Abstract
Background
Low serum 25-hydroxyvitamin D [25(OH)D] levels have been associated with higher risk of many diseases that affect mortality, including cardiovascular disease (CVD) and cancer. The inverse association between serum 25(OH)D and mortality may be modified by excess circulating vitamin A, due to interactions of vitamin A at the level of the vitamin D nuclear receptor. In this prospective cohort study, we investigated whether the association of 25(OH)D with all-cause, cancer, and CVD mortality was modified by circulating vitamin A or preformed vitamin A intake from supplements.
Methods
We analyzed 15,998 adults in the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Mortality data for all-cause (n = 3890), cancer (n = 844), and CVD mortality (n = 1715) were assessed through December 2006. Serum 25(OH)D was measured using a radioimmunoassay kit, vitamin A biomarkers were measured by HPLC, and information on supplement use was obtained by self-report. Multivariable hazard ratios (HRs) and corresponding 95 % confidence intervals (CI) were estimated by proportional hazards regression.
Results
Serum 25(OH)D was significantly inversely associated with all-cause mortality (HR 0.93, 95 % CI 0.89, 0.97, per 10 ng/mL increase) and also with CVD mortality and mortality due to non-cancer/non-cardiovascular causes, but not with cancer mortality. The observed inverse associations remained statistically significant only among participants with serum retinyl esters <7.0 μg/dL. High intake (>5000 IU/day) of preformed vitamin A from supplements attenuated the inverse association of 25(OH)D with overall mortality. The observed interactions were not statistically significant.
Conclusions
25(OH)D was inversely associated with overall mortality, CVD mortality, and mortality due to non-cancer/non-CVD causes, but not with cancer mortality. A possible interaction between vitamin A exposure and 25(OH)D concentration appears to be associated with an attenuation of the inverse association between risk of death and quartile of 25(OH)D concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D is a fat-soluble steroid molecule that plays a pivotal role in the maintenance of musculoskeletal health. For several years, however, vitamin D is emerging as a critical regulator of the pathogenetic process of a number of non-skeletal diseases such as cardiovascular [1] and autoimmune disorders [2, 3], infections [4, 5], and several types of cancers [6, 7], indicating a possible pleiotropic effect across extraskeletal systems. Accordingly, it was suggested that low levels of vitamin D may increase the risk of death due to the wide-ranging anti-inflammatory and immune-modulating effects [8, 9]. A recently published meta-analysis of observational and trial data relating vitamin D to the risk of all-cause and cause-specific mortality found inverse associations of circulating 25-hydroxyvitamin D [25(OH)D] concentration with risks of death due to cardiovascular disease (CVD), cancer, and non-vascular/non-cancer causes [10]. Suggested biological functions in the suspected causal pathway include immune-modulatory properties, induction of cell differentiation, inhibition of angiogenesis and cell proliferation, stimulation of insulin production, and inhibition of rennin production [7, 11–15]. Given the high prevalence of vitamin D deficiency worldwide, this issue is becoming of paramount importance [16].
Most of the biologic activities of vitamin D are mediated by its binding to a high-affinity nuclear receptor (VDR) that acts as a ligand-activated transcription factor. A crucial step in the control of gene transcription by VDR involves heterodimerization with the retinoid X receptor (RXR), in order that high-affinity binding of the heterodimer (RXR–VDR) to specific DNA sites—the vitamin D response elements (VDREs)—can occur [17]. However, excessively high concentrations of 9-cis-retinoic acid, an active metabolite of vitamin A and the ligand of RXR, can lead to the formation of RXR–RXR homodimers instead of heterodimers with VDR. If this highly regulated heterodimerization process is interrupted, vitamin D cannot exert its important transcriptional effects in the human body [18]. Recent data from epidemiological studies suggest that the association between 25(OH)D and cancer incidence or mortality is modified by vitamin A, such that excess circulating vitamin A may attenuate a beneficial association [19–22]. Studies on the association of 25(OH)D with mortality other than cancer mortality so far have not considered a potential vitamin D–vitamin A interaction effect. We tested the hypothesis that inverse associations of serum 25(OH)D with all-cause, cancer, CVD and non-cancer/non-CVD mortality are modified by circulating levels of vitamin A in a prospective cohort of healthy US adults (NHANES III; 1988–1994).
Subjects and methods
Study population
NHANES is a data collection program designed to assess the health and nutritional status of the civilian, non-institutionalized population in the USA. The third NHANES study was conducted by the National Center for Health Statistics (NCHS) between 1988 and 1994 using a stratified, multistage probability design. In order to provide reliable estimates for specific subgroups of the US population, young children, older persons (aged 65 or older), black persons, and Mexican Americans were oversampled. NHANES III collected household interview data including demographics and data on health and nutrition for 33,994 (85.6 %) of the 39,695 invited participants. Subsequent physical and laboratory examinations in mobile examination centers (MEC) or at home visits were conducted for 30,882 (77.8 %) subjects. The NCHS institutional review board approved all procedures, and all subjects were provided a written informed consent sheet. Detailed methods for the NHANES III baseline data collection, including sampling, in-house interview, physical examination, laboratory measurements, mortality linkage, ethics approval, and informed consent have been described elsewhere [23]. All analyses in this report are based on NHANES III data extracted from the publicly available NHANES website.
Our analysis was restricted to the 20,024 adults, defined as 17 years or older in NHANES III. We excluded those who did not complete both the interview and the subsequent MEC examination including a blood draw (n = 1875), women who were pregnant at baseline (n = 280), individuals without reported serum 25(OH)D measurement (n = 1159), and participants without complete information on the study variables (n = 712), resulting in a cohort of 15,998 individuals.
Mortality follow-up
The NHANES III linked mortality file provides mortality follow-up data from the date of survey participation (1988–1994) through December 31, 2006. All participants aged 17 years or older at baseline were eligible for mortality follow-up. Vital status was assessed based primarily upon the results from a probabilistic match between NHANES III and National Death Index (NDI) death certificate records [24]. The follow-up period was calculated as the time from physical examination to either a mortal event or censoring date. Underlying cause of death was coded using the 9th revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-9) for deaths occurring between 1988 and 1998, and the 10th ICD revision (ICD-10) for deaths occurring between 1999 and 2006. All deaths before 1999 were recoded by the NCHS to ICD-10 codes for comparability. Cardiovascular disease mortality was defined as ICD-10 codes I00–I99, corresponding to the ICD-9 codes 390–434 and 436–459, and cancer mortality as ICD-10 codes C00–C34, C37–C41, C43–C49, C50–C52, C54–C65, C67–C80, C82–C85, C88, C90–C95, and C97, corresponding to the ICD-9 codes 140–239. Non-cancer/non-CVD mortality included all deaths with known underlying causes, except CVD and cancer deaths. All-cause mortality included all specified causes of death as well as cases with unknown cause. During the median 14.5 years of follow-up, there were 3890 (24 %) deaths in our analytical cohort, including 1715 CVD-related deaths and 844 cancer-related deaths.
Covariate assessment
Information on age, sex, race/ethnicity, and socioeconomic status was obtained by self-report from the household interview. Race/ethnicity was reported as non-Hispanic white, non-Hispanic black, Mexican American (defined as persons of Mexican origin living in the USA), and others (including multiracial). Age was defined as the age in years at time of recruitment. Socioeconomic status was assessed using the poverty income ratio, a calculated variable based on family income and family size, and self-reported years of schooling (less, equal, more than high school). Information on alcohol consumption was divided into three categories (none, 1–8, and 9+ times/month), and smoking history was classified according to current (1–20 cigarettes/day, >20 cigarettes/day), former, or never smokers. The level of physical activity was categorized into none, 1–3, and 4+ times of moderate physical activity per week. In women, hormone replacement therapy was also assessed. History of obstructive pulmonary disease (defined as any positive response to one of the diagnoses of asthma, emphysema, or chronic bronchitis), as well as myocardial infarction, stroke, heart failure, and cancer, was assessed through self-reporting. Intake and type (product label) of mineral and vitamin supplements were recorded from the 30-day supplement interview. As there is no evidence that pro-vitamin A properties arising from dietary intakes of carotenoids contribute to vitamin A-related toxicity [25, 26], the present study only considered intake of supplements containing preformed vitamin A in the form of retinol and its esters. Supplement use was divided into quartiles based on information on frequency and quantity (units each time) of consumption in the past month.
From physical examination data, height and weight were used to calculate the body mass index (BMI) as kg/m2. Hypertension, diabetes mellitus, and hypercholesterolemia were defined by history/physician’s diagnosis or medication use. Fasting blood samples collected during examination were centrifuged, aliquoted, and frozen to −70 °C before transport on dry ice to central laboratories for analysis. Blood collection in mobile unites was performed in two seasonal groups based generally on latitude, with southern collections undertaken during the winter months (November–March) and northern collections during the summer months (April–October). To account for variability of latitude and season, we divided our sample into two groups: winter/lower latitude and summer/higher latitude. Serum 25(OH)D was measured using a radioimmunoassay kit (Diasorin, Stillwater, MN). Serum levels of retinol and retinyl esters (that is, the sum of retinyl linoleate, retinyl oleate, retinyl palmitate, and retinyl stearate) were assayed by isocratic high-performance liquid chromatography with detection at three different wavelengths (Waters, Milford, MA).
Statistical analysis
All analyses were performed using STATA statistical software version 13 (StataCorp. 2013, College Station, Texas, USA). In order to account for the complex survey design of NHANES III, all analyses were weighted by using the “survey” command, with the “subpop” option to subset data. A two-sided P value of 0.05 was the criterion for statistical significance.
Continuous variables are expressed as mean ± standard deviation (SD); categorical variables are presented as proportions. Cox proportional hazard regression models were used to examine the association between 25(OH)D concentrations and mortality, whereby multivariable-adjusted hazard ratios (HR) and 95 % confidence intervals (CI) were estimated for total and cause-specific mortality. Serum 25(OH)D concentration was modeled continuously (per 10 ng/mL) and in quartiles based on the unweighted distribution in the cohort. For all participants, time at entry was the date of physical examination. Time at exit was either date of death or date of censoring, whichever came first. Covariates included in the multivariable models were selected a priori. The primary analysis focused on the association between baseline serum 25(OH)D concentration and all-cause, cancer, CVD, and non-cancer/non-CVD mortality during follow-up. Three different multivariable models were used, specified a priori, to test for the independent effect of serum 25(OH)D on mortality. The first model adjusted for age, sex, race/ethnicity, and season. Building on the first model, the second model further adjusted for lifestyle and socioeconomic factors, including BMI, smoking status, alcohol consumption, physical activity, and hormone replacement therapy in women as well as poverty income ratio and education level. The third model added potential mediators in the suspected causal pathway to help explain the observed associations. This model also included hypertension, diabetes mellitus, hypercholesterolemia, obstructive pulmonary disease, and history of myocardial infarction, stroke, and cancer. To examine a potential modifying effect of vitamin A on main effects of serum 25(OH)D, we ran stratified analyses by excess circulating vitamin A and preformed vitamin A supplement use. Currently, there is no well-accepted, noninvasive physiological measure of vitamin A excess. Serum retinol is tightly regulated by liver storage and by the production of retinol-binding protein and is likely a better biomarker of vitamin A deficiency rather than excess [27]. While serum retinol may only slightly be elevated when vitamin A intake is excessive, serum retinyl esters are markedly increased [28]. Therefore, fasting retinyl ester levels have been used as a marker of possible vitamin A toxicity or hypervitaminosis A [29, 30]. Under normal conditions, retinyl esters account for <5 % of total serum vitamin A [30] and concentrations ≥7.0 μg/dL have been interpreted as marker of potential toxicity [27]. In our study, excess circulating vitamin A was therefore defined as serum retinyl esters ≥7 μg/dL. Data were also stratified by quartiles of serum retinol and preformed vitamin A intake from supplements. Since a definitive cutoff value to indicate excess vitamin A is lacking for both markers, the 75th percentile was used as threshold in the analyses. Effect modification was assessed on a multiplicative scale by using the Wald test to compare adjusted Cox models with and without an interaction term of serum 25(OH)D and the vitamin A stratification variable. To at least partially preclude reverse causation, we conducted additional analyses by excluding cases during the first 5 years of follow-up. Restricted cubic spline models were used to provide evidence of nonlinear relations between 25(OH)D and mortality.
Results
Table 1 summarizes demographic characteristics and confounding variables of the weighted NHANES III sample according to serum 25(OH)D quartiles. Median 25(OH)D concentration was 28.3 ng/mL (weighted sample); mean age at baseline was 43.4 years. Individuals with low 25(OH)D concentrations were older, less physically active, and more often female. Blacks and Mexican Americans were overrepresented in the lowest quartile of 25(OH)D concentration. Higher 25(OH)D concentration was associated with a higher educational level, higher income, and lower prevalence of diabetes, hypertension, COPD, as well as history of stroke and myocardial infarction.
During the 14.5 years of follow-up, 3890 (24.3 %) of the 15,998 study participants died. Of these, 844 (21.7 %) deaths were related to cancer and 1715 (44.1 %) to CVD. A significant association of serum 25(OH)D with all-cause mortality was observed when adjusting for age, sex, race/ethnicity, and season (HR 0.89; 95 % CI 0.85, 0.94 per 10 ng/mL increase in 25(OH)D; Table 2). The inverse association remained statistically significant even after controlling for potential confounders and intermediate variables [HR 0.93; 95 % CI 0.89, 0.97 per 10 ng/mL increase in 25(OH)D]. A similar pattern was observed in the categorical model, where HRs tended to decrease with increasing quartiles of 25(OH)D. Comparing individuals with a high 25(OH)D concentration of ≥40 ng/mL (100 nmol/L) to those with concentrations <16 ng/mL (25 nmol/L), we observed a HR of 0.70 (95 % CI 0.50, 0.83). Excluding the first 5 years of follow-up did not materially affect the observed associations (data not shown). Results of the restricted cubic spline models did not indicate a nonlinear association between serum 25(OH)D and mortality (P value >0.05; results not shown).
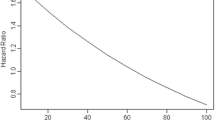
The inverse association between 25(OH)D and all-cause mortality remained statistically significant among participants with serum retinyl ester concentrations <7.0 μg/dL [HR 0.92; 95 % CI 0.88, 0.97 per 10 ng/mL increase in 25(OH)D], but not among those with serum retinyl esters ≥7.0 μg/dL (HR 0.97, 95 % CI 0.85, 1.09; P interaction = 0.59). Similarly, in the categorical model, a statistically significant inverse association of serum 25(OH)D with all-cause mortality was observed for serum retinyl esters <7.0 μg/dL (Fig. 1). When data were stratified by preformed vitamin A supplement use, effect estimates were generally lower among participants taking supplements. However, looking at vitamin A supplement use in more detail, the potential protective effect of 25(OH)D on overall mortality held for individuals taking supplements containing preformed vitamin A at amounts ≤5000 IU [HR 0.87, 95 % CI 0.77, 0.97 per 10 ng/mL increase in 25(OH)D], but not for those taking high amounts (>5000 IU) of preformed vitamin A from supplements (HR 1.01, 95 % CI 0.72, 1.43; P interaction = 0.53). However, these results should be interpreted with caution due to the low case numbers in the subgroups. When stratifying by serum retinol, HR tended to decrease with increasing quartiles of 25(OH)D concentrations in both strata, with the risk reduction being more pronounced at high concentrations (>69 μg/dL) of serum retinol (HR 0.64, 95 % CI 0.48, 0.86 for serum retinol >69 μg/dL and HR 0.82, 95 % CI 0.71, 0.96 for serum retinol ≤69 μg/dL; top vs. bottom quartile; P interaction = 0.16). Generally, the strength of associations was similar when analyses were stratified by sex (Supplementary Table 1).
Association between circulating 25(OH)D concentration and all-cause mortality by vitamin A markers in NHANES III (1988–2006); results are reported as hazard ratios (HR) with 95 % confidence intervals (CI) per 10 ng/mL increase in 25(OH)D concentration (horizontal bar), adjusted for all variables in model 3 [Model 3 was adjusted for age, sex, race/ethnicity, season, lifestyle and socioeconomic factors (poverty income ratio, body mass index, physical activity, smoking status, alcohol consumption, education and hormone replacement therapy) and for potential intermediates (hypertension, diabetes mellitus, hypercholesterolemia, history of myocardial infarction, history of stroke, and history of cancer)]. All estimates were weighted to account for the complex survey design of NHANES III
Overall, there was no significant association between serum 25(OH)D and total cancer mortality (Supplementary Table 2). Similar to associations seen with all-cause mortality, lower 25(OH)D was associated with increased CVD mortality (HR 0.79, 95 % CI 0.67, 0.94; top vs. bottom quartile, model 3; Supplementary Table 2). At serum retinyl ester concentrations <7 μg/dL and serum retinol concentrations >69 μg/dL, 25(OH)D maintained its inverse association with CVD mortality (HR 0.76, 95 % CI 0.61, 0.95 and HR 0.62, 95 % CI 0.44, 0.89, respectively; top vs. bottom quartile). Mortality due to non-cancer/non-CVD causes was significantly inversely associated with 25(OH)D in the multivariable-adjusted model [HR 0.89, 95 % CI 0.82, 0.97 per 10 ng/mL increase in 25(OH)D]. Lower serum retinyl esters as well as preformed vitamin A supplement use were associated with lower mortality [HR 0.87, 95 % CI 0.78, 0.96 and HR 0.79, 95 % CI 0.65, 0.95, respectively, per 10 ng/mL increase in 25(OH)D]. Whether differences in preformed vitamin A intake from supplements modify risk estimates for cause-specific mortality could not be assessed due to the low number of cases in the supplementation group.
Discussion
In this nationally representative sample of US adults, we observed an inverse association of serum 25(OH)D with all-cause mortality, CVD mortality, and mortality due to non-cancer/non-CVD causes. We found these associations to be modified by circulating concentrations of serum retinyl ester, a commonly used biomarker of possible vitamin A excess, in a way that the beneficial associations were attenuated among those with excessively high concentrations (≥7 μg/dL). In addition, high preformed vitamin A intake (>5000 IU) from supplements was found to diminish the inverse association of 25(OH)D with overall mortality. However, there was limited statistical evidence of an interaction between 25(OH)D and vitamin A exposure.
Our results on the relation of serum 25(OH)D and mortality corroborate earlier findings. A recently published patient-level meta-analysis of eight observational studies from Europe and the USA, including the NHANES III survey, has shown that low 25(OH)D is associated with an increase in all-cause and cardiovascular mortality, with a curvilinear inverse association between 25(OH)D concentration and mortality outcomes [31]. These results are similar to previous study-level meta-analyses of observational studies, where vitamin D deficiency has been suggested as an independent risk factor for all-cause and CVD mortality [8, 32–35]. While the association between serum 25(OH)D and CVD mortality appears to be a strong inverse association, findings regarding cancer mortality are heterogeneous. Most prospective studies and meta-analyses have focused on colorectal, breast, and prostate cancer and often yielded different results depending on tumor type [36–43]. Schottker et al. [31] observed an association only among subjects with a history of cancer, and two other recently published meta-analyses showed weak, albeit statistically significant, elevated pooled risk ratios [10, 44]. Studies using data of NHANES III have reported an inverse association between circulating concentrations of 25(OH)D and overall [45] as well as cardiovascular disease mortality [46], but the associations with cancer mortality were not entirely clear [47]. These studies differed from our study in that the length of follow-up was shorter (7.3 [46] and 8.7 years [45]), and one study [46] only included participants aged 65 or older.
We further observed that the inverse associations between 25(OH)D and mortality were diminished among those with excess circulating retinyl esters. The fact that this pattern did not hold for serum retinol strata does not conflict with other reports that suggest that retinol concentrations are under tight homeostatic control and may remain constant or decline to compensate for higher retinyl ester concentrations [29, 48]. The large differences in risk estimates between strata of vitamin A variables did mostly not result in a significant vitamin D–vitamin A interaction, which may be attributed to small case numbers in the respective subgroups. Previous evidence as to whether vitamin A modifies vitamin D’s effect on mortality is limited. To our knowledge, this is the first study to examine a possible vitamin D–vitamin A interaction not only in association with cancer mortality, but also with overall, CVD, and non-cancer/non-CVD mortality. Among the few epidemiological studies investigating the influence of vitamin A on vitamin D-related cancer risks, high intakes of retinol were found to mask a beneficial association of vitamin D with colorectal and pancreatic cancer [21, 22]. Several studies on lung cancer have recently been published, but results are conflicting. Cheng and Neuhouser [19] reported that the inverse association of 25(OH)D with lung cancer mortality seen in non-smokers was more likely to be observed among those with no sign of excess vitamin A exposure in NHANES III. Similarly, a recent study in postmenopausal women reported suggestive evidence that lower vitamin A intake may be important for a beneficial association of vitamin D supplementation with lung cancer risk [20]. However, statistical evidence to support effect modification by vitamin A was limited in both studies. In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC), serum retinol was not found to modify the effect of vitamin D on lung cancer risk [49], and results of the Carotene and Retinol Efficacy Trial (CARET) showed that high-dose vitamin A may be important for the protective effect of vitamin D against lung cancer among smokers [50]. Caution should be exercised when comparing our results to these findings. In most studies, the primary outcome was cancer incidence rather than mortality. In addition, no study but one [19] used retinyl esters as biomarker of vitamin A excess and studies on vitamin A intake usually included pro-vitamin A carotenoids.
Overall, serum 25(OH)D was similarly inversely associated with all-cause and cause-specific mortality when we stratified analyses by sex. Other studies that reported their results for all-cause mortality stratified by sex have found very similar results for both sexes [51, 52], stronger associations in men [53] or stronger associations in women [45]. One study reported a difference between sexes for cause-specific death [54]. Interestingly, we found the amount of supplemental preformed vitamin A intake to significantly modify the effect of vitamin D on all-cause mortality only in men. Low case numbers among supplement users did not allow for gender-specific analyses for cause-specific mortality.
Although we cannot exclude that vitamin D interferes with vitamin A metabolism rather than the other way around, the biological mechanism by which circulating vitamin A is thought to mask a beneficial association of 25(OH)D with mortality seems plausible and has frequently been discussed in the literature [17, 18, 55]. It involves excessively high concentrations of 9-cis-retinoic acid, an active metabolite of vitamin A, leading to intranuclear retinoid X receptor (RXR) homodimers (RXR–RXR) instead of VDR–RXR heterodimers. Serum concentrations of 9-cis-retinoic acid are directly related to dietary vitamin A intake [56], but the precise concentration of vitamin A leading to disturbance of heterodimerization remains unknown. In most developed countries including the USA, consumption of multivitamin or single supplement products commonly consisting of high-dose preformed vitamin A has increased over time and concerns of subclinical vitamin A toxicity have already been raised [57–59]. In our cohort, 21 % of study participants took supplements containing preformed vitamin A and more than 20 % had excess circulating vitamin A, defined as retinyl esters ≥7.0 μg/dL.
The strengths of our study include statistical adjustment for a wide range of factors. Furthermore, NHANES III is a large well-characterized survey, which incorporates a representative sample of the US population. A number of limitations should be considered when interpreting our results. First, serum 25(OH)D was only measured once in NHANES III. Second, serum 25(OH)D data from NHANES III have an inherent season–latitude structure that prevents assessing associations in specific subgroups. In addition, results of sub-analyses should be interpreted with caution because of low number of cases. Moreover, adequate concentrations of vitamin D may reflect a pattern of behavior to minimize threats to one’s health and thus be a proxy for a healthy lifestyle. Although we controlled for numerous confounders, potential residual and unmeasured confounding remains a distinct possibility.
In this study, inverse associations of 25(OH)D with overall, CVD, and non-cancer/non-CVD mortality were found to be diminished if circulating vitamin A was excessively high (serum retinyl esters ≥7.0 μg/dL). The beneficial association between 25(OH)D and all-cause mortality further remained statistically significant only in participants taking preformed vitamin A from supplements in amounts ≤5000 IU. If the interaction effect is real, i.e., vitamin A interferes with the action of vitamin D, our findings underscore the need to assess safety of high intakes of preformed vitamin A in order to prevent toxic levels in the body that potentially undermine a protective effect of vitamin D. Other than with preformed vitamin A intake, a diet rich in red and yellow-orange fruits and vegetables such as carrots and sweet potatoes would supply all the carotenoids the body needs to make retinol without the potential for hypervitaminosis A. Further well-designed studies to more clearly identify a potential causal relationship of vitamin D with overall and cause-specific mortality as well as a potential interaction with vitamin A are warranted.
References
Pilz S, Tomaschitz A, Marz W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB, Holick MF, Dekker JM (2011) Vitamin D, cardiovascular disease and mortality. Clin Endocrinol 75(5):575–584. doi:10.1111/J.1365-2265.2011.04147.X
Arnson Y, Amital H, Shoenfeld Y (2007) Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis 66(9):1137–1142. doi:10.1136/ard.2007.069831
Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guerin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A (2010) Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 9(11):709–715. doi:10.1016/j.autrev.2010.06.009
Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H (2010) Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 91(5):1255–1260. doi:10.3945/ajcn.2009.29094
Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML (2010) Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One 5(6):e11088. doi:10.1371/journal.pone.0011088
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ (2014) The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. doi:10.1038/nrc3691
Welsh J (2012) Cellular and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys 523(1):107–114. doi:10.1016/J.Abb.2011.10.019
Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S (2012) Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 95(1):91–100. doi:10.3945/ajcn.111.014779
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281. doi:10.1056/NEJMra070553
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS, Pan A, Johnson L, Crowe F, Hu FB, Franco OH (2014) Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 348:g1903. doi:10.1136/bmj.g1903
Prietl B, Treiber G, Pieber TR, Amrein K (2013) Vitamin D and immune function. Nutrients 5(7):2502–2521. doi:10.3390/Nu5072502
Hewison M (2012) An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 76(3):315–325. doi:10.1111/j.1365-2265.2011.04261.x
Bikle D (2009) Nonclassic actions of vitamin D. J Clin Endocrinol Metab 94(1):26–34. doi:10.1210/jc.2008-1454
Lee S, Clark SA, Gill RK, Christakos S (1994) 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology 134(4):1602–1610. doi:10.1210/endo.134.4.8137721
Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP (2002) 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J Clin Investig 110(2):229–238. doi:10.1172/JCI15219
Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J, Group IOFCoSANW (2009) Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20(11):1807–1820. doi:10.1007/s00198-009-0954-6
Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, Selznick SH, Whitfield GK (1997) The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol 154(Suppl):S57–S73
Thompson PD, Jurutka PW, Haussler CA, Whitfield GK, Haussler MR (1998) Heterodimeric DNA binding by the vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. Evidence for allosteric receptor interactions. J Biol Chem 273(14):8483–8491
Cheng TY, Neuhouser ML (2012) Serum 25-hydroxyvitamin D, vitamin A, and lung cancer mortality in the US population: a potential nutrient–nutrient interaction. Cancer Causes Control 23(9):1557–1565. doi:10.1007/s10552-012-0033-8
Cheng TY, Lacroix AZ, Beresford SA, Goodman GE, Thornquist MD, Zheng Y, Chlebowski RT, Ho GY, Neuhouser ML (2013) Vitamin D intake and lung cancer risk in the women’s health initiative. Am J Clin Nutr 98(4):1002–1011. doi:10.3945/ajcn.112.055905
Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL (2007) Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol 165(10):1178–1186. doi:10.1093/aje/kwm026
Bao Y, Ng K, Wolpin BM, Michaud DS, Giovannucci E, Fuchs CS (2010) Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer 102(9):1422–1427. doi:10.1038/sj.bjc.6605658
National Center for Health Statistics (1994) Plan and operation of the third National Health and Nutrition Examination Survey, 1988–94. vol 32. Natl Ctr for Health Statistics
Dukas L, Platz EA, Colditz GA, Willet WC, Giovannucci EL (2000) Bowel movement, use of laxatives and risk of colorectal adenomatous polyps among women (United States). Cancer Causes Control 11(10):907–914
European Commission, Scientific Committee on Food (2002) Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Preformed Vitamin A (retinol and retinyl esters)
Blomhoff R, Beckman-Sundh U, Brot C, Solvoll C, Steingrimsdóttir L, Carlsen MH (2003) Health risks related to high intake of preformed retinol (vitamin A) in the Nordic countries. Nordic Council of Ministers
Ballew C, Galuska D, Gillespie C (2001) High serum retinyl esters are not associated with reduced bone mineral density in the third national health and nutrition examination survey, 1988–1994. J Bone Miner Res 16(12):2306–2312. doi:10.1359/jbmr.2001.16.12.2306
Willett W (2013) Nutritional epidemiology, vol 40. Oxford University Press, Oxford
Krasinski SD, Russell RM, Otradovec CL, Sadowski JA, Hartz SC, Jacob RA, McGandy RB (1989) Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol-binding protein, retinyl esters, carotene, alpha-tocopherol, and cholesterol among elderly people and young adults: increased plasma retinyl esters among vitamin A-supplement users. Am J Clin Nutr 49(1):112–120
Smith FR, Goodman DS (1976) Vitamin A transport in human vitamin A toxicity. N Engl J Med 294(15):805–808. doi:10.1056/NEJM197604082941503
Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, Streppel M, Gardiner J, Ordonez-Mena JM, Perna L, Wilsgaard T, Rathmann W, Feskens E, Kampman E, Siganos G, Njolstad I, Mathiesen EB, Kubinova R, Pajak A, Topor-Madry R, Tamosiunas A, Hughes M, Kee F, Bobak M, Trichopoulou A, Boffetta P, Brenner H, Consortium on H, Ageing: Network of Cohorts in E, the United S (2014) Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 348:g3656. doi:10.1136/bmj.g3656
Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C (2014) Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 1:CD007470. doi:10.1002/14651858.CD007470.pub3
Rush L, McCartney G, Walsh D, Mackay D (2013) Vitamin D and subsequent all-age and premature mortality: a systematic review. BMC Public Health 13(1):679. doi:10.1186/1471-2458-13-679
Schottker B, Ball D, Gellert C, Brenner H (2013) Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev 12(2):708–718. doi:10.1016/j.arr.2012.02.004
Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD (2012) Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 5(6):819–829. doi:10.1161/CIRCOUTCOMES.112.967604
Pilz S, Tomaschitz A, Obermayer-Pietsch B, Dobnig H, Pieber TR (2009) Epidemiology of vitamin D insufficiency and cancer mortality. Anticancer Res 29(9):3699–3704
Bikle DD (2014) Vitamin D and cancer: the promise not yet fulfilled. Endocrine 46(1):29–38. doi:10.1007/s12020-013-0146-1
Michaelsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundstrom J, Berglund L, Arnlov J, Hellman P, Blomhoff R, Wolk A, Garmo H, Holmberg L, Melhus H (2010) Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr 92(4):841–848. doi:10.3945/ajcn.2010.29749
Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H (2010) Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat 121(2):469–477. doi:10.1007/s10549-009-0593-9
Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P (2011) Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 128(6):1414–1424. doi:10.1002/ijc.25439
Gilbert R, Martin RM, Beynon R, Harris R, Savovic J, Zuccolo L, Bekkering GE, Fraser WD, Sterne JA, Metcalfe C (2011) Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control 22(3):319–340. doi:10.1007/s10552-010-9706-3
Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H (2011) Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol 29(28):3775–3782. doi:10.1200/JCO.2011.35.7566
Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H (2011) Meta-analysis: serum vitamin D and colorectal adenoma risk. Prev Med 53(1–2):10–16. doi:10.1016/j.ypmed.2011.05.013
Yin L, Ordonez-Mena JM, Chen T, Schottker B, Arndt V, Brenner H (2013) Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med 57(6):753–764. doi:10.1016/j.ypmed.2013.08.026
Melamed ML, Michos ED, Post W, Astor B (2008) 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168(15):1629–1637. doi:10.1001/archinte.168.15.1629
Ginde AA, Scragg R, Schwartz RS, Camargo CA Jr (2009) Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc 57(9):1595–1603. doi:10.1111/j.1532-5415.2009.02359.x
Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI (2010) Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988–2006). Cancer Res 70(21):8587–8597. doi:10.1158/0008-5472.CAN-10-1420
Ragavan VV, Smith JE, Bilezikian JP (1982) Vitamin A toxicity and hypercalcemia. Am J Med Sci 283(3):161–164
Weinstein SJ, Yu K, Horst RL, Parisi D, Virtamo J, Albanes D (2011) Serum 25-hydroxyvitamin D and risk of lung cancer in male smokers: a nested case-control study. PLoS One 6(6):e20796. doi:10.1371/journal.pone.0020796
Cheng TY, Goodman GE, Thornquist MD, Barnett MJ, Beresford SA, Lacroix AZ, Zheng Y, Neuhouser ML (2014) Estimated intake of vitamin D and its interaction with vitamin A on lung cancer risk among smokers. Int J Cancer. doi:10.1002/ijc.28846
Bates CJ, Hamer M, Mishra GD (2012) A study of relationships between bone-related vitamins and minerals, related risk markers, and subsequent mortality in older British people: the National Diet and Nutrition Survey of People Aged 65 Years and Over. Osteopor Int 23(2):457–466. doi:10.1007/s00198-011-1543-z
Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS (2011) Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol 58(14):1433–1441. doi:10.1016/j.jacc.2011.03.069
Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R (2010) Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromso study. Eur J Endocrinol 162(5):935–942. doi:10.1530/EJE-09-1041
Rohrmann S, Braun J, Bopp M, Faeh D, Swiss National C (2013) Inverse association between circulating vitamin D and mortality-dependent on sex and cause of death? Nutr Metab Cardiovasc Dis 23(10):960–966. doi:10.1016/j.numecd.2013.05.005
Zou A, Elgort MG, Allegretto EA (1997) Retinoid X receptor (RXR) ligands activate the human 25-hydroxyvitamin D3-24-hydroxylase promoter via RXR heterodimer binding to two vitamin D-responsive elements and elicit additive effects with 1,25-dihydroxyvitamin D3. J Biol Chem 272(30):19027–19034
Arnhold T, Tzimas G, Wittfoht W, Plonait S, Nau H (1996) Identification of 9-cis-retinoic acid, 9,13-di-cis-retinoic acid, and 14-hydroxy-4,14-retro-retinol in human plasma after liver consumption. Life Sci 59(12):PL169–PL177
Millen AE, Dodd KW, Subar AF (2004) Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: the 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc 104(6):942–950. doi:10.1016/j.jada.2004.03.022
Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF (2011) Dietary supplement use in the United States, 2003–2006. J Nutr 141(2):261–266. doi:10.3945/jn.110.133025
Park YK, Kim I, Yetley EA (1991) Characteristics of vitamin and mineral supplement products in the United States. Am J Clin Nutr 54(4):750–759
Acknowledgments
We thank Aline Richard for her help in initial stages of statistical analysis.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmutz, E.A., Zimmermann, M.B. & Rohrmann, S. The inverse association between serum 25-hydroxyvitamin D and mortality may be modified by vitamin A status and use of vitamin A supplements. Eur J Nutr 55, 393–402 (2016). https://doi.org/10.1007/s00394-015-0860-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0860-y