reduction or inhibition of their activity can be one of the important strategies in management of T2D. Studies in the field of bioactive peptides have shown that dietary proteins could be natural source of alpha-glucosidase and DPP-IV inhibitory peptides.
Purpose
The purpose of this review is to provide an overview of food protein-derived peptides as potential inhibitors of alpha-glucosidase and DPP-IV with major focus on milk proteins.
Methods
Efforts have been made to review the available information in literature on the relationship between food protein-derived peptides and T2D. This review summarizes the current data on alpha-glucosidase and dipeptidyl peptidase IV inhibitory bioactive peptides derived from proteins and examines the potential value of these peptides in the treatment and prevention of T2D. In addition, the proposed modes of inhibition of peptide inhibitors are also discussed.
Results
Studies revealed that milk and other food proteins-derived bioactive peptides play a vital role in controlling T2D through several mechanisms, such as the satiety response, regulation of incretin hormones, insulinemia levels, and reducing the activity of carbohydrate degrading digestive enzymes.
Conclusions
The bioactive peptides could be used in prevention and management of T2D through functional foods or nutraceutical supplements. Further clinical trials are necessary to validate the findings of in vitro studies and to confirm the efficiency of these peptides for applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidences of diabetes are on a perpetual rise around the globe. In 2013, the number of diabetic persons was 382 million, and this is expected to be increased to 592 million by 2035 [1]. Diabetes mellitus (DM), a chronic metabolic disorder, is caused due to defective insulin production or action [2]. It is characterized by hyperglycemia, a condition in which surplus of sugar is present in the blood stream. Prevalence of diabetes mellitus is increasing markedly because of aging, population growth, increasing urbanization, incidences of obesity, and more sedentary lifestyles [3]. Type 1 diabetes (T1D) and Type 2 diabetes (T2D) are the main two types of diabetes. Though the later is much more common and accounts for 90–95 % of all diabetes. A number of factors, such as insulin resistance, hyperinsulinemia, impaired insulin secretion, reduced insulin mediated glucose uptake, and utilization convoluted the treatment of T2D [4]. Postprandial blood glucose levels could be a better indicator of glycemic control than fasting blood glucose levels in T2D patients [5].
It is important to have a normal blood glucose level, both in fasting and postprandial conditions. However, in preventing the T2D, it is important to control postprandial glucose level [6, 7], because in the long term, this will be leading to serious complications, including hypertension, cardiovascular disorders, blindness, and renal failure [8]. For the management of T2D, various strategies are being used such as the regular use of different antidiabetic medications, manipulation of diet, change in lifestyle, and regular physical exercise [9, 10]. One therapeutic approach for management of T2D is through inhibition of carbohydrate hydrolyzing enzymes such as alpha-amylases and alpha-glucosidases in digestive organ [11–14]. Another therapeutic approach for the management of T2D is the use of glucose-lowering agent, i.e., dipeptidyl peptidase-IV (DPP-IV) inhibitors [15, 16]. Currently, in the field of diabetes research, the main focus is on the development of the antihyperglycemic agents that are from natural source and safe without any side effects [17–19]. Nutritional intervention has been established as a main element in the prevention and management of T2D [20]. In humans, several biomarkers of diabetes are affected by dietary intake of food protein and food protein hydrolysates. It was investigated that milk proteins, mainly, milk protein-derived peptides and amino acids have also been associated with the regulation of postprandial glycemia and insulin secretion in normal and T2D patients [21].
Bioactive peptides are specific protein fragments that have a positive impact on body functions or conditions and may ultimately influence health [22]. Fermented dairy products and other foods containing bioactive peptides appear to have the potential to offer specific health benefits to consumers. Milk protein-derived active biological constituents and bioactive peptides have received much attention for their biological significances, and are currently the subject of intensive research. The intrinsic bioactivities of the peptides encrypted in major milk proteins are latent until released and activated by enzymatic hydrolysis in vitro or in vivo [23]. Biologically active peptide fragments are formed during the degradation of the milk proteins (whey proteins and casein) by digestive enzymes in the gastrointestinal tract and by proteolytic lactic acid bacteria (LAB) during fermentation of milk. In these processes, varieties of peptides containing 2–20 amino acid residues are formed. Once liberated and absorbed, these bioactive peptides may exert a physiological effect on the cardiovascular, digestive, endocrine, immune, and nervous systems of the body [24]. The activity of these peptides is based on their inherent amino acid composition and length of the sequence [25]. Milk protein-derived peptides exert multiple physiological activities, including antimicrobial, antioxidant, antithrombotic, opioid, antihypertension activity, modulation of digestive enzymes, nutrient absorption, and immune responses (Fig. 1) [26–32]. Many milk-derived peptides reveal multifunctional properties, i.e., specific peptide sequences may exert two or more different biological activities. These regions known as “strategic zones” are partially protected from further proteolytic breakdown [25]. Because of their physiological and physicochemical versatility, milk peptides are reckoned as very important constituents for incorporation in functional and novel foods, dietary supplements, and even pharmaceuticals with the purpose of targeting specific disease. Recent research has also shown that peptides derived from milk proteins have alpha-glucosidase and DPP-IV inhibitory properties [33, 34].
The purpose of this non-systematic review is to look at the significance of milk protein-derived peptides as potential inhibitors of alpha-glucosidase and DPP-IV. However, other food protein-derived peptide inhibitors have also been taken into consideration for better understanding of the topic, but our primary focus is on milk protein-derived peptide inhibitors. The role of milk protein-derived alpha-glucosidase and DPP-IV inhibitory peptides will be considered in the context of a nutritional strategy for the management of T2D.
Milk protein and T2D
Milk proteins contain approximately 80 % casein and 20 % whey. The casein comprises α-s1, α-s2, β, and κ-casein, while whey comprises β-lactoglobulin, α-lactalbumin, lactoferrin, immunoglobulins, serum albumin, glycomacropeptide, enzymes, and growth factors. Whey proteins and caseins may help in diminishing the physiological effects of T2D and have been revealed to arouse insulin secretion and control blood glucose level in T2D patients [35, 36]. Recently, the role of protein in the diet as a physiological active ingredient has increasingly been acknowledged worldwide. Milk protein is an important source of amino acid is very well accepted, but in current times, it has been recognized that milk protein shows numerous functionalities in vivo by the action of bioactive peptides. Presently, milk proteins are considered as the most vital resource of a range of bioactive peptides [37]. For that reason, there is a growing interest for milk proteins or peptides as potential ingredients of health-promoting functional food targeted at metabolic syndrome, which linked to cardiovascular disease, T2D, and obesity. It seems that milk proteins or peptides may reduce the risk of metabolic syndrome through several mechanisms [32]. It was suggested that the antidiabetic properties of whey protein are primarily attributable to its content of bioactive peptides which, following their release during gastrointestinal digestion, could arouse the secretion of gut-derived hormones and/or inhibit enzymes involved in glucose homeostasis [38, 39].
Effect on insulin secretion
It was found that whey protein absorbed faster than casein. Therefore, plasma amino acid (AA) increased more rapidly after consumption of whey protein and that leads to more rapid secretion of insulin than micellar casein [40]. The absorption rate of casein in its native micellar form is lower because the acidic conditions in the stomach cause casein to clot and thus delay gastric emptying [41]. Insulin is sensitive to both the composition and concentration of plasma AAs, hence both whey and casein ingestion stimulate increased insulin secretion [42, 43]. However, the absorption of AAs and secretion of insulin speed up after the hydrolysis of casein relative to the micellar form of casein [44]. Therefore, different milk proteins may create a remarkable contribution to metabolic effects in insulin-sensitive tissues and in particular skeletal muscle anabolism by the stimulation of insulin secretion [45]. Extended, high fasting glucose is one of the main characteristics of T2D. Increased insulin resistance and defect in insulin secretion causes hyperglycemia [46].
Effect on postprandial glycemia
It is important to decrease the prolonged exposure to high blood glucose levels in individuals both with and without T2D for the management of postprandial glucose level [47]. Milk proteins, i.e., casein and whey stimulate the insulin secretion, have the potential to alter tissue glucose uptake, and suppress postprandial blood glucose excursions [48–50]. The insulinotropic effect of whey protein is mainly contributed by its AA profile. The numbers of insulinotropic AAs present in whey protein (e.g., leucine, isoleucine, valine, lysine, and threonine) are observed to show modified insulinemic and glycemic responses [43].
In a comparative study, the consumption of whey protein (18 g in lunch or breakfast) resulted in greater insulinotropic responses, circulating levels of the gut peptide glucose-dependent insulinotropic polypeptide (GIP), and suppression of postprandial glycemia than non-dairy protein (lean ham) in individual with T2D [35]. In healthy subjects, addition of whey protein supplement to a drink contacting 50 g glucose reduced the postprandial glycemia in a dose-dependent manner [51]. Similarly, whey protein in mice showed increased level of glucagon-like peptide 1 (GLP-1) and inhibit DPP-IV, a peptide that hydrolyze incretin hormones, resulting in an increased and prolonged insulin response [52]. Concerning about the casein, in an overweight subjects with T2D consumption of a casein hydrolysate (~30 g) and leucine (~10 g), beverage after food intake decreased prevalence of hyperglycemia over the period of 24 h [53]. Milk proteins are gaining considerable attention due to their beneficial effect on postprandial blood glucose, which are comparable to insulin secretagogues used for the treatment of hyperglycemia in T2D. Therefore, there is a rationale for regular whey protein consumption before or with meals to control postprandial glycemic responses in individuals with poor metabolic control or T2D [45].
Effect on satiety
It is very well examined that protein is the most satiating component of food [54]. In several clinical studies, dairy products showed a certain satiating effect. In a recent study with 49 overweight and obese adults, the weight loss between the high-dairy diet (1400 mg/day) group and low-dairy diet (750 mg/day) group was similar, but high-dairy diet group was found with slightly higher peptide YY concentrations in plasma and improved feelings of satisfaction [55]. In a comparative study, consumption of both pea protein hydrolysates and whey protein leads to greater satiety and fullness as compared to milk protein. A positive correlation was observed between insulin and both cholecystokinin (CCK) and GLP-1 for whey protein. However, both CCK and GLP-1 were increased by milk protein [56]. In addition, skim milk containing casein and whey protein showed more decreased in food intake than either protein alone in a study of isoenergetic preloads [57]. The mechanisms of action of different protein or peptides on satiety are still under investigation, but it has been proposed that it could be related to a delay of the gastric emptying, an increase in brain amino acids, or the presence of specific peptides or amino acids [58]. The satiating effect of whey protein is mainly due to a high concentration of branch chain amino acids, particularly l-leucine. Regarding the casein fraction of milk, it was proposed that peptides from casein hydrolysates activates the peripheral opioid and cholecystokinin receptors and blocks the antagonist receptors which reduces their effect on food intake [58, 59].
Effect on incretin system
The amino acids or peptides affect both the insulin secretion and release of incretin hormones, i.e., glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide 1 (GLP-1) from gut.
Glucose-dependent insulinotropic peptide
GIP, also known a gastric inhibitory peptide, is synthesized from K cells in the duodenum and jejunum after the food intake [60]. Recent studies show that GIP response was (+80 %) significantly enhanced by a whey drink in healthy subjects, while branched-chain amino acid mixtures did not [43]. Whey or casein hydrolysates elicited about 50 % more gastric secretion than whole protein solutions [44]. It is possible that bioactive peptides and/or other amino acids liberated during whey digestion are the key stimulators of GIP synthesis and secretion, as the same was found for insulin [61]. One more probability is that bioactive peptides released from whey protein may lead to increased half-life of GIP.
Glucagon-like peptide 1
GLP-1 secretion by intestinal L cells is dependent on the presence of nutrients in the lumen of the small intestine. It is a potent antihyperglycemic hormone, inducing glucose-dependent stimulation of insulin secretion while suppressing glucagon secretion. Whey generated the strongest response of active GLP-1 as compared to casein [62]. This stimulatory effect of whey protein on GLP-1 may result into many beneficial effects like increase in the glucose-induced insulin secretion and reduction in postprandial glycemia. Enhanced GLP-1 levels also boost the synthesis of pro-insulin and insulin stores in β-cells [63, 64].
Effect on appetite
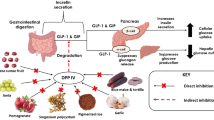
Protein does not only increase energy expenditure, but also manifests reduction in energy intake through mechanisms that influence appetite control [65]. Many studies explained that dairy protein has a stronger effect on suppression of hunger compared to soy and egg albumin [62]. The appetite-suppressing effect of whey protein can be attributed to the high content in branched-chain amino acids (mainly, l-leucine), and the presence of certain peptides, such as CMP, or it can be mediated by the release of satiety hormones [66]. Recently, it has been found that whey protein may exert a central effect on appetite by suppression of food intake [67, 68]. The increase in levels of insulin by ingestion of whey protein not only helps in modifying the glycemic response, but is also strongly associated with satiety and decreased food intake by suppressing appetite [48, 49]. Directly or indirectly, other hormones are also involved in the regulation of food intake, such as ghrelin, CCK, and peptide YY (PYY) [38]. Figure 2 shows the metabolic effect of milk protein in T2D management.
Metabolic effect of milk protein in T2D management. Adapted from Jakubowicz and Froy 2013 [35]
Bioactive food peptides and T2D
The significance of food proteins in the diet has been increasingly recognized because of the functionalities of biologically active peptides. In recent times, many studies reveal that antidiabetic properties of milk are mainly attributed to a variety of peptides derived from milk protein [21, 33, 69–71]. The two enzymes, i.e., dipeptidyl peptidase-IV and alpha-glucosidase play an important role in the development of hyperglycemia in T2D. Accordingly, inhibition of these two enzymes opens up new vistas for the treatment of T2D [33, 34].
Alpha-glucosidase enzyme inhibitory peptides
Enzyme alpha-glucosidase (EC 3.2.1.20) is located in the brush border of the enterocytes of the jejunum in the small intestine and happens to be a key enzyme in carbohydrate synthesis and breakdown [72]. It is an exo-type carbohydrolase, cleaving glycosidic bonds in complex carbohydrate to release absorbable monosaccharides (Fig. 3a) [73]. The hydrolyzed dietary carbohydrate is the main source of increased level of glucose in the blood. After the hydrolysis of dietary carbohydrate by pancreatic alpha-amylase, the subsequent absorption in the intestine is done by alpha-glucosidases [74]. One of the remedial strategies for managing T2D is to decrease postprandial hyperglycemia by retarding the absorption of glucose through inhibition of carbohydrate-hydrolyzing enzymes, e.g., alpha-glucosidase, in the digestive organs [75]. In addition, one interesting aspect of the alpha-glucosidase inhibitor is that, it has the ability to both enhance and extend GLP-1 secretion in normal individuals and patients with T2D [76, 77].
Mechanism of action of alpha-glucosidase inhibitors. Adapted from Arungarinathan et al. [89]. a Action of alpha-glucosidase on carbohydrate in the absence of alpha-glucosidase inhibitor. b Action of alpha-glucosidase on carbohydrate in the presence of alpha-glucosidase inhibitor. AG alpha-glucosidase, AGI alpha-glucosidase inhibitor
Currently alpha-glucosidase inhibitors such as acarbose, miglitol, voglibose, and emiglitate are considered as therapeutic drugs for the treatment of diabetic individuals with postprandial hyperglycemia. However, the chronic use of these agents could result in side effects such as flatulence, abdominal cramping, vomiting, and diarrhea [78–80]. Therefore, a number of studies have been carried out to identify the natural sources of alpha-glucosidase inhibitors. A range of fruits, vegetables, and animals, including blueberry, strawberry, broccoli sprouts, green paper, peptides from sardine muscle, and more recently egg white protein, has been reported to display alpha-glucosidase inhibitory activity [81–85].
Alpha-glucosidase inhibitors act as competitive inhibitors of enzymes needed to digest carbohydrates, specifically alpha-glucosidase [86]. Inhibition of this enzyme in digestive tract delays carbohydrate digestion thus increases overall carbohydrate digestion time. As a result, less glucose gets absorbed, because the carbohydrates are not rapidly hydrolyzed down into glucose molecules and subsequently diminishing the postprandial blood glucose and insulin level (Fig. 3b) [87, 88]. The short time effect of these inhibitors is to decrease the current blood glucose level.
Alpha-glucosidase inhibitors also play a crucial role in secretion of GLP-1 in normal and diabetic patients. GLP-1 is secreted from intestinal L-cells in response to nutrient ingestion. GLP-1 is recognized to be involved in the regulation of insulin secretion [90], glucagon decreation [91], and β-cell turnover [92]. Since, alpha-glucosidase inhibitors slow down carbohydrate absorption, this in turn results in an elevated sugar absorption in the lower gut [93]. Taking into consideration that sugar absorption plays a significant role in GLP-1 secretion [94, 95] and that lower gut is plentiful in GLP-1 producing cells [96, 97], delayed carbohydrate absorption is considered a satisfactory contributing factor for stimulating GLP-1 secretion by alpha-glucosidase inhibition. Hence, alpha-glucosidase inhibitor is a promising therapeutic modality in T2D patients. The accurate mechanism by which peptides can inhibit alpha-glucosidase activity is unknown, but it has been proposed that non-saccharide compounds may apply their inhibitory activity by binding to the enzyme’s active site through hydrophobic interactions [98].
Food protein-derived bioactive peptides inhibiting alpha-glucosidase enzyme
Beyond their nutritional importance, proteins and peptides have a wide variety of physiological functions that may benefit the human health. Many food proteins showed the natural precursor of alpha-glucosidase inhibitory peptides.
Sardine muscle hydrolysates by alkaline protease at 0.3 wt%–17 h hydrolysis showed highest inhibitory activity against alpha-glucosidase with an IC50 value of 48.7 mg/mL. Various proteases-treated sardine muscle hydrolysates exhibited different alpha-glucosidase inhibition activity, which indicated that the main contributor to inhibitory activity was sardine protein hydrolysates. This indicated that differences in the inhibitory activity of hydrolysates by various proteases were due to the difference in the size of the hydrolytic product with their different substrate specificity. Further purification of hydrolysates with DEAE-Sephadex A-25 resin showed the two most potent alpha-glucosidase inhibitory fractions i.e., A and B with the IC50 value of 16.5 and 15.6 mg/mL, respectively [76]. Two peptides Val-Trp (IC50 = 22.6 mM) and Tyr–Tyr–Pro–Leu (IC50 = 3.7 mM) were identified from sardine muscle hydrolysates (Table 1) [99]. Lavigne et al. [100, 101] reported that cod proteins could improve glucose tolerance and insulin sensitivity in high fat-fed rats, and their studies showed that this might be attributed to certain amino acids. Huang and Wu [102] purified and characterized an antidiabetic peptide from shark liver which reduced the fasting plasma glucose level in diabetic mice.
Peptides from egg white protein hydrolysates displayed the potential alpha-glucosidase inhibitory activity. Among the eight synthetic peptides, two peptides, Arg–Val–Pro–Ser–Leu–Met and Thr–pro–Ser–Pro–Arg, demonstrate higher alpha-glucosidase inhibitory activity with an IC50 values at 23.07 and 40.02 µmol/L, respectively (Table 1). These results showed that the potential of bioactive peptides from the egg white protein exhibiting the alpha-glucosidase inhibitory activity could be considered as an ingredient for functional food product with the antidiabetic activity [85]. In another study, the peptides showed antidiabetic activity against alpha-glucosidase. Among the peptides, Lys–Leu–Pro–Gly–Phe displayed the highest inhibitory activity against alpha-glucosidase with the IC50 values of 59.5 µmol/L (Table 1) [103].
Milk protein-derived bioactive peptides inhibiting alpha-glucosidase enzyme
One of the studies conducted in vitro showed that milk and soy milk fermented with L. bulgaricus and L. acidophilus was used for the management of hyperglycemia linked to T2D. During the fermentation process, the alpha-glucosidase inhibition was increased, indicating that possible positive effect was due to the milk-derived bioactive peptides [74]. In another study, whey protein isolate (WPI), α-lactalbumin, β-lactoglobulin, serum albumin, and lactoferrin hydrolysates obtained by peptic digestion were investigated for their potential to serve as natural sources of alpha-glucosidase inhibitors. The peptides generated from whey proteins have dual beneficial effects on glycemic regulation and could be used as functional food ingredients for the management of T2D [33]. The exopolysaccharides and insulin-containing yogurt showed better alpha-glucosidase inhibitory activity vis-a-vis the control yogurt [104]. The alpha-glucosidase inhibitory activity was found in peptic treated whey protein hydrolysates at 2.50 mg/mL. Peptic digested β-lactoglobulin and WPI hydrolysates displayed the highest alpha-glucosidase inhibition, i.e., 33 and 36 %, respectively. The IC50 values for the β-lactoglobulin and WPI hydrolysates were 3.5 and 4.5 mg/mL, respectively (Table 2) [33]. In addition, the alpha-glucosidase inhibitory activity observed in the whey protein hydrolysates obtained by the use of serine protease was isolated from Asian pumpkins. The peptides from six fractions of WPC-80 hydrolysates with a molecular mass below 3 kDa, showed highest inhibitory activity with the IC50 values lower than 2.0 mg/mL (Table 2) [34].
Dipeptidyl peptidase-IV (DPP-IV) enzyme inhibitory peptides
Dipeptidyl peptidase IV (DPP-IV/CD26; EC.3.4.14.5) is a multifunctional transmembrane glyco-protein, involved in different biological processes. It is a 766–amino acid serine protease that contains N-terminal dipeptidases activity, which selectively cleaves dipeptides after proline or alanine residues [105]. It is originally known as the lymphocyte cell surface marker CD26, or as the adenosine deaminase (ADA)-binding protein. The DPP-IV enzyme is widely distributed in human organs and tissue, including exocrine pancreas, sweat glands, salivary and mammary glands, thymus, lymph nodes, intestines and biliary tract, kidney, liver, placenta, uterus, prostate, brain, blood cells, and skin. DPP-IV is present in almost all organs in the body through the attachment to the plasma membrane of the endothelia [106]. The enzyme DPP-IV plays numerous roles in several physiological processes, including enzymatic incretin degradation, including immune and endocrine activity, cell adhesion, and dampening of cancer growth [107, 108]. DPP-IV is renowned for its inactivation of incretin hormones GLP-1 and GIP (Fig. 4).
Many studies reveal that hydrolysate and peptides from different proteins, including those from egg [109], fish [110, 111], amaranth [112], rice bran [113], corn [114], and milk [70, 71, 115–118] have been found to be the source of the DPP-IV inhibitors. Among those proteins, peptides derived from the milk protein i.e., whey and casein fractions of milk have been found to display in vitro DPP-IV inhibitory activity and in vivo reduction in blood glucose level [33]. The new strategy for enhancing incretin action consists in the administration of foods with the potential to inhibit intestinal DPP-IV to prevent incretin degradation [52, 119].
The incretin hormones, GLP-1 and GIP, are gut hormones released into plasma from K cells in the duodenum and L cells in the intestine mucosa, respectively, after the ingestion of food [120]. The incretin hormones enhance meal-induced insulin secretion from β-cells of the islets of langerhans into the bloodstream in glucose-dependent manner and play an important part in the maintenance of normal glucose homeostasis [121–123]. They also have a role in suppression of pancreatic glucagon release, delay gastric emptying, and modulate appetite [124, 125]. Released after the ingestion of the meal, the concentration of active endogenous GLP-1 and GIP increases from two- to threefold. These two hormones enhance approximately 60 % of the postprandial insulin release [126]. The DPP-IV enzyme is responsible for the degradation of both incretin hormones GIP and GLP-1, as these gut-derived hormones are endogenous physiological substrates of DPP-IV [127]. However, both GIP and GLP-1 have very short half-lives (<7 and 2 min, respectively) due to the action of DPP-IV enzyme [106].
A DPP-IV inhibitor enhances the exogenous and endogenous GLP-1 and GIP action by blocking their N-terminal degradation, and hence results in inactivation [128]. It was found that in patients with T2D, there is a decrease in the incretin response, resulting in decreased insulin secretion, increased postprandial glucagon levels, and elevated postprandial glucose [129]. DPP-IV inhibitors extend the half-life and increase the concentrations of circulating intact (active) incretins [130, 131]. As a result, the increased level of incretin causes the inhibition of glucagon, which in turn elevates insulin secretion, diminishes gastric emptying, and decreases blood glucose levels (Fig. 5). Therefore, a DPP-IV inhibitor can improve glucose tolerance, by augmenting the incretin effect in patients with T2D [120, 132]. It was found that canary seed peptides obtained by gastrointestinal digestion showed an inhibition of DPP-IV in a dose-dependent manner; the highest inhibition (43.4 %) was observed at peptide concentration of 1.4 mg/mL. However, the non-hydrolyzed proteins showed a low inhibition (9.3 %). This showed that peptides that are released during gastrointestinal digestion have an effect on blood glucose levels [133]. The presence of Leu and Pro in second and third position in the peptides sequence suggested a potential inhibitory role of these peptides; the flanking amino acids could affect the interaction between them and the enzyme [134]. Most of the DPP-IV inhibitors are competitive in nature with some exceptions. The peptides from amaranth 11S globulin interact with the active site pocket of DPP-IV, thereby blocking access to the substrate. Three peptides larger than 13 residues prevented the formation of the dimeric active form of DPP-IV, resulting in enzyme inhibition [112]. DPP-IV inhibitors signify a new class of oral antihyperglycemic agents to treat patients with T2D [135].
Food protein-derived bioactive peptides inhibiting DPP-IV enzyme
The peptide derived from the Atlantic salmon skin gelatin showed DPP-IV inhibitory activity and the activity was dependent on the type of enzyme used to generate peptides [110]. The three proteases used in the study were alcalase, bromelain, and flavourzyme. At the concentration of 5 mg/mL, the Flavourzyme hydrolysates showed the greatest DPP-IV inhibition activity, i.e., 45.2 % as compared to alcalase and bromelain hydrolysates with the same E/S ratio. Further, hydrolysates fractionation by ultrafiltration gives peptides within the <1 kDa range. This UF fraction had the greatest (61.2 %) DPP-IV inhibitory activity and IC50 value of 1.35 mg/mL. The <1 kDa UF fraction was further separated by RP-HPLC, and most potent F-1 fraction had the highest DPP-IV inhibition rate of 68 % with IC50 value of 0.57 mg/mL. Two peptides Gly–Pro–Ala–Glu and Gly–Pro–Gly–Ala (Table 3) were identified in F-1 fraction, which had IC50 values of 49.6 and 41.9 μM, respectively. In another study, marine collagen peptides (MCPs) from fish hydrolysate in Chinese patients with T2D significantly reduced levels of fasting blood glucose, human glycated hemoglobin A1c (GHbA1c), and fasting blood insulin [136]. Nutripeptin™ a product containing cod hydrolysate and Fortidium Liquamen® a white fish (Molva molva) autolysate are commercialized as postprandial blood glucose-lowering food supplements [137].
Proteolytic or microbial enzymatic hydrolysis of East Asian azuki bean produces hydrolysates with DPP-IV inhibitory activity. The hydrolysate from 10 kDa UF permeate had 52 % DPP-IV inhibition at 1.0 mg/mL, which was generated using Umanizyme G® [138]. The glutelins, main protein fraction of amaranth seeds, contain the peptide with DPP-IV inhibitory activity [139]. Tryptic digested amaranth hydrolysates show the decreased DPP-IV inhibitory activity in a dose-dependent manner with an IC50 ranging from 1.2 to 2.0 mg/mL. The removal of fragments larger than 10 kDa was done by ultrafiltration, and the IC50 value of the ultrafiltrated samples ranged from 1.0 to 1.6 mg/mL. It indicated that peptides smaller than 10 kDa were able to inhibit the DPP-IV enzyme. In silico analysis identified four peptides in amaranth globulins [11S amaranth globulin f (1–13), f (18–39), f (69–81), f (92–143)] and which may be responsible for the DPP-IV inhibitory activity [112]. Hen’s egg hydrolysates also showed the inhibitory activity against the DPP-IV and the activity dependent on the specificity of the enzyme used [125]. The peptides from the rice bran showed the DPP-IV inhibitory activity [113]. It was found that peptides obtained by using the enzyme Umamizyme G were 11 times more effective in inhibiting DPP-IV than those obtained with Bioprase SP and had IC50 values of 2.3 and 26.4 mg/mL, respectively. Further purification of Umamizyme G hydrolysates found two DPP-IV inhibitory dipeptides (Leu–Pro and Ile–Pro) (Table 3).
DPP-IV inhibitory activity was found in tuna cooking juice hydrolysates, and it was produced using two fungal endoproteinases in order to generate DPP-IV inhibitory peptides. Hydrolysates showed the highest inhibition rate of 45.2 % at the enzyme concentration of 2 mg/mL and after 1 h hydrolysis. Further purified by gel filtration chromatography, the highest DPP-IV inhibitory activity was 40 % at 5 mg/mL, and after reversed-phase HPLC, the inhibition rate was ~60 % at 5 mg/mL in order to enrich the peptides with DPP-IV inhibitory activity. Three peptides (Pro–Gly–Val–Gly–Gly–Pro–Leu–Gly–Pro–Ile–Gly–Pro–Cys–Tyr–Glu, Cys–Ala–Tyr–Gln–Trp–Gln–Arg–Pro–Val–Asp–Arg–Ile–Arg and Pro–Ala–Cys–Gly–Gly–Phe–Tyr–Ile–Ser–Gly–Arg–Pro–Gly) isolated from a tuna cooking juice hydrolysate (Table 3). Three peptides showed the IC50 values between the 78 and 116 µM. However, peptides obtained in the study comprised 13–15 amino acid residues which were much longer than the preferable DPP-IV inhibitory peptides. The result reveals that the DPP-IV inhibitory activity is dependent on the composition and sequence of amino acids, but not the length [111].
Peptides derived from the porcine skin gelatin hydrolysates showed in vitro inhibitory activity against the DPP-IV. It was found that the inhibitory activity was increased with the E/S ratio and hydrolysis time. The results exhibited the greater DPP-IV inhibitory activity of hydrolysates with the smaller size of peptides due to the higher degree of hydrolysis. The hydrolysates obtained with the E/S ratio of 3 % and 4 h hydrolysis at the rate of 1 mg/mL were further fractionated by ultrafiltration with different cutoff membranes. The DPP-IV inhibition was highest (30.7 %) for the <1 kDa UF fraction as compared to other UF fractions. As the small-sized peptides passed through the digestive tract without degradation, therefore, <1 kDa UF fraction was selected for further study. The IC50 value of the <1 kDa fraction was 1.50 mg/mL. This fraction was further purified by HPLC, and the most potent F-3 fraction showed the highest DPP-IV inhibition rate of 64.6 % and the IC50 value was 62.9 μg/mL. Within these two peptides, i.e., Gly–Pro–Hyp and Gly–Pro–Ala–Gly were identified in F-3 fraction (Table 3), which inhibited DPP-IV. There were a range of structural resemblance between the two peptides, each peptide contained Pro as the second N-terminal residue, and Pro residue was flanked by Ala and Gly, and moreover, the peptides were composed of mostly hydrophobic amino acid residues [140].
Milk protein-derived bioactive peptides inhibiting DPP-IV enzyme
Casein contains a large amount of Pro residues, which increases the vulnerability to cleavage by DPP-IV and potential for the release of peptides with DPP-IV inhibitory activity. Lacroix and Li-Chan [117] examined the potential of dietary proteins from various food commodities to serve as precursors of DPP-IV inhibitors by using an in silico approach. Caseins from cow’s milk emerged to be the richest potential sources of DPP-IV inhibitors. The β-casein was found to have the greatest potential among milk proteins to serve as a source of DPP-IV inhibitors. A computer-aided analysis of milk proteins as sources of bioactive peptides [141] identified β-casein as the most promising milk protein precursor of DPP-IV inhibitory peptides. On the contrary, alpha-lactalbumin contained a limited number of peptides with DPP-IV inhibitory activity. Dairy protein hydrolysates examined for DPP-IV inhibitory activity. With the time, in vitro pepsin–pancreatin hydrolysis enhances the DPP-IV inhibitory activity of sodium caseinate, skim milk powder, and milk proteins concentrate hydrolysates. The maximum DPP-IV inhibitory activity (IC50 of 0.075 mg/mL) was found in Whey protein isolate (WPI) hydrolysate following peptic digestion. With the use of different proteases, hydrolysates generated from sodium caseinate showed the highest inhibitory activity than the majority WPI hydrolysates [69]. Milk protein-derived hydrolysates and dipeptides exhibited the inhibitory activity against the DPP-IV. Eight dipeptides showed the inhibitory activity, and the IC50 values for the dipeptides were ranging from 65.29 to 3216 µM (Table 5). Among all potent peptides, Trp–Val was found to be most potent, but paradoxically, the reverse peptide did not inhibit the DPP-IV. This indicated that N-terminal residue of peptide plays an important role in the inhibition of DPP-IV. Lactoferrin, casein, and WPH hydrolysates showed the inhibitory activity against DPP-IV. Of these, lactoferrin and casein hydrolysates were the most potent inhibitors (Table 4). The result indicated that both hydrolysates and dipeptides acted as competitive inhibitors of DPP-IV [21]. Antihyperglycemic effect was found in water-soluble extract of Gouda-type cheese, and several types of DPP-IV inhibitory peptides were identified (Table 5). Of all, cheese octapeptide Leu–Pro–Gln–Asn–Ile–Pro–Pro–Leu (β-CN f70-77) showed the highest inhibitory activity (IC50 of 46 µM), which increased during ripening period and played an important role in inhibiting DPP-IV in vivo [71]. Patent WO 2006/068480 [142] has demonstrated that the small peptides from casein hydrolysates possessed DPP-IV inhibitory activity.
Whey protein hydrolysates also showed peptides with DPP-IV inhibitory activity. The DPP-IV inhibitory activity of β-lactoglobulin hydrolysates from goat/sheep and bovine has been compared. Combined in silico examination and in vitro studies verified that caprine/ovine whey is abundant in the content of short peptides carrying weak DPP-4 inhibitory activity than bovine whey [115]. The bioactive peptide Ile-Pro-Ala (IPA) from bovine β-lactoglobulin showed inhibitory activity against the DPP-IV with IC50 value of 49 µM (Table 5) [70]. Similarly, the hexapeptide was generated from trypsin-treated β-lactoglobulin and identified as Val–Ala–Gly–Thr–Trp–Tyr (β-lactoglobulin f15–20) (Table 5). This hexapeptide showed a concentration-dependent DPP-IV inhibitory activity with IC50 value of 174 µM [116]. The most potent fractions from whey protein hydrolysates were the 2 kDa permeate with the lowest IC50 value of 0.48 mg/mL (Table 4). When WPH was subjected to stimulated gastrointestinal digestion, there was a significant decrease in the IC50 value i.e., 1.02 mg/mL [118]. The study demonstrated the release of DPP-IV substrate like peptide sequences by gastrointestinal enzymes using an in silico digestion of the milk proteins, and these peptides behave as a substrate or prodrug-type inhibitors to DPP-IV. The DPP-IV inhibitory activity was determined for the identified peptides (Table 5). The lowest IC50 value was found for the Ile–Pro–Ile (3.4 µM) [143]. In addition, peptides from trypsin-treated β-lactoglobulin showed DPP-IV inhibitory activity (Table 5). The notable DPP-IV inhibitory activity was shown by the peptide Ile–Pro–Ala–Val–Phe with IC50 value of 44.7 µM [144]. Whey protein hydrolysates obtained by peptic hydrolysis exhibited the inhibitory activity against the DPP-IV (Table 4). The maximum DPP-IV inhibitory activity was shown by the α-lactalbumin and WPI hydrolysates, with 91 and 82 % inhibition, whereas the IC50 value measured for both was found in the same order of magnitude, i.e., 0.036 mg/mL [33]. In another study, the DPP-IV inhibitory activity was shown by the peptides from the hydrolysates of β-lactoglobulin and WPC-80 obtained by serine protease isolated from C. ficifolia. The peptide fractions below 3 kDa from WPC-80 hydrolysates showed the highest inhibition with IC50 value <0.55 mg/mL (Table 4) [34].
Production of antidiabetic peptides from milk protein
Although consumption of low-fat milk and dairy products have a beneficial effect on the prevention or treatment of T2D [145], research has been focused on milk protein-derived peptides. Peptides may be released from their parent protein by following four ways [26, 146]:
-
1.
Enzymatic hydrolysis during gastrointestinal digestion
-
2.
Fermentation of milk with proteolytic starter cultures
-
3.
Hydrolysis by enzymes obtained from microorganisms or plants
-
4.
Combination of fermentation and hydrolysis
If the sequence of the peptide is known, it is also possible to synthesize peptides by chemical route, recombinant DNA technology, or enzymatic amide synthesis [147, 148]. After releasing from origin, bioactive peptides must reach the target receptor in the intestinal lumen or in other peripheral organs, passing via the systematic circulation [25].
Gastrointestinal digestion
During gastrointestinal digestion, physiologically active peptides are produced from several milk proteins. Hydrolysis may occur in various stages after ingestion of the protein. In the gastrointestinal tract, ingested proteins are hydrolyzed by gastrointestinal enzymes, usually pepsin, trypsin, chymotrypsin, and other membrane peptidases [23]. Other digestive enzymes and different enzyme combinations of proteinases, i.e., alcalase, chymotrypsin, pancreatin, pepsin, and thermolysin have also been utilized to produce bioactive peptides from various proteins [149, 150]. Absorption of peptides can be through the gastrointestinal wall by different mechanisms, such as by passive diffusion through the enterocytes, and para-cellularly through cytosis or through a carrier [151]. After absorption, bioactive peptides must reach their target sites at the luminal side of the intestinal tract or at specific peripheral organs to exert their physiological effects [152].
It has been hypothesized that peptides released from whey proteins during their transit through gastrointestinal tract might be responsible for the postmeal glycemic response produced after whey intake [48]. It has been suggested that the antidiabetic property of whey proteins is mainly due to its content of bioactive peptides which, following their release during gastrointestinal digestion, could stimulate the secretion of gut-derived hormones and/or inhibit enzymes involved in glycemic homeostasis [38, 39].
Microbial fermentation
Lactic acid bacteria utilize milk protein as their key source of essential and growth-stimulating amino acids [153]. Many industrially important dairy starter cultures are highly proteolytic in nature. Bioactive peptides can, thus, be generated by the proteolytic activities of the strains of starter and non-starter lactic acid bacteria (LAB) in fermented dairy products [154]. Besides, to live microorganisms, proteolytic enzymes isolated from LAB have also been successfully employed to release bioactive peptides from milk proteins. The production of a variety of bioactive peptides in fermented dairy products, e.g., yoghurt, sour milk, and dahi has been well documented in many studies [151]. Apostolidis et al. [74] observed that milk and soy milk fermented with L. bulgaricus and L. acidophilus generated α-glucosidase inhibitory activity. The activity suggested that peptides were responsible for the management of hyperglycemia linked to T2D. Uenishi et al. [71] identified several DPP-IV inhibitory peptides from Gouda cheese. The DPP-IV inhibitory activity was observed in the water-soluble fraction of a Gouda-type cheese, and this inhibitory activity increased during ripening. This inhibitory activity positively correlated with the blood glucose improving effect of Gouda cheese.
Consumption of water kefir in (10–30 %) concentrations for 5 weeks has shown beneficial effect on blood glucose level in animal model [155]. Similarly, the water-soluble fraction of Kefram–Kefir showed glucose uptake in skeletal muscle cells specifically in vitro [156]. In another study, the probiotic fermented milk (kefir) consumption causes the decline of fasting blood glucose and Glycated hemoglobin (HbA1C) in comparison with conventional fermented milk [157]. The results of Maeda et al. [158] showed that kefiran had hypoglycemic effects in KKAy mice. Fermented soymilk extract showed the suppression of postprandial blood glucose levels in diabetics through inhibition of alpha-glucosidase and alpha-amylase [159]. The probiotic dahi-supplemented diet significantly delayed the onset of glucose intolerance, hyperglycemia, and hyperinsulinemia in high-fructose-induced diabetic rats which indicates a lower risk of diabetes [160].
Enzymatic hydrolysis
The most common way to obtain bioactive peptides in vogue is by enzymatic hydrolysis of protein [23]. Proteases derived from microorganisms, animals, or plants have also been successfully employed in the proteolytic process to release peptides from milk proteins. In addition, enzyme combinations including alcalae, chymosin, pancreatin, trypsin, and thermolysin can be used to release bioactive peptides [146]. The commonly used enzymes of plant and animal origin are α-chymotrypsin, papain, neutrase, thermolysin, pepsin, alcalase, pronase, carboxypeptidase A, and trypsin [23, 161, 162]. Generation of DPP-IV inhibitory milk peptides by enzymatic hydrolysis from milk protein are more studied [21, 69, 70, 116]. Similarly, alpha-glucosidase inhibitory milk peptides have been produced from the milk protein by enzymatic hydrolysis [33, 34].
Clinical studies
So far, only some human studies have evaluated the role of milk protein hydrolysates or peptides in T2D. The impact of co-ingestion of intact or hydrolyzed protein with carbohydrate on postprandial plasma insulin and glucose responses was evaluated in T2D patients. Patients participated in a study received the single bolus of carbohydrate (0.7 g/kg: CHO) with or without an intact casein protein (0.3 g/kg: PRO) or its hydrolysate (0.3 g/kg: PROh). Results showed that protein co-ingestion strongly increased postprandial insulin release, with the insulin response +99 and +110 % greater in the CHO + PRO and CHO + PROh experiments when compared with the CHO experiment. The concomitant plasma glucose responses were 22 and 23 % lower in the CHO + PRO and CHO + PROh experiments, respectively [163]. Similarly, 11 long standing T2D patients and 11 healthy control subjects received a beverage containing casein hydrolysate (0.3 g/kg)/leucine (0.1 g/kg) mixture (PRO) or a placebo (PLA). In the PRO trial, glucose was significantly lower compared with PLA trial at 24 h. PRO resulted in a 11 % decline in the overall glucose response in diabetic patients [53].
Meric et al. [164] study the effect of four different beverages containing 6 % w/v whey protein isolate (WPI), whey protein hydrolysate (WPH), soy protein isolate (SPI), and 2.66 % WPI or a control (no protein added) on the glucose and insulin response in 25 healthy men. Result demonstrated that only beverages containing 6 % (w/v) of whey protein increased insulin response and decreased glucose level compared with control. Furthermore, in the Goudarzi and Madadlou [165] study, they demonstrated that whey protein hydrolysates showed distinctly better effectiveness in blood glucose control than intact whey protein. The effect of WPH on blood glucose level might be due to faster digestion and availability of its insulinotropic peptides and amino acids in the blood [166].
There are increasing evidences that milk protein, protein hydrolysates or bioactive peptides, and amino acids could be used as valuable nutritional strategies to improve blood glucose levels in T2D patients. Milk protein-derived peptides are commercially available as functional food, food supplements, and nutraceuticals with certain health claims. There is a need to validate the associated functional attributes of milk protein-derived alpha-glucosidase and DPP-IV inhibitors through well-designed clinical trials. At the same time, the challenge to the food industry remains to incorporate these bioactive peptides without adversely affecting the sensory profile, convenience, bioavailability, and safety.
Conclusions
Epidemiological evidences reveal that a diet rich in dairy products has been associated with the prevention and treatment of metabolic-related disorders, while evidence from observational studies points toward milk protein-derived peptides as dietetic ingredients which may aid prevention of T2D. One of the new promising therapeutic strategies for the treatment of T2D is the use of inhibitors of alpha-glucosidase and DPP-IV. However, synthetic drug inhibitors have been associated with some side effects and limitations. Therefore, there is an increasing attention toward natural, safe, food-derived peptide inhibitors as these are without any side effects. Milk protein-derived peptides with alpha-glucosidase and DPP-IV inhibitory traits potentially regulate the postprandial hyperglycemia in healthy and T2D subjects by inhibiting both the inactivation of the incretin hormones and the carbohydrate hydrolyzing enzymes. These antidiabetic peptides are liberated from milk protein by gastrointestinal digestion of milk, fermentation of milk with proteolytic starter cultures, or hydrolysis by proteolytic enzymes from plant or animal. Milk protein-derived alpha-glucosidase and DPP-IV inhibitory peptides could be used as ingredients for functional food, nutraceuticals, and pharmaceuticals applications for the management of T2D. However, there is a need to unravel the molecular mechanisms of action of these peptides on alpha-glucosidase and DPP-IV. Besides, clinical studies are necessary to validate the most in vitro and some in vivo data and to confirm the efficacy and bioavailability of milk protein-derived peptides in humans.
References
IDF (2013) IDF diabetes atlas, 6th edn. International Diabetes Federation, Brussels
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431
UK Prospective Diabetes Study (UKPDS) Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317:703–713
Tiwari A, Rao JM (2002) Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci 83:30–38
Avignon A, Radauceanu A, Monnier L (1997) Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 20:1822–1826
Abrahaamson MJ (2004) Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med 164:486–491
Jermendy G (2005) Can type 2 diabetes mellitus be considered preventable? Diabetes Res Clin Pract 68:S73–S81
Ben-Avraham S, Harman-Boehm I, Schwarzfuchs D (2009) Dietary strategies for patients with type 2 diabetes in the era of multi-approaches review and results from the dietary intervention randomized controlled trial (DIRECT). Diabetes Res Clin Pract 86:S41–S48
Bantle Jp, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ (2008) Nutrition recommendations and interventions for diabetes: a position statement of the American diabetes association. Diabetes Care 31:S61–S78
Hawley JA, Gibala MJ (2012) What’s new since Hippocrates? Preventing type 2 diabetes by physical exercise and diet. Diabetologia 55:535–539
Holman RR, Cull CA, Turner RC (1999) A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (UK Prospective Diabetes Study 44). Diabetes Care 22:960–964
Toeller M (1994) α-Glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. Eur J Clin Invest 24:31–35
Clissold SP, Edwards C (1988) A preliminary review of its pharmacodynamic and pharmacokinetics properties, and therapeutic potential. Drugs 35:214–243
Saito N, Sakai H, Sekihara H, Yajima Y (1998) Effect of an α-glucosidase inhibitor (voglibose) in combination with sulphonilureas, on glycemic control in type 2 diabetes patients. J Int Med Res 26:219–232
Bennett WL, Wilson LM, Bolen S et al (2011) Oral diabetes medications for adults with type 2 diabetes: an update. Comparative Effectiveness Rev. 27. http://www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed 09 Nov 2014
Tahrani AA, Bailey CJ, Del Prato S, Barnett AH (2011) Management of type 2 diabetes: new and future development. Lancet 378:182–197
American Diabetes Association (2015) Standards of medical care in diabetes-2015. Diabetes Care 38:S1–S98
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35:1364–1379
Agius R (2014) An update on pharmacotherapy for type 2 diabetes. Malta Med J 26:29–38
Evert AB, Boucher JL, Cypress M et al (2014) Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 37(Suppl 1):S120–S143
Nongonierma AB, FitzGerald RJ (2013) Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 39:157–163
Kitts DD, Weiler K (2003) Bioactive proteins and peptide from food sources: application of bioprocesses used in isolation and recovery. Curr Pharm Des 9:1309–1323
Korhonen H, Pihlanto A (2006) Bioactive peptides: production and functionality. Int Dairy J 16:945–960
Phelan M, Kerins D (2011) The potential role of milk-derived peptides in cardiovascular disease. Food Funct 2:153–167
Tidona F, Criscione A, Guastella AM, Zuccaro A, Bordonaro S, Marletta D (2009) Bioactive peptides in dairy products. Ital J Anim Sci 8:315–340
Korhonen H (2009) Milk-derived bioactive peptides: from science to applications. J Funct Foods 1:177–187
Seppo L, Jauhiainen T, Poussa T, Korpela R (2003) A fermented milk high in bioactive peptides has a blood pressure lowering effect in hypertensive subjects. Am J Clin Nutr 77:326–330
Haug A, Hostmark AT, Harstad OM (2007) Bovine milk in human nutrition–a review. Lipids Health Dis 6:1–16
Ebringer L, Ferenčík M, Krajčovič J (2008) Beneficial health effects of milk and fermented dairy products-review. Folia Microbiol 53:378–394
Atanasova J, Ivanova I (2010) Antibacterial peptides from goat and sheep milk proteins. Biotechnol Biotechnol Equip 24:1799–1803
Meisel H (1998) Overview on milk protein-derived peptides. Int Dairy J 8:363–373
Ricci-Cabello I, Olalla Herrera M, Artacho R (2012) Possible role of milk derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr Rev 70:241–255
Lacroix IM, Li-Chan ECY (2013) Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J Agric Food Chem 61:7500–7506
Konrad B, Anna D, Marek S, Marta P, Aleksandra Z, Jozefa C (2014) The evaluation of dipeptidyl peptidase (DPP)-IV, α-Glucosidase and angiotensin converting enzyme (ACE) inhibitory activities of whey proteins hydrolyzed with serine protease isolated from asian pumpkin (Cucurbita ficifolia). Int J Pept Res Ther 20:483–491
Frid AH, Nilsson M, Holst JJ, Björck IME (2005) Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 82:69–75
Manders RJF, Wagenmakers AJM, Koopman R (2005) Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am J Clin Nutr 82:76–83
Korhonen H, Pihlanto A (2007) Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr Pharm Des 13:829–843
Jakubowicz D, Froy O (2013) Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and type 2 diabetes. J Nutr Biochem 24:1–5
Graf S, Egert S, Heer M (2011) Effects of whey protein supplements on metabolism: evidence from human intervention studies. Curr Opin Clin Nutr Metab Care 14:569–580
Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci 94:14930–14935
Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P (2001) The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 280:E340–E348
Nilsson M, Stenberg M, Frid AH, Holst JJ, Björck IME (2004) Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 80:1246–1253
Nilsson M, Holst JJ, Björck IM (2007) Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr 85:996–1004
Calbet JA, Holst JJ (2004) Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr 43:127–139
McGregor RA, Poppitt SD (2013) Milk protein for improved metabolic health:a review of the evidence. Nutr Metab 10:46
Pistrosch F, Natali A, Hanefeld M (2011) Is hyperglycemia a cardiovascular risk factor? Diabetes Care 34:S128–S131
Gerich JE (2003) Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med 16:1306–1316
Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH (2010) Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr 91:966–975
Pal S, Ellis V (2010) The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br J Nutr 104:1241–1248
Claessens M, Calame W, Siemensma AD, Van Baak MA, Saris WHM (2009) The effect of different protein hydrolysate/carbohydrate mixtures on postprandial glucagon and insulin responses in healthy subjects. Eur J Clin Nutr 63:48–56
Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V (2009) A whey protein supplement decreases post-prandial glycemia. Nutr J 8:1475–2891
Drucker DJ (2006) Enhancing the action of incretin hormones: a new whey forward? Endocrinology 147:3171–3172
Manders RJF, Praet SFE, Meex RCR, Koopman R, de Roos AL, Wagenmakers AJM, Saris WHM, van Loon LJ (2006) Protein hydrolysate/leucine co-ingestion reduces the prevalence of hyperglycemia in type 2 diabetic patients. Diabetes Care 29:2721–2722
Fromentin G, Darcel N, Chaumontet C, Marsset-Baglieri A, Nadkarni N, Tomé D (2012) Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr Res Rev 25:29–39
Jones KW, Eller LK, Parnell JA, Doyle-Baker PK, Edwards AL, Reimer RA (2013) Effect of a dairy and calcium rich diet on weight loss and appetite during energy restriction in overweight and obese adults: a randomized trial. Eur J Clin Nutr 67:371–376
Diepvens K, Häberer D, Westerterp-Plantenga M (2008) Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes 32:510–518
Lorenzen J, Frederiksen R, Hoppe C, Hvid R, Astrup A (2012) The effect of milk proteins on appetite regulation and diet-induced thermogenesis. Eur J Clin Nutr 66:622–627
Hernández-Ledesma B, García-Nebot MJ, Fernández-Tomé S, Amigo L, Recio I (2013) Dairy protein hydrolysates: peptides for health benefits. Int Dairy J 38:82–100. doi:10.1016/j.idairyj.2013.11.004
Hall WL, Millward DJ, Long SJ, Morgan LM (2003) Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr 89:239–248
Kazafeos K (2011) Incretin effect: GLP-1, GIP, DPP4. Diabetes Res Clin Pract 93(Suppl 1):S32–S36
Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Bjorck I (2012) The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on beta-cells. Nutr Metab 9:48
Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP (2009) Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav 96:675–682
Nauck MA, Vilsboll T, Gallwitz B, Garber A, Madsbad S (2009) Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care 32(Suppl 2):S223–S231
Portha B, Tourrel-Cuzin C, Movassat J (2011) Activation of the GLP-1 receptor signaling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res. doi:10.1155/2011/376509
Halton TL, Hu FB (2004) The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 23:373–385
Anderson GH, Tecimer SN, Shah D, Zafar TA (2004) Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr 134:3011–3015
Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A (2005) Brain amino acid requirements and toxicity: the example of leucine. J Nutr 135:1531S–1538S
Morrison CD, Xi X, White CL, Ye J, Martin RJ (2007) Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab 293:E165–E171
Lacroix IME, Li-Chan ECY (2012) Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int Dairy J 25:97–102
Tulipano G, Sibilia V, Caroli AM, Cocchi D (2011) Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 32:835–838
Uenishi H, Kabuki T, Seto Y, Serizawa A, Nakajima H (2012) Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int Dairy J 22:24–30
Yao Y, Sang W, Zhou M, Ren G (2010) Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J Agric Food Chem 58:770–774
Jaiswal N, Srivastava SP, Bhatia V, Mishra A, Sonkar AK (2012) Inhibition of alpha-glucosidase by acacia nilotica prevents hyperglycemia along with improvement of diabetic complications via aldose reductase inhibition. J Diabetes Metab 6:1–7
Apostolidis E, Kwon YI, Ghaedian R, Shetty K (2007) Fermentation of milk and soymilk by Lactobacillus bulgaricus and Lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension. Food Biotechnol 21:217–236
Slama G, Elgrably F, Mbemba J, Larger E (2006) Postprandial glycaemia:a plea for the frequent use of delta postprandial glycaemia in the treatment of diabetic patients. Diabetes Metab 32:187–192
Lee A, Patrick P, Wishart J, Horowitz M, Morley JE (2002) The effects of miglitol on glucagon-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes Obes Metab 4:329–335
Yusuke Moritoh, Takeuchi Koji, Hazama Masatoshi (2009) Voglibose, an alpha-glucosidase inhibitor, to increase active glucagon-like peptide-1 levels. Mol Cell Pharmacol 1:188–192
Campbell LK, Baker DE, Campbell RK (2000) Miglitol: assessment of its role in the treatment of patients with diabetes mellitus. Ann Pharmacother 34:1291–1301
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents:current role in type 2 diabetes mellitus. Drugs 65:385–411
Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B (2006) Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 49:1711–1721
McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D (2005) Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J Agric Food Chem 53:2760–2766
da Silva Pinto M, Kwon YI, Apostolidis E, Lajolo FM, Genovese MI, Shetty K (2008) Functionality of bioactive compounds in Brazilian strawberry (Fragaria ananassa Duch.) cultivars: evaluation of hyperglycemia and hypertension potential using in vitro models. J Agric Food Chem 56:4386–4392
McCue P, Kwon YI, Shetty K (2005) Anti-amylase, anti-glucosidase and anti-angiotensin I-converting enzyme potential of selected foods. J Food Biochem 29:278–294
Matsui T, Yoshimoto C, Osajima K, Oki T, Osajima Y (1996) In vitro survey of α-glucosidase inhibitory food components. Biosci Biotechnol Biochem 60:2019–2022
Yu Z, Yin Y, Zhao W, Yu Y, Liu B, Liu J, Chen F (2011) Novel peptides derived from egg white protein inhibiting alpha-glucosidase. Food Chem 129:1376–1382
Aschenbrenner DS, Venable SJ (2009) Drug therapy in nurshing, 3rd edn. Wolters Kluwer Health, New York, pp 589–592
Ross SA, Gulve EA, Wang M (2004) Chemistry and biochemistry of type 2 diabetes. Chem Rev 104:1255–1282
Chiasson JL, Rabasa-Lhoret R (2004) Prevention of type 2 diabetes:insulin resistance and beta-cell function. Diabetes 53:S34–S38
Arungarinathan G, McKay GA, Fisher M (2011) Drugs for diabetes: part 4 acarbose. Br J Cardiol 18:78–81
Holst JJ (2004) On the physiology of GIP and GLP-1. Horm Metab Res 36:747–754
Gromada J, Rorsman P (2004) New insights into the regulation of glucagon secretion by glucagon-like peptide-1. Horm Metab Res 36:822–829
Perfetti R, Hui H (2004) The role of GLP-1 in the life and death of pancreatic beta cells. Horm Metab Res 36:804–810
Goto Y, Yamada K, Ohyama T, Matsuo T, Odaka H, Ikeda H (1995) An alpha-glucosidase inhibitor, AO-128, retards carbohydrate absorption in rats and humans. Diabetes Res Clin Pract 28:81–87
Gribble FM, Williams L, Simpson AK, Reimann F (2003) A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52:1147–1154
Tolhurst G, Reimann F, Gribble FM (2009) Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol 587:27–32
Moritoh Y, Takeuchi K, Hazama M (2009) Chronic administration of voglibose, an alpha-glucosidase inhibitor, increases active glucagon-like peptide-1 levels by increasing its secretion and decreasing dipeptidyl peptidase-F4 activity in ob/ob mice. J Pharmacol Exp Ther 329:669–676
Nauck MA (1998) Glucagon-like peptide 1 (GLP-1): a potent gut hormone with a possible therapeutic perspective. Acta Diabetol 35:117–129
Bharatham K, Bharatham N, Park KH, Lee KW (2008) Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives, a new class of α-glucosidase inhibitors. J Mol Graph Model 26:1202–1212
Matsui T, Oki T, Osajima Y (1999) Isolation and identification of peptidic α-glucosidase inhibitors derived from sardine muscle hydrolyzate. Z Naturforsch C 54:259–263
Lavigne C, Marette A, Jacques H (2000) Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol 278:E491–E500
Lavigne C, Tremblay F, Asselin G, Jaques H, Marette A (2001) Prevention of skeletal muscle insulin resistance by dietary cod protein in high fat-fed rats. Am J Physiol Endocrinol Metab 281:E62–E71
Huang FJ, Wu WT (2010) Purification and characterization of a new peptide (s-8300) from shark liver. J Food Biochem 34:962–970
Yu Z, Yin Y, Zhao W, Liu J, Chen F (2012) Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem 135:2078–2085
Ramchandran L, Shah NP (2009) Effect of exopolysaccharides and inulin on the proteolytic, angiotensin-I-converting enzyme- and α-glucosidase-inhibitory activities as well as on textural and rheological properties of low-fat yogurt during refrigerated storage. Dairy Sci Technol 89:583–600
Fan H, Yan S, Stehling S, Marguet D, Schuppan D, Reutter W (2003) Dipeptidyl peptidase IV/CD26 in T cell activation, cytokine secretion and immunoglobulin production. Adv Exe Med Biol 524:165–174
Shubrook J, Colucci R, Guo A, Schwartz F (2011) Saxagliptin: a selective DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes 4:1–12
Kushner P, Gorrell M (2010) DPP-4 inhibitors in type 2 diabetes: importance of selective enzyme inhibition and implications for clinical use. J Fam Pract 59(2):1
Wang XM, Yu DM, McCaughan GW (2005) Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cellline. Hepatology 42:935–945
Amerongen AV, Beelen MJC, Wolbers LAM, Gilst WH, Buikema JH, Nelissen JWPM (2009) Egg protein hydrolysates. WO 2009/128713 A1 (Patent)
Li-Chan ECY, Huang SL, Jao CL, Ho KP, Hsu KC (2012) Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J Agric Food Chem 60:973–978
Huang SL, Jao CL, Ho KP, Hsu KC (2012) Dipeptidyl peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 35:114–121
Velarde-Salcedo AJ, Barrera-Pacheco A, Lara-González S, Montero-Morán GM, Díaz-Gois A, Gonzá lez de Mejia E, Barba de la Rosa A (2013) In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem 136:758–764
Hatanaka T, Inoue Y, Arima J, Kumagai Y, Usuki H, Kawakami K, Kimura M, Mukaihara T (2012) Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem 134:797–802
Mochida T, Hira T, Hara H (2010) The corn protein, zein hydrolysate, administered into the ileum attenuates hyperglycemia via its dual action on glucagon-like peptide-1 secretion and dipeptidyl peptidase-IV activity in rats. Endocrinology 151:3095–3104
Tulipano G, Cocchi D, Caroli AM (2012) Comparison of goat and sheep β-lactoglobulin to bovine β-lactoglobulin as potential source of dipeptidyl peptidase IV (DPP-4) inhibitors. Int Dairy J 24:97–101
Uchida M, Ohshiba Y, Mogami O (2011) Novel dipeptidyl peptidase-4-inhibiting peptide derived from β-lactoglobulin. J Pharmacol Sci 117:63–66
Lacroix IME, Li Chan ECY (2012) Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J Funct Food 4:403–422
Nongonierma AB, FitzGerald RJ (2013) Dipeptidyl peptidase IV inhibitory properties of a whey protein hydrolysate: influence of fractionation, stability to simulated gastrointestinal digestion and food-drug interaction. Int Dairy J 32:33–39
Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahrèn B (2006) Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology 147:3173–3180
Demuth HU, McIntosh CH, Pederson RA (2005) Type 2diabetes—therapy with dipeptidyl peptidase IV inhibitors. Biochim Biophys Acta 1751:33–44
Deacon CF (2005) What do we know about the secretion and degradation of incretin hormones? Regul Pept 128:117–124
Kim W, Egan JM (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60:470–512
Kalra S (2011) Emerging role of dipeptidyl peptidase-IV (DPP-4) inhibitor vildagliptin in the management of type 2 diabetes. J Assoc Physicians India 59:237–245
Addison D, Aguilar D (2011) Diabetes and cardiovascular disease: the potential benefit of incretin-based therapies. Curr Atheroscler Rep 13:115–122
Holst JJ, Deacon CF (2004) Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus. Curr Opin Pharmacol 4:589–596
Thornberry NA, Gallwitz B (2009) Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab 23:479–486
Thoma R, Löffler B, Stihle M, Huber W, Ruf A, Hennig M (2003) Structure basis of proline specific exopeptidase activity as observed in human dipeptidyl peptidase-IV. Structure 11:947–959
Rajput R (2009) Dipeptidyl Peptidase-IV Inhibitors: a new drug in the Therapeutic Armamentarium for treatment of Type 2 Diabetes Mellitus. J Indian Acad Clin Med 10:128–133
Deacon CF (2007) Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 9:23–31
Weber AE (2004) Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem 47:4135–4141
Deacon CF, Ahren B, Holst JJ (2004) Inhibitors of dipeptidyl peptidase-IV: a novel approach for the prevention and treatment of Type 2 diabetes? Expert Opin Investig Drugs 13:1091–1102
Herman GA, Bergman A, Stevens C (2006) Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 91:4612–4619
Estrada-Salas PA, Montero-Moran GM, Martinez-Cuevas PP, Gonzalez C, Barba de la Rosa AP (2014) Characterization of antidiabetic and antihypertensive properties of canary seed (Phalaris canariensis L.) peptides. J Agric Food Chem 62(2):427–433
Pieter BJW (2006) Protein hydrolysate enriched in peptides inhibiting DPP-IV and their use. WO 2006/068480 200 (Patent)
Green BD, Flatt PR, Bailey CJ (2006) Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc Dis Res 3:159–165
Zhu CF, Li GZ, Peng HB, Zhang F, Chen Y, Li Y (2010) Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl Physiol Nutr Metab 35:797–804
Guerard F, Decourcelle N, Sabourin C, Floch-Laizet C, Le Grel L, Le Floch P, Gourlay F, Le Delezir R, Jaouen P, Bourseau P (2010) Recent developments of marine ingredients for food and nutraceutical applications: a review. Journal des Sciences Halieutiques et Aquatiques 2:21–27
Tominaga Y, Yokota S, Tanaka H et al (2012) Inventors Kaneka Corporation, assignee. Dipeptidyl peptidase-4 inhibitor. United States US 2012 0189611 (Patent)
Silva-Sánchez C, de la Rosa APB, León-Galván MF (2008) Bioactive peptides in amaranth (Amaranthus hypo-chondriacus) seed. J Agric Food Chem 56:1233–1240
Hsu KC, Tung YS, Huang SL Jao CL (2013) Dipeptidyl peptidase-IV inhibitory activity of peptides in porcine skin gelatin hydrolysates. In Hernández-Ledesma B (ed) Bioactive food peptides in health and disease, pp 205–218. doi:10.5772/51264
Dziuba M, Dziuba B, Iwaniak A (2009) Milk proteins as precursors of bioactive peptides. Acta Sci Pol Technol Aliment 8:71–90
Boots J (2006) Inventor Campina Nederland Holding BV assignee. Protein hydrolysates enriched in peptides inhibiting DPP IV and thier use. WO 2006/068480 200 (Patent)
Nongonierma AB, FitzGerald RJ (2014) Susceptibility of milk protein derived peptides to dipeptidyl peptides IV (DPP-IV) hydrolysis. Food Chem 145:845–852
Silveira ST, Martínez-Maqueda D, Recio I, Hernández-Ledesma B (2013) Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem 141:1072–1077
Tremblay A, Gilbert JA (2009) Milk products, insulin resistance syndrome and type 2 diabetes. J Am Coll Nutr 28(Suppl 1):91S–102S
Phelan M, Aisling A, FitzGerald RJ, O’Brien NM (2009) Casein-derived bioactive peptides: biological effects, industrial uses, safety aspects and regulatory status. Int Dairy J 19:643–654
Gill I, López-Fandiño R, Jorba X, Vulfson EN (1996) Biologically active peptides and enzymatic approaches to their production. Enzyme Microb Technol 18:163–183
Madureira AR, Tavares T, Gomes MP, Malcata FX (2010) Physiological properties of bioactive peptides obtain from whey proteins. J Dairy Sci 93:437–455
Kilara A, Panyam D (2003) Peptides from milk proteins and their properties. Crit Rev Food Sci Nut 43:607–633
Korhonen H, Pihlanto A (2003) Food-derived bioactive peptides-opportunities for designing future foods. Curr Pharm Des 9:1297–1308
Haque E, Chand R, Kapila S (2008) Biofunctional properties of bioactive peptides of milk origin. Food Rev Int 25:28–43
Urista CM, Fernández RA, Rodríguez FR, Cuenca AA, Jurado AT (2011) Review: production and functionality of active peptides from milk. Food Sci Technol Int 17:293–317
Juillard V, Le Bars D, Kunji ER, Konings WN, Gripon JC, Richard J (1995) Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl Environ Microbiol 61:3024–3030
Christensen JE, Dudley EG, Pederson JA, Steele JL (1999) Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 76:217–246
Alsayadi M, Jawfi AL et al (2014) Evaluation of anti-hyperglycemic and anti-hyperlipidemic activities of water kefir as probiotic on streptozotocin-induced diabetic wistar rats. J Diabetes Mellit 4:85–95
Teruya K, Yamashita M et al (2002) Fermented milk, Kefram-Kefir enhances glucose uptake into insulin-responsive muscle cells. Cytotechnology 40:107–116
Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, Gheshlaghi ZB, Vahedjabbari M (2015) Effect of probiotic fermented milk (Kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health 44:228–237
Maeda H, Zhu X, Mitsuoka T (2004) Effects of an exopolysaccharide (kefiran) from Lactobacillus kefiranofaciens on blood glucose in KKAy mice and constipation in SD rats induced by a low-fiber diet. Biosci Microflora 23:149–153
Yi N, Hwang JY, Han JS (2009) Hypoglycemic effect of fermented soymilk extract in STZ-induced diabetic mice. J Food Sci Nutr 14:8–13
Yadav H, Jain S, Sinha PR (2007) Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 23:62–68
Kim SK, Wijesekara I (2010) Development and biological activities of marine-derived bioactive peptides: a review. J Funct Foods 2:1–9
Pedroche J, Yust MM, Lqari H, Megias C, Girón-Calle J, Alaiz M (2007) Obtaining of Brassica carinata protein hydrolysates enriched in bioactive peptides using immobilized digestive proteases. Food Res Int 40:931–938
Manders RJF, Hansen D, Zorenc AHG, Dendale P, Kloek J, Saris WHM, van Loon LJC (2014) Protein co-ingestion strongly increases postprandial insulin secretion in type 2 diabetes patients. J Med Food 17:758–763
Méric E, Lemieux S, Turgeon SL, Bazinet L (2014) Insulin and glucose responses after ingestion of different loads and forms of vegetable or animal proteins in protein enriched fruit beverages. J Funct Foods 10:95–103
Goudarzi M, Madadlou A (2013) Influence of whey protein and its hydrolysate on prehypertension and postprandial hyperglycaemia in adult men. Int Dairy J 33:62–66
Jonker JT, Wijngaarden MA et al (2011) Effect of low doses of casein hydrolysate on post-challenge glucose and insulin levels. Eur J Intern Med 22:245–248
Acknowledgments
The authors thank the Director of NDRI for supporting the work. There are no conflicts of interest whatsoever among the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, P., Mandal, S., Tomar, S.K. et al. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur J Nutr 54, 863–880 (2015). https://doi.org/10.1007/s00394-015-0974-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0974-2