Abstract
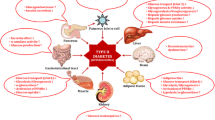
The increasing prevalence of diabetes has led to reducing hyperglycemia through mechanisms other than the conventional mechanism, such as α-amylase and α-glucosidase inhibition. In recent years, medicinal drugs focusing on inhibiting dipeptidyl peptidase IV (DPP IV) and protein tyrosine phosphatase 1B (PTP1B) enzymes have emerged and are being used for type 2 diabetes management. DPP IV inhibitors reduce blood glucose levels by preventing the degradation of incretin hormones such as glucagon-like peptide and glucose-dependent insulinotropic polypeptide. PTP1B has also been known to play a crucial role in reducing insulin resistance and is one of the most promising targets for managing Type 2 diabetes. Inhibition of these two enzymes is also expected to benefit other metabolic conditions such as cancer, obesity, lowered immunity, etc. The existing synthetic DPP IV and PTP1B inhibitors have been known to cause side effects. Inhibitors from natural sources are expected to be safer. The search for PTP1B inhibitors is especially necessary since the primary treatment for type 2 diabetes is to reduce insulin resistance. None of the existing PTP1B inhibitors are clinically well-approved to date. Hence, searching for antihyperglycemic components from natural sources such as foods has become a pressing need. This review has attempted to collate and analyze the existing scientific evidence to identify plant foods and their phytochemicals with in vitro and in vivo DPP IV and PTP1B inhibitory activity comprehensively. With further scientific validation and safety studies, the identified phytochemicals could be used for pharmacological applications. The foods and their extracts could be advantageous in formulating functional foods and diets suitable for type 2 diabetes, along with other physiological benefits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hyperglycemia is the most characteristic symptom in all forms of diabetes. Uncontrolled hyperglycemia is associated with complications such as fluid and electrolyte disturbances and increased infection risk. Studies have reported impairment of host defenses, including decreased polymorphonuclear leukocyte mobilization, chemotaxis, and phagocytic activity related to hyperglycemia1. Chronic hyperglycemia has also been associated with delayed wound healing in the diabetic population2. Acute hyperglycemia is also known to cause tissue injury in various organs. Consequently, glucose toxicity causes microvascular or diabetic complications such as neuropathy, retinopathy, and nephropathy3.
Hence, the primary goal in diabetes management is reduced glycemic response to foods. Diabetic drug therapy often focuses on inhibiting enzymes implicated in diabetes to stabilize blood glucose levels. Some important target enzymes for diabetes therapy include α-amylase, α-glucosidase, dipeptidyl peptidase IV (DPP IV), and protein tyrosine phosphatase 1B (PTP1B). Specific drugs exist in the market to reduce hyperglycemia via specific mechanisms4. DPP IV inhibitor drugs in the market, such as sitagliptin, vildagliptin, and saxagliptin, are commonly prescribed to control hyperglycemia and reduce HbA1c levels. These drugs, however, have been reported to cause side effects such as fever, ocular hyperemia, nasopharyngitis, upper respiratory tract infection, streaming eyes, and gastrointestinal disturbances5. Sodium orthovanadate has been reported to be a potent inhibitor of the PTP1B enzyme. However, the safety of sodium orthovanadate remains questionable due to a lack of clinical studies6. Another common problem with different synthetic drugs in diabetes treatment is the occurrence of hypoglycemia7. Hence, the search for safer alternatives from natural sources continues to date.
Plant foods are one such natural source that has been investigated extensively for their inhibitory capacity against different target diabetic enzymes. These plant foods have several phytochemicals which exhibit significant biological activity, including DPP IV and PTP1Benzyme inhibition . Incorporating plant foods with health functionality is being recognized as a promising innovative dietary strategy for the management of type 2 diabetes and its complications8. There are few recent scientific reviews on DPP IV inhibitory activities of food-derived bioactive peptides9 and some medicinal foods10. Synthetic and naturally isolated flavonoids have been reviewed for both DPP IV and PTP1B activities11. Marine-derived bioactive molecules have been reviewed for PTP1B inhibition12. These studies have focussed on identifying lead compounds for pharmacological applications. Activities of plant foods and their extracts have not been reviewed to date to our knowledge. This review, hence, is an attempt to collate and review the scientific information reported in the last decade on plant food extracts and their phytochemicals with DPP IV and PTP1B inhibitory activities in an attempt to scientifically identify foods that could be beneficial in a diabetic diet.
2 Plant Foods and Their Phytochemicals with DPP IV Inhibitory Activity
One of the common antihyperglycemic mechanisms observed in diabetic drugs is the inhibition of the dipeptidyl peptidase IV (DPP IV/CD26) enzyme. DPP IV is a cell-surface protease belonging to the prolyl oligopeptidase family. It prevents the degradation of two key glucoregulatory incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1)13. These incretin hormones are secreted in the intestine in response to the intake of nutrients, which in turn produce glucose-induced insulin response. DPP IV selectively removes the N-terminal dipeptide from peptides with proline or alanine in the second position. In certain metabolic conditions, GLP-1, which contains alanine, serves as a substrate for DPP IV, resulting in its inactivation. Therefore, DPP IV inhibitors have been reported to improve glucose tolerance and pancreatic islet cell function in animal models of type 2 diabetes and in diabetic patients. The DPP IV inhibitors present in functional plant foods are expected to prevent GLP-1 from being inactivated by acting as an alternative substrate for DPP IV14. This will result in an improvement in blood glucose levels and increased insulin production with the inhibition of glucagon secretion10.
2.1 DPP IV Inhibitory Plant Foods
This section reviews the scientific information available in the last decade for DPP IV inhibitory activities of different foods/food products under different food groups (Table 1). For a more comprehensive idea of their glucose regulatory potential, the other mechanisms of antidiabetic action reported in the same study have also been mentioned in the descriptive text.
2.1.1 Cereals, Pulses, and Their Functional Food Products
Pigmented rice: Rice is the staple food of more than half of the world’s population. Rice seeds (paddy rice) have outer shells called husk (woody, siliceous and non-edible in nature), which are removed by dehulling/de-husking process. De-husked rice with its bran layers (nucellar, testa, and pericarp) and germ (embryo) intact is whole-grain rice, normally also referred to as brown rice, due to the color of the bran. Pigmented rice refers to whole grain rice with the bran layers varying in hues and intensities from brown, red, black, purple, green, and yellow in different varieties. In contrast, in white rice, also referred to as milled rice/polished rice, the bran layers and germ are completely removed on milling15. Recent studies16,17 have reported the DPP IV inhibitory activity (IC50) of methanolic extracts of Bamboo seed rice (4.2 µg/ml) and four pigmented rice varieties from India—Karungkuruvai (1.7 µg/ml), Garudan samba (1.8 µg/ml), Kattuyanam (2.2 µg/ml), and Chennangi (5.7 µg/ml). The activity was comparable to sitagliptin, the positive control (1.2 µg/ml), and significantly much higher than in white rice (IC5072.6 µg/ml). The studies identified these pigmented rice varieties to be rich sources of bioactive phenolics such as gallic acid, 4-hydroxybenzaldehyde, caffeic acid 4-O-glucoside, p-coumaric acid, cirsimaritin, eriocitrin, etc. They further reported the good binding potential of these metabolites at the active site of the DPP IV enzyme using molecular docking. Therefore, the DPP IV inhibitory property of pigmented rice was attributed to the presence of these phenolic compounds. Additionally, Bamboo seed rice and Garudan samba were reported to inhibit the α-amylase enzyme16, and the extracts of Kattuyanam, Karungkuruvai, and Chennangi were reported to inhibit α-amylase, α-glucosidase, and PTP IB enzymes17.
Red rice extruded product: Another study18 reported the in vitro DPP IV inhibitory activity of Indian red rice from Arunachal Pradesh, its bran, and extruded product. The highest inhibition was exhibited by the bran (70.5%), followed by whole red rice (42.6%) and polished red rice (35.9%). The optimized extruded product (red rice combined with 11.25% passion fruit powder) exhibited a higher (25.5%) inhibition than the control extruded product (red rice extruded alone − 13.6%).
Chickpea-fortified maize tortillas: One of the popular grains consumed worldwide is maize. Due to the presence of anthocyanins, blue and purple maize have been investigated for their anti-inflammatory, anti-adipogenic, and antidiabetic potential19. The maize/corn-based snacks industry has been increasing steadily, and tortillas are the most popular corn-based product20. To enhance the suitability of this staple food for diabetes, a recent study evaluated the effect of fortifying white and blue maize tortillas with chickpea protein hydrolysate (CPH) on in vitro DPP IV inhibition. The DPP IV inhibition increased significantly from 11 and 26% (0% fortification) to 91% and 95% in white and blue maize tortillas, respectively, when fortified with 15% CPH21. After simulated gastrointestinal digestion, the activity was retained but decreased to 41% and 61% in white and blue maize tortillas, respectively. Physicochemical characteristics such as texture and color were, however, found to be affected adversely.
Herb-enriched wheat and/ barley flour-based products: Wheat and/barley-based products enriched with three herbs (T. chebula, T. bellerica, and E. officinalis) were tested for their in vitro and in vivo DPP IV inhibitory capacity22. The methanolic extracts showed better in vitro DPP IV inhibition (IC50 4.3–4.6 mg/mL) than the water extracts (IC50 5.6–30.5 mg/mL). Studies in streptozotocin-induced diabetic rats showed a significant decrease in DPP-IV enzyme activity (p moles pNA/min/mg protein) from 1.4 to 0.64, 0.71, and 0.91 when fed with herb-enriched barley, wheat, and wheat-barley products. These products were also found to exhibit good in vitro and in vivo amylase and glucosidase inhibitory activity, low estimated glycemic index, and lower in vivo glycemic response.
Pre-cooked sorghum flour fortified with sorghum peptides: Pre-cooked sorghum flour added with 3 g/100 g of angiotensin-converting enzyme 1 (ACE-I) and DPP IV inhibitory sorghum peptides had high total peptide dialysability after simulated gastrointestinal digestion23. The bioaccessible peptides from the fortified product exhibited lower IC50values, indicating a higher inhibitory activity than unfortified pre-cooked sorghum flour against ACE-I (1.04 ± 0.12 vs. 1.82 ± 0.09 mg protein mL − 1, respectively) and DPP IV (0.86 ± 0.02 vs. 2.12 ± 0.08 mg protein mL − 1, respectively) enzymes.
2.1.2 Fruits, vegetables, and spices
Amla: Emblica officinalis, also known as Indian gooseberry or amla in Ayurveda, is considered a powerful rejuvenator for delaying aging and degenerative process. A study24 reported amla fruit extract to exhibit a concentration-dependent inhibitory activity in vitro against human DPP IV enzyme with the highest inhibition (53.2%) at 4 mg/ml. The fruit extract was also reported to contain phytochemicals such as β-glucogallin, and hydrolyzable tannins, including mucic acid 1,4-lactone 5-O-gallate, mucic acid 2-O-gallate, mucic acid 6-methyl ester 2-O-gallate, mucic acid 1-methyl ester2-O-gallate, and ellagic acid. The presence of these metabolites was considered the basis of the DPP IV inhibitory potential of amla. The study also reported good α-amylase and α-glucosidase inhibitory activity of amla fruit extract.
Pomegranate: In some traditional medical systems, pomegranate fruit is used in the treatment of diabetes. Pomegranate polyphenols are well-known for their antioxidant properties, which have advantages including lowering oxidative/inflammatory stress and raising protective signaling molecules like antioxidant enzymes, neurotrophic factors, and cytoprotective proteins. Some of the major compounds present in pomegranate juice, such as ellagic acid and punicalagin, inhibited α-amylase, α-glucosidase, and dipeptidyl peptidase-4 in free-cell systems and adipocyte cell lines25. The results from a recent study in women with type 2 diabetes26 showed a nonsignificant decrease in DPP IV from 475.77 to 390.86 IU/L after the consumption of 100 ml of pomegranate juice for 8 weeks. When combined with resistance training, a significant decrease (p < 0.05) was observed in DPP IV levels (432.74–216.63 IU/L). The combination treatment also significantly reduced LDL and glucose levels (p = 0.0001) and improved GLP-1, HDL, and insulin levels (p = 0.0001).
Rhus chinensis Mill.: Several bioactivities, including antioxidant and pancreatic lipase inhibitory actions in vitro, prevention of non-alcoholic fatty liver, and alcoholic fatty liver, have been associated with R. chinensis fruit, popularly known as Chinese sumac27. Liu et al.28 studied the in vitro DPP IV inhibitory potential of Rhus chinensis Mill. fruit phenolic-rich extracts. The free phenolic extract showed the highest inhibitory activity with an IC50 value of 66 µg/ml. Phytochemical correlation analysis indicated di-O-galloyl-glucoside and its isomer to contribute to the above activity. The fruit phenolic-rich extracts were also found to inhibit α-glucosidase and advanced glycation end products.
Garlic: Garlic (Allium sativum) is a well-known spice and member of the Alliaceae family. It is also utilized as a treatment for various illnesses and physiological conditions. Garlic has been reported to have positive effects on blood pressure, cholesterol, and infections, as well as on treating metabolic disorders and cancer prevention. The antidiabetic, hepatoprotective, anthelmintic, anti-inflammatory, antioxidant, antifungal, and wound-healing properties of garlic extracts have also been reported29. Methanolic extract from garlic has been reported to show good inhibition of the DPP IV enzyme with an IC50 value of 70.9 µg/ml30. The study also reported the presence of phytochemicals such as catechin, caffeic acid 3-glucoside, calenduloside E, and malonylgenistin. The DPP IV inhibitory potential of garlic was attributed to the presence of these phenolic compounds, which exhibited good inhibition against DPP IV as evidenced through in silico virtual screening and molecular docking simulations studies.
2.1.3 Edible algae
Both eastern and western nations have utilized marine macroalgae, such as Sargassum polycystum, Sargassum wightii, etc., for food and as folk medicine. Numerous phytochemicals with diverse bioactivities, such as antidiabetic and antioxidant activities, are present in natural marine products. Significant contributions to the control of glucose-induced oxidative stress and regulation of starch-digesting enzymes have been documented for bioactive components found in these edible seaweeds31. Apart from these other antidiabetic mechanisms, recent studies reported the DPP IV inhibitory potential of edible algae. Sargassum polycystum and Sargassum wightii methanolic extracts exhibited IC50 values of 36.9 and 38.3 µg/ml, respectively32.
Calderwood et al.33 compared the in vitro DPP IV inhibitory activity of eleven types of red and brown algae. Alaria esculenta (ethanolic extract- 91%) and Laminaria digitata (aqueous extract-90%) exhibited the highest inhibitory potential comparable to berberine, the positive control. Extracts of F. vesivulosus, A. nodosum, and A. esculenta also exhibited glucosidase inhibition in vitro. Water and ethanolic extracts of A. esculenta and U. rigida were reported to also significantly increase GLP secretion from STC-1 cells at 25 mg/ml concentration. The proposed mechanistic action of plant food extracts against the DPP IV enzyme is presented in Fig. 1.
2.2 Food-Derived Metabolites with DPP IV Inhibitory Activity
In this section, we have reviewed the DPP IV inhibitory potential of peptides and metabolites extracted from food sources and not reported in recent earlier review articles.
2.2.1 Peptides
Peptides isolated from various food sources have been studied extensively for their DPP IV inhibitory. Two recent review articles by Liu et al.13 and Zhang et al.34 have reported the DPP IV inhibitory potential of peptides from several foods. In this review, we have updated the information from recent in vitro studies (Table 2).
Two peptides (IPI and IPV) hydrolyzed from quinoa exhibited DPP IV inhibitory activity with IC50 values of 30 μM35. In another study, nine peptides from oats were found to have DPP IV IC50 values ranging from 25.2 to 283 μM36. Three peptides from sorghum bicolor seed protein, LSICGEESFGTGSDHIR, SLGESLLQEDVEAHK, and QLRDIVDK, were reported to have DPP IV inhibitory potential with IC50 values of 73.5, 82.5, and 8.6 µM, respectively37. In the same study, molecular docking analysis revealed the peptides to bind at the active and secondary sites of DPP IV. The peptide QLRDIVDK exhibited the highest binding affinity. A study by Zhang et al.38 reported the IC50 of pea protein peptide (IPYWTY) to be 11.04 μM. The in vitro results were further confirmed using molecular docking studies. Peptides from germinated soybean of 5 to 10 KDa were found to have 0.91 mg/ml IC50, and peptides > 10 KDa exhibited 1.18 mg/ml IC5039. Cian et al.40 reported the IC50 values of protein hydrolysates from Ulva spp. NPF hydrolysate exhibited 2.7 mg/ml, and APF hydrolysate exhibited 1.21 mg/ml. Lu et al.41 reported the IC50 value of a black tea peptide (AGFAGDDAPR) to be 1 mg/ml.
2.2.2 Phenolics
Dietary polyphenols have drawn interest because they act as indirect insulin secretion stimulants and dipeptidyl peptidase-IV (DPP-IV) inhibitors. The DPP IV inhibitory potential of dietary polyphenols is due to their structural affinity for the active site of the DPP IV enzyme42. O-Ethyl-4-[(α-I-rhamnosyloxy-benzyl] carbamate, a phenolic glucoside derivative from Moringa oleifera leaves, exhibited a very low IC50 value of 798 nM, indicating good DPP IV inhibitory potential43. The phenolic metabolites, oleuropein, oleacein, oleocanthal, hydroxytyrosol, and tyrosol extracted from extra virgin olive oil exhibited DPP IV inhibition with IC50 ranging from 187 to 741.6 µM44.
3 Plant Foods and Their Phytochemicals with PTP1B Inhibitory Activity
Protein tyrosine phosphatase 1B (PTP1B) is a non-transmembrane phosphatase enzyme belonging to PTPs enzymes class and is expressed in tissues targeted by insulin, such as the liver, muscle, and fat. It catalyzes the dephosphorylation of the activated insulin receptor, insulin receptor substrate-1, thereby downregulating insulin signaling through the Akt/PI3K pathway. It also negatively regulates leptin signaling, contributing to obesity and metabolic disorders. Thus, inhibition of PTP1B is considered a promising method for treating type 2 diabetes and preventing obesity45.
3.1 PTP1B Inhibitory Plant Food Extracts
3.1.1 Cereals
Pigmented rice: Methanolic extracts of three Indian pigmented rice varieties, namely, Kattuyanam, Karungkuruvai, and Chennangi, were found to inhibit in vitro PTP1B enzyme with IC50 values ranging from 30.9 to 38.6 µg/ml (Table 3)17. Molecular docking analysis of seven phenolic metabolites abundant in these rice varieties (3′,4′,7-trihydroxyisoflavone, 3′-O-methylviolanone, catechin, cirsimaritin, dihydroquercetin, isorhamnetin, and isoxanthohumol) reported in the same study demonstrated good binding potential to the active site of PTP1B enzyme. Thus, these phytochemicals were opined to contribute to the PTP1B inhibitory potential of the pigmented rice samples.
3.1.2 Vegetables and Spices
Onion: Allium cepa is one of the most widely grown and consumed crops and is most frequently utilized in Indian cuisine. Onion peel has been reported to be a rich source of phenolics such as quercetin and its derivatives, protocatechuic acid, isorhamnetin, coumaric acid, vanillic acid, anthocyanins, etc. and tannins46. In a recent study, Yang et al.47 reported the in vitro PTP1B inhibitory potential of red and yellow onion peel extracts (Table 3). The IC50 values of water extracts (red—0.33, yellow—0.3) were lower than the ethanolic extracts (red—0.76, yellow—0.86 µg/ml). The water extract also showed greater (90%) glucose uptake than ethanolic extract (70%) at 1.25 μg/ml in insulin-resistant HepG2 cells. Further, treatment of insulin-resistant HepG2 cells with water extracts was reported to significantly reduce the expression of PTP1B. The study also reported the onion peel extracts to have good antioxidant (DPPH and ABTS), α-glucosidase, and AGEs inhibitory activity. The water extract showed no toxicity in HepG2 cells up to a concentration of 100 µg/ml.
Moringa oleifera: A highly nourishing vegetable with several potential benefits for treating rheumatism, poisonous bites, and pathogenic infections is the commonly cultivated species of Moringa oleifera in India48. Saidu et al.49 reported 41% inhibition of PTP1B in vitro at a concentration of 100 μg/ml of methanolic extract of M. oleifera leaves. Sierra-Campos et al.50 also reported in vitro inhibition of the PTP1B enzyme by methanolic extract of M. oleifera leaves with an IC50 value of 346.8 µg/ml (Table 3). The study also reported it is in vitro aldose reductase activity.
Cinnamon: Consumption of cinnamon is linked to statistically significant reductions in fasting plasma glucose, total cholesterol, LDL cholesterol, and triglyceride levels, as well as an elevation of HDL cholesterol51. Lin et al.52 found that different solvents and aqueous extracts of cinnamon twigs inhibited PTP 1B with IC50 values ranging from 1.7 to 3.2 µg/ml (Table 3). Cinnamon twig was also reported to inhibit α-amylase and α-glucosidase in the same study.
Honey: The oldest known natural sweetener is honey. Besides its sweetening property, honey is also known to be a rich source of vitamins, amino acids, proteins, mineral salts, trace elements, and other metabolites such as flavonoids, phenolic and organic acids53. Honey extracts from twenty samples belonging to four different floral varieties, such as honeydew, chestnut, wildflowers, and acacia, were found to inhibit PTP1B (Table 3) with IC50 values ranging from 3.1 to 42.8 µg/ml54. Highest in vitro inhibitory activity was observed in honey obtained from honeydew flowers. The honey extracts, when treated with HepG2 cells, also enhanced the expression of insulin receptors and stimulated glucose uptake.
Tea: Epidemiological research has shown that tea use lowers the risk of developing diabetes and its complications. Its antidiabetic mechanisms of action include improving insulin resistance, activating the insulin signaling pathway, playing an insulin-like role, improving oxidative stress, and alleviating inflammatory response55. Ma et al.56 found that tea extracts inhibited PTP1B activity in vitro. The IC50 values were found to be 0.4 mg/ml (black tea), 2 mg/ml (Oolong tea), and 4 mg/ml (green tea) (Table 3). HPLC analysis revealed that polyphenols, specifically catechins, were the major bioactive compounds in the tea. When applied to cultured cells, the tea extracts also induced tyrosine phosphorylation of cellular proteins.
Pomegranate rinds: Punica granatum (Pomegranate) rind methanolic extract has been reported to exhibit an IC50 value of 26.25 μg/ml against PTP 1B enzyme57. Administration of 200 and 400 mg/kg of the extract to diabetic rats significantly reduced the blood glucose levels from 268.51 to 132.44 mg/dl and from 272.4 to 115.27 mg/dl, respectively, after 21 days. The extract was also reported to inhibit α-amylase and aldose reductase.
3.1.3 Mushrooms
Yang et al.58 reported the PTP1B inhibitory potential of Ganoderma lucidum ethanolic extract (Table 3). It was found to reduce the expression of PTP1B mRNA in L6 cells in a dose-dependent manner until saturation (100 μg/ml). Ganoderma lucidum ethanolic extract also increased the glucose uptake in L6 cells, upregulated the expression of GLUT4 mRNA, and improved the phosphorylation of IRS1 on Tyr612 in PTP1B-cells in the same study. Morchella conica methanolic extract has been reported to exhibit PTP1B inhibition in vitro with an IC50 value of 26.5 μg/ml59. The study also reported a gradual reduction in the blood sugar level in M. conica-treated diabetic mice from 448 to 148 mg/dl. The extract also improved liver and kidney damage in the rats by normalizing the serum glutamic pyruvic transaminase, serum glutamic oxaloacetate, alkaline phosphatase, serum creatinine, and urea levels. The antidiabetic activity of M. conica was attributed to the presence of metabolites such as diethofencarb, glycyl-prolyl-lysine, enterodiol, S-Nitroso-L-glutathione, acenocoumarol, lagochiline, 16,16-dimethylprostaglandin, and quercetin 3,5,7,3′,4′-pentamethyl ether.
3.1.4 Edible Algae
Solvent extracts of edible brown alga Hizikia fusiformis inhibited PTP1B in vitro activity with IC50 values ranging from 1.69 to 32 µg/ml60. The extracts also exhibited good α-glucosidase and reactive oxygen species inhibition (Table 3). Another study61 reported the IC50 values of Gracilaria lemaneiformis hexane and ethanolic extracts against PTP1B to be 17 and 68.6 µg/ml, respectively. The study attributed the inhibitory potential of Gracilaria lemaneiformis extracts to the presence of 2,2′-methylenebis-6-(1,1-dimethylethyl)-4-methylphenol, 2,4-di-tert-butylphenol and palmitoleic acid using molecular docking analysis. The proposed mechanistic action of plant food extracts against the PTP 1B enzyme is presented in Fig. 2.
3.2 Food-Derived Metabolites with PTP1B Inhibitory Activity
In this section, we have reviewed the PTP1B inhibitory potential of metabolites extracted from food sources (Table 4).
3.2.1 Mushroom Alkaloids and Terpenoids
Several studies have reported the PTP 1B inhibitory potential of different varieties of mushrooms. Wang et al.62 reported the alkaloids from the edible mushroom, Hericium erinaceus to have good PTP1B inhibitory activity with IC50 values ranging from 24 to 50 μM. Sesquiterpenoids from the edible mushroom Pleurotus citrinopileatus were found to have IC50 values ranging from 30 to 100 μM63. In another study, Tao et al.64 reported the IC50 values of sesquiterpenoids from the edible mushroom Pleurotus cystidiosus to range from 38 to 50 μM. Lanostane triterpenoids from the edible mushroom, Fomitopsis pinicola, exhibited IC50 values ranging from 21 to 53 μM65.
3.2.2 Edible Algae Terpenoids
Phlorotannins from edible brown algae, Ecklonia stolonifera, and Eisenia bicyclis were found to have IC50 values ranging from 0.18 to 1.69 μg/ml66. Jung et al.67 reported fucoxanthin from edible brown algae Eisenia bicyclis and Undaria pinnatifida to have an IC50 value of 4.80 μM. The IC50 values of terpenoids and organic acids from edible green alga Caulerpa racemosa ranged from 2.3 to 50 μM68. Fatty acids, sterols, phenolic compounds, homo-monoterpene, and triterpenoid glycosides from Hizikia fusiformis exhibited IC50 values ranging from 6 to 23 μM69.
3.2.3 Metabolites from Other Food Sources
Valoneic acid dilactone (VAD) extracted from the rinds of Punica granatum (Pomegranate) has an IC50 value of 12.4 μg/ml against PTP 1B57. Administration of VAD at 10, 25, and 50 mg/kg for 21 days significantly reduced blood glucose levels from 259.87 to 124.66 mg/dl, 264.51 to 98.26 mg/dl, and 274.56 to 95.12 mg/dl, respectively, in alloxan-induced diabetic rats. Another study reported the PTP1B inhibitory activity of two metabolites isolated from wasted lychee seed. The IC50 values of pavetannin B2, procyanidin A2, and the positive control, ursolic acid, were reported to be 450.295, 338.257, and 19.686 μM, respectively70. Lychee seed extracts in other in vivo studies have been found to reduce insulin resistance by increasing the expression of PI3K, AKT, and mTOR and triggering the PI3K/AKT/mTOR signaling pathway71. However, the safety of Lychee seed is under deliberation because it contains Hypoglycin A, which has been reported to cause hypoglycemia and fatal encephalopathy in humans72. Cucurbitane triterpenoids from Momordica charantia L. exhibited IC50 values ranging from 10 to 28.5 μM73. Eleftheriou et al.45 reported the PTP1B inhibitory activity of acetoside (IC50 9.3 μM) from Sideritis L. (mountain tea). Procyanidins isolated from Lotus (Nelumbo nucifera Gaertn.) seed pods exhibited significant in vitro PTP1B inhibitory activity with an IC50 value of 0.33 μg/ml74.
4 Conclusions and Future Directions
Managing diabetes through foods and bioactive food phytochemicals is gaining more emphasis due to the associated safety aspects coupled with their diverse mechanisms of action. Diabetic drugs target enzymes involved in glucose homeostasis pathways that include DPP IV and, more recently, PTP1B. This review has collated the scientific evidence for DPP IV inhibitory activity of select traditional Indian pigmented rice varieties, common fruits such as gooseberry/amla, pomegranate, and Chinese sumac, and spices such as garlic assessed by in vitro/in vivo assays. Few edible algae have also emerged as potent DPP IV inhibitors. Foods such as oats, quinoa, sorghum, pea, soybean, Moringa, and a few others have shown inhibitory activity through compounds derived/extracted from them. This review also has helped to identify plant foods such as pigmented rice varieties, onion peels, Moringa oleifera, cinnamon, honey, tea, mushrooms, and edible algae with (in vitro/in vivo) PTP1B inhibitory activity. Screening of the scientific literature also revealed compounds isolated from a few mushrooms and edible algae to exhibit PTP1B inhibitory activity. Among the phytochemicals, peptides were found to contribute most to DPP IV inhibitory activity. For PTP1B inhibitory activity, terpenoids have been evaluated more widely. However, comparatively the few phenolic compounds studied have shown lower IC50 and, thereby, greater potency. Few of the foods, such as Moringa, pomegranate, and select pigmented rice varieties reviewed here, have inhibitory activity against both the target DPP IV and PTP1B enzymes. Several of them also have been reported to exhibit inhibitory properties against other diabetic target enzymes, alter plasma biomarkers, and maintain glucose homeostasis. Foods with multi-mechanistic glucose regulatory potential are expected to be promising candidates for planning preventive and therapeutic diets for diabetes.
DPP IV has also been proposed as a diagnostic or prognostic marker for various tumors, hematological malignancies, immunological, inflammatory, psychoneuroendocrine disorders, and viral infections75. Similarly, PTP1B’s has been proposed as an ideal therapeutic target for obesity, cancer, and immune modulation apart from type II diabetes76. Hence, the foods identified through this review could be promising natural candidates for the management of various other disorders.
The inhibitory activity has been attributed to several bioactive phytochemicals present in these foods. These compounds could be changed chemically and may reduce during food processing. However, few interesting studies show that the addition of phytochemicals-rich plants, herbs, and protein hydrolysates to processed food products resulted in moderate to good enzyme inhibitory activity even after simulated intestinal digestion. But the organoleptic acceptability of a few of these products appears to be a challenge.
There appears to be an urgent need as well as wide scope for the development of food products enriched with antidiabetic extracts/compounds to aid in the dietary management of this chronic disease. These will have to be further evaluated in in vivo models, and their inhibitory activity will be confirmed in human clinical trials. In line with the ancient dictum “Food is medicine”, the nutrition, food, and metabolism nexus is gaining prominence and being scientifically revisited for lifestyle metabolic disorders such as diabetes. At this juncture, this review would serve as a small accelerator to further in-depth research in this area of medicinal foods for diabetes and related disorders. A healthy diet-associated metabolic signature has been reported to be inversely associated with future risk for type 2 diabetes and coronary artery disease. The association with diabetes was independent of traditional risk factors suggesting an independent beneficial effect of health-conscious dietary intake77. Hence, assessment using metabolomics biomarkers can be a potential futuristic area of research to provide stronger clinical evidence and validate the role of functional foods/therapeutic diets in diabetes.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Butler SO, Btaiche IF, Alaniz C (2005) Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy 25(7):963–976. https://doi.org/10.1592/phco.2005.25.7.963
Blakytny R, Jude E (2006) The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 23(6):594–608. https://doi.org/10.1111/j.1464-5491.2006.01773.x
Kawahito S, Kitahata H, Oshita S (2009) Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol 15(33):4137–4142. https://doi.org/10.3748/wjg.15.4137
Lankatillake C, Huynh T, Dias DA (2019) Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 15:105. https://doi.org/10.1186/s13007-019-0487-8
Zhou XJ, Ding L, Liu JX, Su LQ, Dong JJ, Liao L (2019) Efficacy and short-term side effects of sitagliptin, vildagliptin and saxagliptin in Chinese diabetes: a randomized clinical trial. Endocr Connect 8(4):318–325. https://doi.org/10.1530/EC-18-0523
Rana D, Kumar A (2019) Is there a role for sodium orthovanadate in the treatment of diabetes? Curr Diabetes Rev 15(4):284–287. https://doi.org/10.2174/1573399814666180903162556
Chaudhury A, Duvoor C, Reddy Dendi VS et al (2017) Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 8:6. https://doi.org/10.3389/fendo.2017.00006
Mirmiran P, Bahadoran Z, Azizi F (2014) Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: a review. World J Diabetes 5(3):267–281. https://doi.org/10.4239/wjd.v5.i3.267
Nongonierma AB, FitzGerald RJ (2019) Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J Food Biochem 43(1):e12451. https://doi.org/10.1111/jfbc.1245
Kazeem M, Bankole H, Ogunrinola O, Wusu A, Kappo A (2021) Functional foods with dipeptidyl peptidase-4 inhibitory potential and management of type 2 diabetes: a review. Food Front 2(2):153–162
Proença C, Ribeiro D, Freitas M, Carvalho F, Fernandes E (2022) A comprehensive review on the antidiabetic activity of flavonoids targeting PTP1B and DPP-4: a structure-activity relationship analysis. Crit Rev Food Sci Nutr 62(15):4095–4151. https://doi.org/10.1080/10408398.2021.1872483
Ezzat SM, Bishbishy MHE, Habtemariam S et al (2018) Looking at marine-derived bioactive molecules as upcoming anti-diabetic agents: a special emphasis on PTP1B inhibitors. Molecules 23(12):3334. https://doi.org/10.3390/molecules23123334
Liu R, Cheng J, Wu H (2019) Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: a review. Int J Mol Sci 20(3):463. https://doi.org/10.3390/ijms20030463
Lacroix IM, Li-Chan EC (2016) Food-derived dipeptidyl-peptidase IV inhibitors as a potential approach for glycemic regulation–current knowledge and future research considerations. Trends Food Sci Technol 54:1–6
Eyarkai Nambi V, Manickavasagan A, Shahir S (2017) Rice milling technology to produce brown rice. In: Brown rice. Springer International Publishing, pp 3–21
Haldipur AC, Srividya N (2021) A comparative evaluation of in vitro antihyperglycemic potential of Bamboo seed rice (Bambusa arundinacea) and Garudan samba (Oryza sativa): an integrated metabolomics, enzymatic and molecular docking approach. J Cereal Sci 99:103200
Haldipur AC, Srividya N (2021) Multi-mechanistic in vitro evaluation of antihyperglycemic, antioxidant and antiglycation activities of three phenolic-rich Indian red rice genotypes and in silico evaluation of their phenolic metabolites. Foods 10(11):2818. https://doi.org/10.3390/foods10112818
Samyor D, Calderwood D, Carey M, Das AB, Green BD, Deka SC (2022) Dipeptidyl peptidase-4 (DPP-4) inhibitory activity and glucagon-like peptide (GLP-1) secretion in arsenically safe pigmented red rice (Oryza sativa L.) and its product. J Food Sci Technol 59(10):4016–4024. https://doi.org/10.1007/s13197-022-05444-x
Zhang Q, Gonzalez de Mejia E, Luna-Vital D et al (2019) Relationship of phenolic composition of selected purple maize (Zea mays L.) genotypes with their anti-inflammatory, anti-adipogenic and anti-diabetic potential. Food Chem 289:739–750. https://doi.org/10.1016/j.foodchem.2019.03.116
Sánchez-Vega LP, Espinoza-Ortega A, Thomé-Ortiz H, Moctezuma-Pérez S (2021) Perception of traditional foods in societies in transition: the maize tortilla in Mexico. J Sens Stud 36(2):e12635
Acevedo-Martinez KA, Gonzalez de Mejia E (2021) Fortification of maize tortilla with an optimized chickpea hydrolysate and its effect on DPPIV inhibition capacity and physicochemical characteristics. Foods 10(8):1835. https://doi.org/10.3390/foods10081835
Das A, Naveen J, Sreerama YN, Gnanesh Kumar BS, Baskaran V (2022) Low-glycemic foods with wheat, barley and herbs (Terminalia chebula, Terminalia bellerica and Emblica officinalis) inhibit α-amylase, α-glucosidase and DPP-IV activity in high fat and low dose streptozotocin-induced diabetic rat. J Food Sci Technol 59(6):2177–2188. https://doi.org/10.1007/s13197-021-05231-0
Cian RE, Albarracín M, Garzón AG, Drago SR (2022) Precooked sorghum flour as proper vehicle of ACE‐I and DPP‐IV inhibitory sorghum peptides. Int J Food Sci Technol
Majeed M, Majeed S, Mundkur L et al (2020) Standardized Emblica officinalis fruit extract inhibited the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase-4 and displayed antioxidant potential. J Sci Food Agric 100(2):509–516. https://doi.org/10.1002/jsfa.10020
Les F, Arbonés-Mainar JM, Valero MS, López V (2018) Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J Ethnopharmacol 220:67–74. https://doi.org/10.1016/j.jep.2018.03.029
Akbarpour M, Fathollahi Shoorabeh F, Mardani M, Amini MF (2021) Effects of eight weeks of resistance training and consumption of pomegranate on GLP-1, DPP-4 and glycemic statuses in women with type 2 diabetes: a randomized controlled trial. Nutr Food Sci Res 8(1):5–10
Djakpo O, Yao W (2010) Rhus chinensis and Galla Chinensis folklore to modern evidence: review. Phytother Res 24(12):1739–1747. https://doi.org/10.1002/ptr.3215
Liu X, Fu Y, Ma Q, Yi J, Cai S (2021) Anti-diabetic effects of different phenolic-rich fractions from Rhus Chinensis Mill. fruits in vitro. eFood 2(1):37–46
Bayan L, Koulivand PH, Gorji A (2014) Garlic: a review of potential therapeutic effects. Avicenna J Phytomed 4(1):1–14
Kalhotra P, Chittepu VCSR, Osorio-Revilla G, Gallardo-Velazquez T (2020) Phytochemicals in garlic extract inhibit therapeutic enzyme DPP-4 and induce skeletal muscle cell proliferation: a possible mechanism of action to benefit the treatment of diabetes mellitus. Biomolecules 10(2):305. https://doi.org/10.3390/biom10020305
Agarwal S, Singh V, Chauhan K (2022) Antidiabetic potential of seaweed and their bioactive compounds: a review of developments in last decade. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.2024130
Unnikrishnan PS, Suthindhiran K, Jayasri MA (2015) Antidiabetic potential of marine algae by inhibiting key metabolic enzymes. Front Life Sci 8(2):148–159
Calderwood D, Rafferty E, Fitzgerald C, Stoilova V, Wylie A, Gilmore BF, Green BD (2021) Profiling the activity of edible European macroalgae towards pharmacological targets for type 2 diabetes mellitus. Appl Phycol 2(1):10–21
Zhang M, Zhu L, Wu G, Liu T, Qi X, Zhang H (2022) Food-derived dipeptidyl peptidase IV inhibitory peptides: production, identification, structure-activity relationship, and their potential role in glycemic regulation. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2022.2120454
You H, Wu T, Wang W, Li Y, Liu X, Ding L (2022) Preparation and identification of dipeptidyl peptidase IV inhibitory peptides from quinoa protein. Food Res Int 156:111176. https://doi.org/10.1016/j.foodres.2022.111176
Wang W, Liu X, Li Y et al (2022) Identification and characterization of dipeptidyl peptidase-IV inhibitory peptides from oat proteins. Foods 11(10):1406. https://doi.org/10.3390/foods11101406
Majid A, Lakshmikanth M, Lokanath NK, Poornima Priyadarshini CG (2022) Generation, characterization and molecular binding mechanism of novel dipeptidyl peptidase-4 inhibitory peptides from sorghum bicolor seed protein. Food Chem 369:130888. https://doi.org/10.1016/j.foodchem.2021.130888
Zhang M, Zhu L, Wu G, Liu T, Qi X, Zhang H (2022) Rapid screening of novel dipeptidyl peptidase-4 inhibitory peptides from Pea (Pisum sativum L.) protein using peptidomics and molecular docking. J Agric Food Chem 70(33):10221–10228. https://doi.org/10.1021/acs.jafc.2c03949
González-Montoya M, Hernández-Ledesma B, Mora-Escobedo R, Martínez-Villaluenga C (2018) Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-glucosidase enzymes. Int J Mol Sci 19(10):2883. https://doi.org/10.3390/ijms19102883
Cian RE, Nardo AE, Garzón AG, Añon MC, Drago SR (2022) Identification and in silico study of a novel dipeptidyl peptidase IV inhibitory peptide derived from green seaweed Ulva spp. hydrolysates. LWT 154:112738
Lu Y, Lu P, Wang Y, Fang X, Wu J, Wang X (2019) A novel dipeptidyl peptidase IV inhibitory tea peptide improves pancreatic β-Cell function and reduces α-Cell proliferation in streptozotocin-induced diabetic mice. Int J Mol Sci 20(2):322. https://doi.org/10.3390/ijms20020322
Jia Y, Cai S, Muhoza B, Qi B, Li Y (2021) Advance in dietary polyphenols as dipeptidyl peptidase-IV inhibitors to alleviate type 2 diabetes mellitus: aspects from structure-activity relationship and characterization methods. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1989659
Yang Y, Shi CY, Xie J, Dai JH, He SL, Tian Y (2020) Identification of potential dipeptidyl Peptidase (DPP)-IV Inhibitors among Moringa oleifera phytochemicals by virtual screening, molecular docking analysis, ADME/T-based prediction, and in vitro analyses. Molecules 25(1):189. https://doi.org/10.3390/molecules25010189
Lammi C, Bartolomei M, Bollati C et al (2021) Phenolic extracts from extra virgin olive oils inhibit dipeptidyl peptidase IV activity: in vitro, cellular, and in silico molecular modeling investigations. Antioxidants (Basel) 10(7):1133. https://doi.org/10.3390/antiox10071133
Eleftheriou P, Therianou E, Lazari D, Dirnali S, Micha A (2019) Docking assisted prediction and biological evaluation of Sideritis L. components with PTP1b inhibitory action and probable anti-diabetic properties. Curr Top Med Chem 19(5):383–392. https://doi.org/10.2174/1568026619666190219104430
Kumar M, Barbhai MD, Hasan M et al (2022) Onion (Allium cepa L.) peels: a review on bioactive compounds and biomedical activities. Biomed Pharmacother 46:112498. https://doi.org/10.1016/j.biopha.2021.112498
Yang SJ, Paudel P, Shrestha S, Seong SH, Jung HA, Choi JS (2018) In vitro protein tyrosine phosphatase 1B inhibition and antioxidant property of different onion peel cultivars: a comparative study. Food Sci Nutr 7(1):205–215. https://doi.org/10.1002/fsn3.863
Padayachee B, Baijnath H (2020) An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S Afr J Bot 129:304–316
Saidu Y, Muhammad SA, Abbas AY, Onu A, Tsado IM, Muhammad L (2016) In vitro screening for protein tyrosine phosphatase 1B and dipeptidyl peptidase IV inhibitors from selected Nigerian medicinal plants. J Intercult Ethnopharmacol 6(2):154–157. https://doi.org/10.5455/jice.20161219011346
Sierra-Campos E, Sarabia-Sanchez MJ, Sanchez-Munoz MA, Perez-Velazquez JR, Valdez-Solana MA, Avitia-Dominguez C (2017) Modes of inhibition of human protein-tyrosine phosphatase 1b and aldose reductase by Moringa oleifera Lam leaves extract. MOJ Biorg Org Chem 1(1):00005
Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ (2013) Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med 11(5):452–459. https://doi.org/10.1370/afm.1517
Lin GM, Chen YH, Yen PL, Chang ST (2015) Antihyperglycemic and antioxidant activities of twig extract from Cinnamomum osmophloeum. J Tradit Complement Med 6(3):281–288. https://doi.org/10.1016/j.jtcme.2015.08.005
Mandal MD, Mandal S (2011) Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 1(2):154–160. https://doi.org/10.1016/S2221-1691(11)60016-6
Lori G, Cecchi L, Mulinacci N et al (2019) Honey extracts inhibit PTP1B, upregulate insulin receptor expression, and enhance glucose uptake in human HepG2 cells. Biomed Pharmacother 113:108752. https://doi.org/10.1016/j.biopha.2019.108752
Meng JM, Cao SY, Wei XL et al (2019) Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: an updated review. Antioxidants (Basel) 8(6):170. https://doi.org/10.3390/antiox8060170
Ma J, Li Z, Xing S, Ho WT, Fu X, Zhao ZJ (2011) Tea contains potent inhibitors of tyrosine phosphatase PTP1B. Biochem Biophys Res Commun 407(1):98–102. https://doi.org/10.1016/j.bbrc.2011.02.116
Jain V, Viswanatha GL, Manohar D, Shivaprasad HN (2012) Isolation of antidiabetic principle from fruit rinds of Punica granatum. Evid Based Complement Altern Med. https://doi.org/10.1155/2012/147202
Yang Z, Wu F, He Y et al (2018) A novel PTP1B inhibitor extracted from Ganoderma lucidum ameliorates insulin resistance by regulating IRS1-GLUT4 cascades in the insulin signaling pathway. Food Funct 9(1):397–406. https://doi.org/10.1039/c7fo01489a
Begum N, Nasir A, Parveen Z et al (2021) Evaluation of the hypoglycemic activity of Morchella conica by targeting protein tyrosine phosphatase 1B. Front Pharmacol 12:661803. https://doi.org/10.3389/fphar.2021.661803
Han YR, Ali MY, Woo MH, Jung HA, Choi JS (2015) Anti-Diabetic and anti-inflammatory potential of the edible brown algae Hizikia Fusiformis. J Food Biochem 39(4):417–428
Guo X, Gu D, Wang M et al (2017) Characterization of active compounds from Gracilaria lemaneiformis inhibiting the protein tyrosine phosphatase 1B activity. Food Funct 8(9):3271–3275. https://doi.org/10.1039/c7fo00376e
Wang K, Bao L, Ma K, Liu N, Huang Y, Ren J, Wang W, Liu H (2015) Eight new alkaloids with PTP1B and α-glucosidase inhibitory activities from the medicinal mushroom Hericium erinaceus. Tetrahedron 71(51):9557–9563
Tao QQ, Ma K, Bao L et al (2016) Sesquiterpenoids with PTP1B inhibitory activity and cytotoxicity from the edible mushroom Pleurotus citrinopileatus. Planta Med 82(7):639–644. https://doi.org/10.1055/s-0041-111629
Tao QQ, Ma K, Bao L et al (2016) New sesquiterpenoids from the edible mushroom Pleurotus cystidiosus and their inhibitory activity against α-glucosidase and PTP1B. Fitoterapia 111:29–35. https://doi.org/10.1016/j.fitote.2016.04.007
Zhang J, Chen B, Liang J et al (2020) Lanostane triterpenoids with PTP1B Inhibitory and glucose-uptake stimulatory activities from mushroom Fomitopsis pinicola collected in North America. J Agric Food Chem 68(37):10036–10049. https://doi.org/10.1021/acs.jafc.0c04460
Moon HE, Islam N, Ahn BR et al (2011) Protein tyrosine phosphatase 1B and α-glucosidase inhibitory Phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci Biotechnol Biochem 75(8):1472–1480. https://doi.org/10.1271/bbb.110137
Jung HA, Islam M, Lee CM, Jeong HO, Chung HY, Woo HC, Choi JS (2012) Promising antidiabetic potential of fucoxanthin isolated from the edible brown algae Eisenia bicyclis and Undaria pinnatifida. Fish Sci 78(6):1321–1329
Yang P, Liu DQ, Liang TJ et al (2015) Bioactive constituents from the green alga Caulerpa racemosa. Bioorg Med Chem 23(1):38–45. https://doi.org/10.1016/j.bmc.2014.11.031
Seong SH, Nguyen DH, Wagle A, Woo MH, Jung HA, Choi JS (2019) Experimental and computational study to reveal the potential of non-polar constituents from Hizikia fusiformis as dual protein tyrosine phosphatase 1B and α-glucosidase inhibitors. Mar Drugs 17(5):302. https://doi.org/10.3390/md17050302
Choi SA, Lee JE, Kyung MJ, Youn JH, Oh JB, Whang WK (2017) Anti-diabetic functional food with wasted litchi seed and standard of quality control. Appl Biol Chem 60(2):197–204. https://doi.org/10.1007/s13765-017-0269-9
Zhang Y, Jin D, An X, Duan L, Duan Y, Lian F (2021) Lychee seed as a potential hypoglycemic agent, and exploration of its underlying mechanisms. Front Pharmacol 2:737803. https://doi.org/10.3389/fphar.2021.737803
Spencer PS, Palmer VS (2017) The enigma of litchi toxicity: an emerging health concern in southern Asia. Lancet Glob Health 5(4):e383–e384. https://doi.org/10.1016/S2214-109X(17)30046-3
Yue J, Xu J, Cao J, Zhang X, Zhao Y (2017) Cucurbitane triterpenoids from Momordica charantia L. and their inhibitory activity against α-glucosidase, α-amylase and protein tyrosine phosphatase 1B (PTP1B). J Funct Foods 37:624–631
Xiang J, Raka RN, Zhang L, Xiao J, Wu H, Ding Z (2022) Inhibition of three diabetes-related enzymes by procyanidins from Lotus (Nelumbo nucifera Gaertn.) seedpods. Plant Foods Hum Nutr 77(3):390–398. https://doi.org/10.1007/s11130-022-00987-y
Lambeir AM, Durinx C, Scharpé S, De Meester I (2003) Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40(3):209–294. https://doi.org/10.1080/713609354
Sharma B, Xie L, Yang F et al (2020) Recent advance on PTP1B inhibitors and their biomedical applications. Eur J Med Chem 199:112376. https://doi.org/10.1016/j.ejmech.2020.112376
Smith E, Ericson U, Hellstrand S et al (2022) A healthy dietary metabolic signature is associated with a lower risk for type 2 diabetes and coronary artery disease. BMC Med 20(1):122. https://doi.org/10.1186/s12916-022-02326-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or other conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srividya, N., Haldipur, A.C. & Sanjeevi, C.B. Plant Foods and Their Phytochemicals as DPP IV and PTP1B Inhibitors for Blood Glucose Regulation: A Review. J Indian Inst Sci 103, 149–165 (2023). https://doi.org/10.1007/s41745-023-00371-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-023-00371-y