Abstract
Purpose
To investigate the association between serum 25-hydroxyvitamin D [25(OH)D] concentration, a marker of vitamin D status, and risk of all-cause and cardiovascular mortality in a general older population with relatively low average serum 25(OH)D concentrations.
Methods
The study population included 552 men and 584 women aged 53–73 years who were free of CVD and cancer at baseline in 1998–2001 from the prospective, population-based Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. Deaths were ascertained by a computer linkage to the national cause of death register. All deaths that occurred from the study entry to December 31, 2008, were included. Cox proportional hazards regression models were used to analyze the association between serum 25(OH)D and risk of death.
Results
The mean serum 25(OH)D concentration was 43.7 nmol/L (SD 17.8), with a strong seasonal variation. During the average follow-up of 9.1 years, 87 participants died, 35 from cardiovascular disease (CVD). After multivariable-adjustments, the hazard ratios (HR) for all cause death in the tertiles of serum 25(OH)D were 1, 1.68 (95% CI: 0.92, 3.07) and 2.06 (95% CI: 1.12, 3.80), p for trend = 0.02.
Conclusions
Our study supports the accumulating evidence from epidemiological studies that vitamin D deficiency is associated with increased risk of death. Large-scale primary prevention trials with vitamin D supplementation are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of vitamin D deficiency is getting recognized throughout the world [1]. In addition to the classic role of vitamin D in bone metabolism, vitamin D deficiency has recently been associated with several major chronic diseases, such as cardiovascular disease (CVD), cancer, diabetes, hypertension, and infectious diseases [2–4]. Several [5–13], although not all [14, 15], epidemiological studies have also observed an inverse association with total mortality, and a recent meta-analysis of 18 randomized controlled trials (RCT) found that vitamin D supplementation was associated with decreases in total mortality rates [16].
Because of the northern location of Finland (between 60 and 70° N), skin is able to synthesize vitamin D only during the few summer months [17]. Some foods in Finland are fortified with vitamin D, and although this has raised the average serum 25-hydroxyvitamin D [25(OH)D] levels in the population, a large proportion still has serum levels <50 nmol/L [18], generally agreed to indicate deficiency [2] (to convert to nanograms per milliliter, divide by 2.496).
Previously, low serum 25(OH)D levels were associated with an increased risk of CVD death in a general Finnish population [19], but the relationship with total mortality risk has not been investigated in Finland. The aim of our study was thus to evaluate the association between serum 25(OH)D and risk of death in older men and women with relatively low serum 25(OH)D levels from the prospective, population-based Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study.
Methods
The KIHD study is an ongoing population-based study designed to investigate risk factors for CVD and other chronic diseases in middle-aged men and women from eastern Finland [20]. The study protocol was approved by the Research Ethics Committee of the University of Kuopio. All subjects gave their written informed consent.
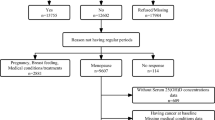
The baseline examinations of the KIHD study were conducted between 1984 and 1989 to a random sample of men living in the city of Kuopio and neighboring rural communities. A total of 2,682 men who were 42, 48, 54, or 60 years old at baseline (82.9% of those eligible) were recruited in two cohorts. The first cohort consisted of 1,166 men who were 54 years old, enrolled between 1984 and 1986, and the second cohort included 1,516 men who were 42, 48, 54, or 60 years old, enrolled between 1986 and 1989. During the years 1998–2001, all men from the second cohort were invited to the 11-year re-examinations of the study and 854 men participated. At these examinations also a random sample of 920 postmenopausal women from the same area, aged 53–73 years, entered the study. Of those eligible, 85.6% of men and 78.4% of women participated in the 11-year examinations. These 1,774 men and women were used as the study population for the current study. We excluded participants with ischemic heart disease, stroke or cancer at baseline (n = 604). We also excluded those without information on stroke history (n = 21) or without data on serum 25(OH)D (n = 13), leaving 552 men and 584 women, a total of 1,136 participants for the analyses.
Measurements
The subjects gave fasting blood samples between 8 and 10 AM at the baseline examinations. They were instructed to abstain from ingesting alcohol for 3 days and from smoking and eating for 12 h prior to giving the sample. Detailed descriptions of the determination of serum lipids and lipoproteins [21], serum fatty acids [22], blood glucose [21], plasma fibrinogen [23], assessment of medical history and medications [21], family history of diseases [21], smoking [21], alcohol consumption [21], and blood pressure [21] have been published. Serum C-reactive protein was measured with an immunometric assay (Immulite High Sensitivity CRP Assay, DPC, Los Angeles, CA, USA). Education was assessed in years by using self-administered questionnaire. Annual income was obtained from a self-administered questionnaire. Diabetes was defined as self-reported diabetes mellitus or fasting blood glucose of 6.7 mmol/L or more. Physical activity was assessed using the KIHD 12-Month Leisure-Time Physical Activity Questionnaire, and this detailed quantitative questionnaire covers the type, frequency, duration, and intensity of the activity [24]. Body mass index (BMI) was computed as the ratio of weight in kilograms to the square of height in meters, both measured during the study visit. Dietary intake of foods and nutrients was assessed at the time of blood sampling using 4-day food recording [25]. Mercury in hair was determined by flow injection analysis-cold vapor atomic absorption spectrometry and amalgamation, as described [26].
Determination of serum 25(OH)D
Blood samples for 25(OH)D analysis were drawn into serum gel tubes at normal laboratory conditions. After 30 min time for clotting, serum was separated and stored at –70 °C for 9–11 years prior to 25(OH)D measurement. Serum 25(OH)D concentration was determined with an HPLC (Shimadzu, Kyoto, Japan) using diode array detector (Beckman, USA) [27]. Isocratic elution was carried out with 77% methanol (v/v) and chromatographic separation with a Discovery HS F5 column (4 × 250 mm; 5 μm particles, Supelco, Sigma–Aldrich, USA). Total run time was 35 min and flow rate 0.8 mL/min. Mobile phase was recycled about a week (2.5 L for approximately 200 sample run), which decreased the volume of solvent waste by 50%. Injection volume was 50 μL, and samples were stored at +4 °C in the autosampler.
Sample pretreatment was modified from the method published by Turpeinen and co-workers [28]. Briefly, to 1.0 mL of serum was added 700 μL of methanol-isopropanol (80:20 v/v) and sample was mixed 30 s with Vortex mixer. 25-Hydroxyvitamin D was extracted 3 times with 3 mL of hexane by shaking 1 min. Phases were separated by centrifugation, and hexane phase was transferred into another tube. Combined extracts were evaporated under N2, and dry sample was dissolved in 100 μL of 80% methanol. After an isocratic separation, both 25-hydroxyvitamin D3 and D2 were possible to quantify. Concentrations of 25-hydroxyvitamin D2 were mainly below the quantification limit or that metabolite was not detected at all. Therefore, results for 25-hydroxyvitamin D3 are reported.
Intra and inter assay variation was monitored by analyzing in each assay duplicates of 2 control samples, a self-made serum pool 1 or 2, and a vitamin D control serum (Chromsystems GmbH, Germany). Inter assay variation for serum pool 1 (53 nmol/L) was 15% and for vitamin D control serum (68 nmol/L) 16%. Variation for serum pool 2 (99 nmol/L) was 8.7%.
Ascertainment of follow-up events
Deaths were ascertained by a computer linkage to the national cause of death register using the Finnish personal identification code (social security number) given to all residents of Finland since 1967. There were no losses to follow-up. All deaths were coded according to the Tenth International Classification of Disease (ICD) codes. CVD deaths were coded according to Tenth ICD code numbers I00 to I99. All deaths that occurred from the study entry to December 31, 2008, were included. Of the 87 deaths, 35 were CVD deaths, 28 were cancer deaths, and 24 were other deaths.
Statistical analysis
Tests of linear trend were conducted by assigning the median values for each category of exposure variable and treating those as a single continuous variable. Linear (for continuous variables) or logistic (for binary variables) regression was used to evaluate the trend across the tertiles of serum 25(OH)D in both cross-sectional and longitudinal analyses. In the longitudinal analyses, Cox proportional hazards regression models were used to estimate hazard ratios (HR) in tertiles of serum 25(OH)D concentration; the highest tertile was used as the reference. Cox model assumptions were validated by looking at the distribution of Schoenfeld residuals and Martingale residuals of the associations. Absolute risk increase (ARI) was calculated by multiplying the absolute risk (AR) in the reference group by the multivariable-adjusted hazard ratio increase in the comparison group. The multivariable-adjusted models included age, examination year (a single dummy variable), examination month (a single dummy variable), gender, diabetes, treated hypertension, body mass index, smoking, education years, and medication for hyperlipidemia. Further adjustments for leisure-time physical activity, serum C-reactive protein, plasma fibrinogen, blood glucose, history of kidney stones, family history of heart disease or cancer, common carotid artery intima-media thickness, income, serum LDL or HDL cholesterol or triglycerides, serum long-chain n-3 polyunsaturated fatty acids, hair mercury, systolic or diastolic blood pressure, use of multivitamin or calcium supplements, thiazide-type diuretic use, or intakes of alcohol, energy, fruits, berries, vegetables, milk and milk products, meat and meat products, fiber, or vitamin A did not change the associations (hazard ratio change <5%). Cohort mean was used to replace missing values in covariates. Statistical significance of the interactions on a multiplicative scale was assessed by likelihood ratio tests using a cross-product term. All p-values were 2-tailed (α = 0.05). Data were analyzed using SPSS 14.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
The mean age of the subjects was 61.8 years (SD 6.3, range 53.4–72.7 years). The mean serum 25(OH)D concentration was 43.7 nmol/L (SD 17.8, range 8.9–112.8 nmol/L) in the whole population, 42.4 nmol/L (SD 18.0) in men and 45.0 nmol/L (SD 17.6) in women. Serum 25(OH)D concentrations <50 nmol/L were observed in 64.8% of the participants, 15.1% had concentrations <25 nmol/L, and 4.8% (n = 56) had concentrations ≥75 nmol/L. There were significant seasonal variations in the serum 25(OH)D concentrations (Fig. 1). The lowest average concentrations were found among those participants that had given the blood sample in March (mean 32.2 nmol/L, SD 14.7) and the highest among those who had given the blood sample in July (mean 65.0 nmol/L, SD 13.2) (p for difference: <0.001). The concentrations also decreased rapidly after the summer months. The mean serum 25(OH)D concentration was 39.5 nmol/L (SD 17.0) among those who died during the follow-up and 44.1 nmol/L (SD 17.9) among those who survived (P for difference: 0.02).
Those with a higher serum 25(OH)D concentration were more likely to be female, have lower body mass index, lower systolic and diastolic blood pressure, lower serum triglycerides, lower serum C-reactive protein, and higher dietary vitamin D intake, higher serum HDL cholesterol, higher serum long-chain n-3 PUFA concentration, and higher leisure-time physical activity (Table 1). They were also less likely to smoke tobacco and have a treated hypertension and more likely to have a family history of heart disease and use hyperlipidemia medication and multivitamin supplements.
In the model adjusted for all variables in the Table 1, men, current smokers, and those with low leisure-time physical activity had increased odds of being in the lowest serum 25(OH)D tertile (Table 2). Those with high serum long-chain n-3 polyunsaturated fatty acid concentrations and those who had given the blood sample in summer or fall had lower odds of being in the lowest tertile.
During the average follow-up time of 9.1 years, 87 participants died. After adjustment for age, gender, examination year, and examination month, those in the lowest serum 25(OH)D tertile (<34.1 nmol/L) had 123% [95% confidence interval (CI): 22, 307%] increased risk of death, compared to those in the highest tertile (>50.8 nmol/L) (p for trend across tertiles: 0.01, AR in the reference group: 4.5%, ARI in the lowest tertile: 5.5%) (Model 1 in Table 3). Further multivariable adjustments for diabetes, treated hypertension, body mass index, smoking, education years, and medication for hyperlipidemia slightly attenuated the association; the risk of death was increased by 106% (95% CI: 12, 280%, p for trend: 0.02, ARI: 4.8%) in the lowest vs. the highest tertile (Model 2 in Table 3; Fig. 2).
Survival curves for death among 1,136 older men and women in tertiles of serum 25(OH)D. Model adjusted for age, examination year, gender, diabetes, treated hypertension, body mass index, smoking, education years, and medication for hyperlipidemia. (1) Lowest tertile, < 34.1 nmol/L; (2) middle tertile, 34.1–50.8 nmol/L; (3) highest tertile, nmol/L. To convert the values to nanograms per milliliter, divide by 2.496
We also assessed the associations with the risk of CVD mortality, the most common cause of death in this study population. The numbers of CVD events from the highest to the lowest tertile of serum 25(OH)D were 9 (25.7% of the events), 5 (14.3%), and 21 (60.0%), and the multivariable-adjusted hazard ratios (95% CI) in the tertiles were 1, 0.44 (0.14, 1.35) and 1.79 (0.75, 4.28) (p for trend: 0.09). Because of the low number of events, we also compared the lowest serum 25(OH)D tertile to the other two tertiles; the multivariable-adjusted hazard ratio was 2.70 (95% CI: 1.31, 5.56).
In the case of all-cause mortality risk, no significant effect modification was observed with gender, age, diabetes, treated hypertension, leisure-time physical activity, body mass index, or smoking (p for interactions: >0.10). We had too few cases to assess effect modification with CVD mortality.
Conclusion
The results of this study showed that low concentration of serum 25(OH)D, a marker of vitamin D status of the body, was associated with increased risk of death from all causes and from cardiovascular causes in older men and women without CVD or cancer at baseline.
The results support the accumulating evidence for the role of vitamin D deficiency as a risk factor for death from major chronic diseases. Earlier ecologic studies have suggested that vitamin D could explain differences in mortality rates from ischemic heart disease. Higher mortality rates were observed with, for example, increased distance from the equator and with less amount of sunlight [29, 30]. Similarly, ecologic studies have shown higher cancer mortality in regions with less exposure to UVB radiation from the sun [31–33]. In epidemiological studies, low serum 25(OH)D concentration has been associated with increased risk of death in hemodialysis patients [5] and in those referred for angiography [6], and also in general populations [7–12]. In a Norwegian study, low serum 25(OH)D was associated with increased risk of death only in non-smokers, but not in smokers [13]. We did not find such difference in our study population. In contrast, no association was found among patients of whom 80% had an end-stage heart failure [15] or among patients with a stable CHD [34]. Two studies among community-dwelling participants did not find an association, either [14, 35]. In the Dutch study, low serum 25(OH)D concentration was associated with an increased risk of death after multivariable adjustments for potential confounders, but further adjustments for frailty indicators (mobility performance, low serum albumin concentration and low serum total cholesterol concentration) attenuated the association [14]. However, as the authors state, this may have been overadjustment, because these frailty indicators may be mediators of the association between low vitamin D status and risk of death.
A recent Finnish prospective study, the Mini-Finland Health Survey, found an inverse association between serum 25(OH)D concentration and risk of CVD death [19]. Our results support these findings and also the majority of the results from other epidemiological studies regarding the association with the risk of CVD death [6, 8, 10]. An inverse association with CVD death was observed also in the Third National Health and Nutrition Examination Survey (NHANES III) after adjustment for age, gender, race and season, but further multivariable adjustments attenuated the association [7].
A meta-analysis in 2007 summarized the findings between vitamin D supplements and total mortality from 18 RCTs [16]. Most trials were designed to examine the effect of vitamin D on fracture prevention and the doses ranged from 7.5 to 50 μg/day (300–2,000 IU/day). The summary relative risk for all-cause mortality was 0.93 (95% CI: 0.87, 0.99). Two RCTs have reported on the effect of vitamin D supplementation on primary prevention of clinical CVD and neither found statistically significant reductions in the risk of CVD endpoints [36, 37], although the results from the UK trial favored protection [36]. However, the doses used in these trials, 250 μg every 4 months for 5 years in the UK trial [36] and 10 μg/day for 7 years in the Women’s Health Initiative [37], may have been too low to sufficiently raise serum 25(OH)D concentrations, which may at least partly explain the findings.
Exact mechanisms by which vitamin D sufficiency may protect against mortality are still to be elucidated. However, in addition to CVD [38], low vitamin D status has been associated with several diseases which contribute to mortality, for example, type 2 diabetes, hypertension, metabolic syndrome, infections, and various cancers [4, 39–42]. Low 25(OH)D levels have also been related to coronary calcification, increased carotid artery intima-media thickness, obesity, and higher serum triglyceride levels [43–45]. Vitamin D is also a potent suppressor of the renin-angiotensin system [46], which is important in blood pressure regulation; recent meta-analyses of RCTs have found a modest effect of vitamin D supplementation on blood pressure [47, 48]. On the other hand, vitamin D supplementation with 40,000 IU/week (1,000 μg/week) or 20,000 IU/week (500 μg/week) for 1 year did not improve glucose tolerance, blood pressure or serum lipids in overweight or obese subjects despite significant increase in serum 25(OH)D levels [49].
Our study has several strengths, but also potential limitations. Strengths of the study are the population-based recruitment, homogenous population, high participation rate, prospectively collected data, extensive examinations of potential risk factors, use of registry-based mortality data, and there was no loss to follow-up. All women were also post-menopausal.
The potential limitation of the study is the relatively low number of deaths, which reduces the power to find associations. The average serum 25(OH)D concentrations were similar to other Finnish cohorts [19, 50], but lower than in other studies from Japan, Europe or the USA [8–10]. This prevented investigation of a broader range of values. Only 56 participants (4.8%) in our study had serum 25(OH)D concentration ≥75 nmol/L, a suggested minimum level for optimal health [51], and only four participants died during the follow-up in this group (two from CVD, one from cancer and one from other causes). On the other hand, our results suggest that low serum 25(OH)D is a risk factor for mortality even in a population with relatively low range of serum 25(OH)D concentrations, although stronger associations might have been found with broader range of values. We did not have information on parathyroid hormone (PTH) levels or renal function. Elevated PTH has been shown to be an important mediator of the adverse effects of vitamin D deficiency and also be associated with increased mortality risk [52, 53]. Vitamin D deficiency is common in patients with chronic kidney disease, and it is also associated with increased mortality risk in those patients [54]. In addition to supplements and fortified foods, UVB light and fish consumption are main sources of vitamin D. Low serum 25(OH)D concentration may thus only be a marker of low physical activity outdoors or of low intake of long-chain polyunsaturated fatty acids (EPA + DHA) from fish. Both the low physical activity and low intake of long-chain polyunsaturated fatty acids from fish are associated with increased risk of CVD in this study population [24, 55] and both were also independent predictors of low serum 25(OH)D concentrations in this study, as was smoking (Table 2). However, adjustment for these factors did not appreciably change the associations. The multivitamins sold in Finland contain at most 5–10 μg of vitamin D, which may explain why low intake of multivitamins was not an independent predictor of low serum 25(OH)D. Only one participant used vitamin D supplements.
In conclusion, our study supports the accumulating evidence from epidemiological studies that vitamin D deficiency is associated with increased risk of all cause death also in a low vitamin D status country, Finland. Based on short-term studies, vitamin D supplementation is considered safe even in relatively high doses [56]. However, currently there is no data on the potential benefits (and possible risks) of long-term, high-dose vitamin D supplementation in a large general population. There is an urgent need for a large-scale RCT to test the effects of vitamin D supplementation in primary prevention of CVD and cancer. However, already the current knowledge has been considered sufficient to warrant supplementation in those with or at risk for falls and fractures, CVD, autoimmune disease and cancer [57].
References
Mithal A, Wahl DA, Bonjour JP et al (2009) Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20:1807–1820
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080S–1086S
Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF (2008) Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 52:1949–1956
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
Wolf M, Shah A, Gutierrez O et al (2007) Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72:1004–1013
Dobnig H, Pilz S, Scharnagl H et al (2008) Independent association of low serum 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 168:1340–1349
Melamed ML, Michos ED, Post W, Astor B (2008) 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168:1629–1637
Pilz S, Dobnig H, Nijpels G et al (2009) Vitamin D and mortality in older men and women. Clin Endocrinol 71:666–672
Kuroda T, Shiraki M, Tanaka S, Ohta H (2009) Contributions of 25-hydroxyvitamin D, co-morbidities and bone mass to mortality in Japanese postmenopausal women. Bone 44:168–172
Ginde AA, Scragg R, Schwartz RS, Camargo CA Jr (2009) Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc 57:1595–1603
Semba RD, Houston DK, Ferrucci L et al (2009) Low serum 25-hydroxyvitamin D concentrations are associated with greater all-cause mortality in older community-dwelling women. Nutr Res 29:525–530
Semba RD, Houston DK, Bandinelli S et al (2010) Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr 64:203–209
Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R (2010) Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromso study. Eur J Endocrinol 162:935–942
Visser M, Deeg DJ, Puts MT, Seidell JC, Lips P (2006) Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr 84:616–622
Zittermann A, Schleithoff SS, Frisch S et al (2009) Circulating calcitriol concentrations and total mortality. Clin Chem 55:1163–1170
Autier P, Gandini S (2007) Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737
Webb AR, Kline L, Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67:373–378
Lamberg-Allardt C, Viljakainen H, and a working group (2006) Follow-up study on the vitamin D status in the Finnish population 2002 and 2004. Reports of the Ministry of Social Affairs and Health. Helsinki, Finland
Kilkkinen A, Knekt P, Aro A et al (2009) Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol 170:1032–1039
Salonen JT (1988) Is there a continuing need for longitudinal epidemiologic research? The Kuopio Ischaemic Heart Disease risk factor study. Ann Clin Res 20:46–50
Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R (1992) High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 86:803–811
Nyyssönen K, Kaikkonen J, Salonen JT (1996) Characterization and determinants of an electronegatively charged low-density lipoprotein in human plasma. Scand J Clin Lab Invest 56:681–689
Salonen R, Seppänen K, Rauramaa R, Salonen JT (1988) Prevalence of carotid atherosclerosis and serum cholesterol levels in eastern Finland. Arteriosclerosis 8:788–792
Lakka TA, Venäläinen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT (1994) Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med 330:1549–1554
Voutilainen S, Rissanen TH, Virtanen J, Lakka TA, Salonen JT (2001) Low dietary folate intake is associated with an excess incidence of acute coronary events: the Kuopio Ischemic Heart Disease risk factor study. Circulation 103:2674–2680
Salonen JT, Seppanen K, Nyyssonen K et al (1995) Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 91:645–655
Karppi J, Nurmi T, Olmedilla-Alonso B, Granado-Lorencio F, Nyyssonen K (2008) Simultaneous measurement of retinol, alpha-tocopherol and six carotenoids in human plasma by using an isocratic reversed-phase HPLC method. J Chromatogr B Analyt Technol Biomed Life Sci 867:226–232
Turpeinen U, Hohenthal U, Stenman UH (2003) Determination of 25-hydroxyvitamin D in serum by HPLC and immunoassay. Clin Chem 49:1521–1524
Fleck A (1989) Latitude and ischaemic heart disease. Lancet 1:613
Grimes DS, Hindle E, Dyer T (1996) Sunlight, cholesterol and coronary heart disease. QJM 89:579–589
Grant WB (2002) An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 94:1867–1875
Grant WB (2003) Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res 164:371–377
Mizoue T (2004) Ecological study of solar radiation and cancer mortality in Japan. Health Phys 87:532–538
Grandi NC, Breitling LP, Vossen CY et al (2010) Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J 159:1044–1051
Cawthon PM, Parimi N, Barrett-Connor E, et al. (2010) Serum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab. doi: 10.1210/jc.2010-0638
Trivedi DP, Doll R, Khaw KT (2003) Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326:469
Hsia J, Heiss G, Ren H et al (2007) Calcium/vitamin D supplementation and cardiovascular events. Circulation 115:846–854
Pilz S, Tomaschitz A, Drechsler C, Dekker JM, Marz W (2010) Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res 54:1103–1113
Forman JP, Giovannucci E, Holmes MD et al (2007) Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49:1063–1069
Garland CF, Gorham ED, Mohr SB, Garland FC (2009) Vitamin D for cancer prevention: global perspective. Ann Epidemiol 19:468–483
Laaksi I, Ruohola JP, Tuohimaa P et al (2007) An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr 86:714–717
Cannell JJ, Vieth R, Umhau JC et al (2006) Epidemic influenza and vitamin D. Epidemiol Infect 134:1129–1140
Watson KE, Abrolat ML, Malone LL et al (1997) Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 96:1755–1760
Targher G, Bertolini L, Padovani R et al (2006) Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 65:593–597
Martins D, Wolf M, Pan D et al (2007) Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 167:1159–1165
Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J (2004) Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89–90:387–392
Witham MD, Nadir MA, Struthers AD (2009) Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens 27:1948–1954
Pittas AG, Chung M, Trikalinos T et al (2010) Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med 152:307–314
Jorde R, Sneve M, Torjesen P, Figenschau Y (2010) No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med 267:462–472
Tuohimaa P, Tenkanen L, Ahonen M et al (2004) Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer 108:104–108
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28
Pilz S, Tomaschitz A, Drechsler C et al (2010) Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J 31:1591–1598
Hagstrom E, Hellman P, Larsson TE et al (2009) Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119:2765–2771
Doorenbos CR, van den Born J, Navis G, de Borst MH (2009) Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol 5:691–700
Virtanen JK, Voutilainen S, Rissanen TH et al (2005) Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol 25:228–233
Hathcock JN, Shao A, Vieth R, Heaney R (2007) Risk assessment for vitamin D. Am J Clin Nutr 85:6–18
Souberbielle JC, Body JJ, Lappe JM et al (2010) Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 9:709–715
Acknowledgments
We wish to thank our staff at the Research Institute of Public Health for helping with subject recruitment and data collection. The research was supported by the Academy of Finland grants #121206 to J.K. Virtanen and #114526 to T. Nurmi. None of the authors have conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Virtanen, J.K., Nurmi, T., Voutilainen, S. et al. Association of serum 25-hydroxyvitamin D with the risk of death in a general older population in Finland. Eur J Nutr 50, 305–312 (2011). https://doi.org/10.1007/s00394-010-0138-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-010-0138-3