Abstract
Background
Impaired renal function (IRF) is associated with increased risks of both ischemic and bleeding events. Ticagrelor has been shown to provide greater absolute reduction in ischemic risk following acute coronary syndrome (ACS) in those with versus without IRF.
Methods
A pre-specified sub-analysis of the randomized GLOBAL LEADERS trial (n = 15,991) comparing the experimental strategy of 23-month ticagrelor monotherapy (after 1-month ticagrelor and aspirin dual anti-platelet therapy [DAPT]) with 12-month DAPT followed by 12-month aspirin after percutaneous coronary intervention (PCI) in ACS and stable coronary artery disease (CAD) patients stratified according to IRF (glomerular filtration rate < 60 ml/min/1.73 m2).
Results
At 2 years, patients with IRF (n = 2171) had a higher rate of the primary endpoint (all-cause mortality or centrally adjudicated, new Q-wave myocardial infarction [MI](hazard ratio [HR] 1.64, 95% confidence interval [CI] 1.35–1.98, padj = 0.001), all-cause death, site-reported MI, all revascularization and BARC 3 or 5 type bleeding, compared with patients without IRF. Among patients with IRF, there were similar rates of the primary endpoint (HR 0.82, 95% CI 0.61–1.11, p = 0.192, pint = 0.680) and BARC 3 or 5 type bleeding (HR 1.10, 95% CI 0.71–1.71, p = 0.656, pint = 0.506) in the experimental versus the reference group. No significant interactions were seen between IRF and treatment effect for any of the secondary outcome variables. Among ACS patients with IRF, there were no between-group differences in the rates of the primary endpoint or BARC 3 or 5 type bleeding; however, the rates of the patient-oriented composite endpoint (POCE) of all-cause death, any stroke, MI, or revascularization (pint = 0.028) and net adverse clinical events (POCE and BARC 3 or 5 type bleeding) (pint = 0.045), were lower in the experimental versus the reference group. No treatment effects were found in stable CAD patients categorized according to presence of IRF.
Conclusions
IRF negatively impacted long-term prognosis after PCI. There were no differential treatment effects found with regard to all-cause death or new Q-wave MI after PCI in patients with IRF treated with ticagrelor monotherapy.
Clinical trial registration
The trial has been registered with ClinicalTrials.gov, number NCT01813435.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Impaired renal function (IRF) is an independent predictor of ischemic and bleeding events [1,2,3,4,5,6]. Despite registries suggesting a progressive increase in the number of patients with IRF, these patients still tend to be under represented or excluded from clinical trials, and undertreated in real life [1, 2]. Antiplatelet treatment in patients with IRF is, therefore, complex because IRF can effect thrombocyte function and coagulation [7], and this is further complicated by the change in drug pharmacokinetics in chronic kidney disease [2, 7, 8]. In the PLATO study, the combination of ticagrelor with aspirin substantially reduced cardiovascular death, myocardial infarction (MI), or stroke compared with clopidogrel plus aspirin in patients with acute coronary syndrome (ACS), with a consistent relative risk reduction in patients with and without IRF and a greater absolute risk reduction for patients with IRF [9]. This benefit was not associated with a significant increase in major bleeding; however, numerically more non–procedure–related bleeding events were observed among patients with IRF [9].
In an attempt to mitigate bleeding risk whilst preserving ischemic efficacy, aspirin-free antiplatelet regimens utilizing more potent P2Y12 antagonists have been advocated [10]. The first and largest trial to date evaluating this concept—GLOBAL LEADERS—failed to show superiority of ticagrelor monotherapy starting one month post percutaneous coronary intervention (PCI), compared to standard dual antiplatelet therapy (DAPT) followed by aspirin monotherapy in an all comer patient population [11]. Nevertheless, understanding the impact of IRF on long-term outcomes after PCI in this large all-comer contemporary trial is of clinical interest.
Given this background, we report the results of this pre-specified analysis according to an estimated glomerular filtration rate (eGFR) of 60 ml/min/1.73 m2 and the five major categories of renal impairment, defined by the Kidney Disease: Improving Global Outcomes (KDIGO) classification [12]. In addition, as the randomization in this trial was stratified according to clinical presentation (ACS vs. stable coronary artery disease [CAD]), we assessed the experimental treatment effects in relation to baseline renal function specifically in ACS and stable CAD patients.
Methods and patients
This is a pre-specified subgroup analysis of the GLOBAL LEADERS trial (NCT01813435). GLOBAL LEADERS was an investigator-initiated, randomized, multi-center, open-label trial designed to evaluate two strategies of antiplatelet therapy after PCI using uniformly bivalirudin and biolimus A9-eluting stents (Biomatrix) in an all-comers population [13, 14]. In the experimental treatment strategy, patients received aspirin 75–100 mg once daily in combination with ticagrelor 90 mg twice daily for one month; followed by ticagrelor 90 mg twice daily alone for 23 months (irrespective of the clinical presentation). In the reference treatment strategy, patients received aspirin 75–100 mg daily in combination with either clopidogrel 75 mg once daily in patients with stable CAD or ticagrelor 90 mg twice daily in patients with ACS for 1 year; followed by aspirin 75–100 mg once daily alone for the following 12 months (from 12 to 24 months after PCI).
The trial was approved by the institutional review board at each participating institution. All patients provided informed consent. The study complied with the Declaration of Helsinki and Good Clinical Practices. An independent data and safety monitoring committee oversaw the safety of all patients.
In the present analyses, patients were stratified according to an eGFR cut-off of 60 ml/min/1.73 m2, calculated according to the MDRD equation [15], as pre-specified in the trial protocol. In addition, exploratory analyses were performed stratifying the overall population, and specifically the ACS and stable CAD subgroups, according to the KDIGO classification with chronic kidney disease stage I, II, III, IV, and V defined as respective eGFRs of ≥ 90, 60–89, 44–59,15–29 and < 15 ml/min/1.73 m2 [12].
Patients were followed up at 30 days and 3, 6, 12, 18, and 24 months after the index procedure. Electrocardiogram (ECG) was obtained at discharge, 3-month and 2-year follow-up and during the follow-up if there was suspected ischemic event or repeat revascularization. All ECGs were analyzed at the core laboratory (Cardialysis, Rotterdam, Netherlands) by technicians who were blinded to the treatment assignments.
Study endpoints
The primary endpoint of the present study was the composite of all-cause mortality and new Q-wave myocardial infarction (MI) within 2 years after the index procedure. The survival status of the patients lost to follow-up or those who withdrew their consent was obtained via public civil registries in all but eight patients; complete vital status at 2 years was available in 99.95% [11]. Minnesota classification was used to define the new Q-wave MI which was centrally adjudicated by an independent ECG core lab [16]. The key secondary safety endpoint was investigator-reported bleeding academic research consortium (BARC) type 3 or 5 [17]. Further secondary endpoints included the following: individual components of the primary endpoint (all-cause death, new Q-wave MI), individual components of key secondary safety endpoint (BARC defined bleeding type 3 and type 5) any stroke, site-reported MI, any revascularization, target vessel revascularization (TVR), definite stent thrombosis (ST) and the composite of the definite or probable ST, defined according to the Academic Research Consortium criteria [18].
Finally, the patient-oriented composite endpoint (POCE)—advocated by academic research consortium (ARC)-2, and net adverse clinical events (NACE) were analyzed up to 2 years [17, 19, 20]. The POCE was defined as the composite of all-cause death, any stroke, site-reported MI (including periprocedural or spontaneous with ST elevation MI [STEMI] or non-ST-segment elevation MI [NSTEMI]) and any revascularization (re-PCI or coronary artery bypass graft surgery [CABG] in the target or non-target vessel) [19], whereas NACE combined POCE and BARC 3 or 5 type bleeding [21, 22].
The trial was monitored for event under-reporting and event definition consistency. There were seven on-site monitoring visits performed at individual sites, with 20% of reported events checked against source documents. There was no independent adjudication of clinical events [11, 13].
Statistical analysis
All the analyses were performed on the intention-to-treat population. Continuous variables are expressed as mean ± standard deviation and were compared using independent t test. Categorical variables are presented as counts and percentage and were compared using Chi square test. Kaplan–Meier method was used to estimate the cumulative rates of events and Log-rank test was performed to examine the differences between groups. The effect of IRF on the outcomes was assessed in the univariable and multivariable Cox proportional hazards model. The covariates in the multivariable model included age, gender, diabetes, presentation of ACS, diabetes mellitus, hypertension, hypercholesterolemia, history of stroke, MI, PCI, peripheral vascular disease, COPD and previous major bleeding and current smoking. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated from the model and interaction test was performed to evaluate the differences in the treatment effect of antiplatelet strategy in IRF and non-IRF patients. A schematic summary of the performed analyses in the overall cohort and specifically among stable CAD and ACS patients is presented in Table 1. No procedures were prespecified for multiple testing for subgroup analyses of the trial and, therefore, all presented findings should be considered as exploratory. Analyses were performed in SPSS 25. A two-sided p value less than 0.05 was considered as statistical significance.
Results
Study population
The GLOBAL LEADERS trial recruited and randomly assigned 15,991 participants; as 23 patients subsequently withdrew consent and requested deletion of their data from the database, a total of 15,968 patients remained in the study [11]; of these, 15,883 patients (99.5%) had a baseline serum creatinine level available.
There were 2171 patients with IRF identified using MDRD-derived eGFR threshold of 60 ml/min/1.73 m2 in this study (Suppl. Figure 1). Patients with IRF were older, were more often female, diabetic, hypertensive or hypercholesterolemic, more often had a history of prior stroke, prior PCI or CABG, prior MI, COPD, or peripheral vascular disease or a history of previous major bleeding and were less frequently smoking. Patients with IRF presented more often with stable CAD (Table 2). Patients with IRF had more often left main coronary artery treated and had a larger number of stents implanted. Direct stenting and aspiration thrombectomy were performed less often in patients with IRF, compared with patients without IRF (Table 3).
The baseline clinical characteristics were balanced between the experimental and the reference arm for both IRF and non-IRF subgroups, except for a higher proportion of hypertensive patients in the IRF subgroup receiving the experimental strategy, and lower proportion of patients with peripheral vascular disease among non-IRF patients in the experimental arm (Suppl. Table 1).
Patients with IRF had a consistently lower treatment adherence at each follow-up visit (Suppl. Table 2). At 1 year, among IRF patients 769 out of 1013 (75.9%) versus 828 out of 969 (85.4%) adhered to the experimental and reference strategy, respectively. Among non-IRF patients, these proportions were 5372 out of 6684 (82.7%) versus 5863 out of 6527 (89.8%), respectively. At 2 years, 718 out of 999 (71.9%) versus 859 out of 949 (90.5%) patients with IRF, and 5062 out of 6445 (78.5%) versus 6092 out of 6513 (93.5%) non-IRF patients adhered to the experimental and reference strategies, respectively (Suppl. Table 3).
Clinical outcomes in relation to renal function
Patients with IRF had a significantly higher rate of the primary endpoint (HR 1.64, 95% CI 1.35–1.98, p adjusted = 0.001), all-cause death (HR 1.82, 95% CI 1.46–2.26, p adjusted = 0.001), MI (HR 1.55, 95% CI 1.22–1.96, p adjusted = 0.001), all revascularization (HR 1.19, 95% CI 1.02–1.37, p adjusted = 0.023), TVR (HR 1.22, 95% CI 1.01–1.49, p adjusted = 0.044), BARC type 3 bleeding (HR 1.45, 95% CI 1.09–1.92, p adjusted = 0.012), BARC type 3 or 5 bleeding (HR 1.40, 95% CI 1.07–1.85, p = 0.016), and BARC type 2,3,5 bleeding (HR 1.22, 95% CI 1.03–1.44, p adjusted = 0.019) (Table 4).
Clinical outcomes in relation to renal function and randomized treatment strategy
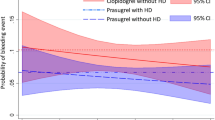
At 2 years, among patients with IRF, the primary endpoint occurred in 79 patients (7.2%) in the experimental arm and in 93 patients (8.7%) in the reference group (HR 0.82, 95% CI 0.61–1.11, p = 0.192, pint = 0.680). Among patients with IRF there were no significant between-group differences in the rates of all-cause death (HR 0.76; 95% CI 0.55–1.06; p = 0.105), POCE (HR 0.86, 95% CI 0.71–1.04, p = 0.128), NACE (HR 0.89, 95% CI 0.74–1.07, p = 0.228) and BARC type 3 or 5 type (HR 1.10, 95% CI 0.71–1.71, p = 0.656) (Table 5). No significant interactions were found between IRF and treatment effect for any of the outcome variables at 1- and 2-year follow-up (Fig. 1, Suppl. Figure 2, Suppl. Table 4). However, when treating eGFR as a continuous variable, there was a differential treatment effect with regard to rates of BARC 3 type (pint = 0.019) and BARC 3 or 5 type bleeding (pint = 0.006), being less frequently observed in the experimental than in the reference arm by decreasing eGFR (Fig. 2).
Two-year clinical outcomes in patients stratified according to presence of impaired renal function* and randomized treatment. The primary endpoint was a composite of 2-year all-cause mortality or nonfatal, centrally adjudicated, new Q-wave myocardial infarction (MI). Patient-oriented clinical outcome (POCE) included all-cause mortality or any MI, revascularization or stroke, whereas net adverse clinical events (NACE) comprised POCE, BARC 3 or 5 type bleeding. ST—stent thrombosis. * based on modification of diet in renal disease (MDRD) equation, using a prespecified cut-off of 60 ml/min
Interaction of treatment safety (BARC 3 or 5 type bleeding) with baseline eGFR (the experimental vs. the reference treatment group). The line represents the hazard ratios, the colored areas represent the 95% confidence intervals. The p value denotes the interaction term between the randomized treatment effects on BARC 3 or 5 type bleeding and the estimated glomerular filtration rate, treated as a continuous variable. Cox proportional hazard model was used. Blue line/area—the experimental treatment, Red line/area—the reference treatment
Clinical outcomes in relation to extent of renal dysfunction and randomized treatment strategy
The experimental treatment strategy was associated with a lower rates of the primary endpoint, all-cause mortality, any revascularizations, TVR, POCE and NACE versus the reference treatment, with progressively decreasing point estimates of the HR with decreasing cut-off values of eGFR from 90 to 15 ml/min; however, no significant interactions were found between KDIGO defined eGFR categories and treatment effect for any of the outcome variables (Suppl. Figure 2).
Clinical outcomes in ACS and stable CAD patients with impaired renal function
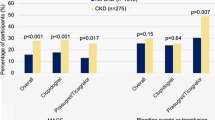
Among ACS patients, individuals with impaired renal function had similar rates of the primary endpoint (HR 0.71; 95% CI 0.47–1.06; p = 0.094, pint = 0.305) and BARC 3 or 5 type bleeding (HR 0.68; 95% CI 0.36–1.27; p = 0.227; pint = 0.841) in both treatment groups, but there was a lower rate of POCE (HR 0.71, 95% CI 0.53–0.93, p = 0.014, pint = 0.028) and NACE (0.71, 95% CI 0.54–0.92, p = 0.010, pint = 0.045) in the experimental arm (Fig. 3). No treatment effects were seen in stable CAD patients categorized according to presence of IRF (Fig. 3). The results of KDIGO-stratified analysis of clinical outcomes in the experimental versus the reference group in the overall population and specifically for stable CAD and ACS patients are presented in the Suppl. Figure 2 and Suppl. Table 5.
Impact of the randomized treatment on 2-year clinical outcomes according to prespecified eGFR cut off of 60 ml/min (according to MDRD equation) in stable CAD and ACS patients. The primary endpoint was a composite of 2-year all-cause mortality or nonfatal, centrally adjudicated, new Q-wave myocardial infarction (MI). Patient oriented clinical outcome (POCE) included all-cause mortality or any MI, revascularization or stroke, whereas net adverse clinical events (NACE) comprised POCE, BARC 3 or 5 type bleeding. ST—stent thrombosis, MDRD—modification of diet in renal disease
Discussion
The main findings of this prespecified sub-analysis of the GLOBAL LEADERS trial can be summarized as follows:
-
(1)
The incidence of IRF in this large, contemporary, unselected patient population undergoing PCI, was 13.7% (13% in patients undergoing PCI for ACS and 14.3% in patients undergoing PCI for stable CAD).
-
(2)
Among patients undergoing PCI, any degree of IRF is associated with a higher risk of mortality, ischemic and bleeding events.
-
(3)
In the overall population, there was no differential treatment effect on safety or efficacy with long-term ticagrelor monotherapy after 1-month DAPT among patients with and without IRF. Nevertheless, post hoc exploratory analyses including eGFR as a continuous variable showed a differential treatment effect on BARC 3 or 5 type bleeding, with less BARC 3 or 5 type bleeding in the experimental group.
This study is currently the largest baseline eGFR-stratified analysis of ischemic and bleeding outcomes in PCI patients receiving monotherapy with a potent P2Y12 antagonist, following 1 month DAPT.
Based on the PLATO study, which demonstrated ticagrelor’s superiority over clopidogrel in patients with ACS regardless of baseline renal function, and its increasing advantage in reducing major adverse cardiac events in cohorts with worsening renal dysfunction, it was of interest to evaluate whether similar findings could be replicated with ticagrelor monotherapy after 1 month of DAPT in an unselected patient population undergoing PCI. Indeed, compared to the reference treatment, the experimental treatment group had non-significantly lower rates of the primary endpoint and all-cause mortality, with progressively decreasing point estimates of the HR with decreasing cutoff values of eGFR from 90 to 30 ml/min. However, no significant interaction term was detected between the randomized treatment and IRF for any of the outcomes. The safety profile of ticagrelor in this large contemporary PCI cohort may facilitate better informed clinical decisions on the use of the more potent P2Y12 antagonists in patients with IRF undergoing PCI. Further research may also establish whether the experimental treatment strategy represents a good alternative in selected patients with IRF, such as those in whom standard DAPT is contra-indicated due to expected excess bleeding risk. Of note, IRF is considered as a major or minor bleeding risk criterion based on the degree of renal dysfunction, as described in the recent consensus document from the Academic Research Consortium for High Bleeding Risk [23].
However, neither the analysis stratifying patients according to baseline IRF status nor the KDIGO categories were powered to detect between-group differences or treatment-by-subgroup interactions. Thus, the present analysis should be considered strictly exploratory, and interpreted in the context of the neutral primary analysis of the parent trial [11] and the limitations inherent to subgroup analyses [24].
Reported clinical outcomes, in particular bleeding rates, should also be interpreted in light of the lower adherence to the randomized treatment in the experimental arm, in particular among patients with IRF who had a consistently lower attendance at each follow-up visit. Importantly, however, discontinuation rates in GLOBAL LEADERS were comparable to other trials evaluating ticagrelor [25, 26].
Reassuringly, there was no excess in bleeding risk related to the experimental therapy among patients with moderate IRF (eGFR = 30–59 ml/min/1.73 m2: n = 2055); however, in patients with an eGFR of < 30 ml/min/1.73 m2 (n = 116) BARC 3 or 5 type bleeding occurred in 4 out of 61 patients in the experimental arm and in 1 out of 55 patients in the reference arm; all but one event occurred in patients presenting with stable CAD.
This corresponds with previous pharmacodynamic studies showing that exposure to ticagrelor was approximately 20% lower, and exposure to the active metabolite approximately 17% higher, in patients with severe renal impairment (eGFR < 30 ml/min/1.73 m2) compared to subjects with normal renal function.
Exploratory analyses suggested that the potential net clinical benefit of the experimental strategy in ACS patients with IRF was mainly observed in patients with grade 3 renal impairment (eGFR = 30–59 ml/min/1.73 m2). A plausible explanation is that the selection of chronic kidney disease patients is an effective way to identify high-risk patients with high event rates, and the subgroup with moderate renal impairment has the greatest reduction in ischemic events, without a corresponding increased bleeding risk [2, 7]. This further underscores the prominent role of IRF as a component of ischemia and bleeding prediction scores used for antiplatelet therapy planning [4, 11, 27].
The present results obtained in the ACS population are very consistent with the PLATO data in showing the excess risk in IRF patients [(19.0% IRF vs. 12.8% no IRF, for POCE in GLOBAL LEADERS) vs. (19.7% vs. 8.4% for death/MI/stroke in PLATO)] with incremental risk with more severe renal dysfunction. In the two studies also, there was a greater absolute all-cause mortality risk reduction found among IRF patients, as compared to that of patients with normal renal function [9]. In both studies also, the bleeding excess with ticagrelor appears to be of similar magnitude in IRF and no IRF patients. This leads to a favorable net clinical benefit with ticagrelor in the two sub-studies. The combined effect of IRF and ACS on ischemic outcomes appears to benefit from ticagrelor use, probably related to the inadequate platelet inhibition achieved with earlier generation P2Y12 inhibitors (e.g., clopidogrel) in patients having both conditions [3, 8]. Whether this is ticagrelor-specific is uncertain as close findings have been reported with prasugrel [28] and in a metaanalysis [29]. In addition, recently among patients who presented with ACS with or without ST-segment elevation enrolled in the randomized open-label ISAR REACT 5 trial, the incidence of death, myocardial infarction, or stroke was significantly lower in the group receiving prasugrel, compared with the group treated with ticagrelor, and the incidence of major bleeding (bleeding BARC 3, 4 or 5 type) was not significantly different between the two groups [30]. Nevertheless, among the elderly ≥ 70 years of age being treated for a non-ST-segment elevation ACS the results of POPular AGE trial showed that clopidogrel was associated with less bleeding and similar ischemic events versus more potent P2Y12 inhibitors (ticagrelor or prasugrel) [31].
The poorer long-term prognosis of patients with IRF is possibly explained by more prevalent pre-existing cardiovascular disease, more extensive atherosclerosis, more frequent high-risk presentations of ACS, lower rates of complete revascularization, and underutilization of guideline-recommended therapies [2, 32]. Renal disease can alter thrombocyte function, coagulation and cause endothelial dysfunction [7, 33, 34]. In this context, it is noteworthy that among patients with ACS, ticagrelor monotherapy, after 1-month DAPT, reduced the rates of POCE and NACE among patients with IRF, without an increase in BARC 3 or 5 type bleeding, compared to standard 12-month DAPT after PCI.
In the present analysis, stable CAD patients with IRF had a similar rate of ischemic events, and a non-significantly higher relative risk of BARC 3 or 5 type bleeding in the experimental versus the reference group. In the PEGASUS-TIMI 54 trial, ticagrelor was associated with an increase in Thrombolysis in Myocardial Infarction (TIMI) major bleeding in stable outpatients with prior MI12. Nevertheless, as GLOBAL LEADERS also enrolled patients with acute MI, and a generally lower cardiovascular risk, as demonstrated by the overall all-cause mortality, the presented findings cannot be directly compared to prior studies evaluating ticagrelor in relation to baseline renal function. A patient cohort with a risk profile which is higher than GLOBAL LEADERS, and comparable to PEGASUS-TIMI 54, has been recently evaluated in the TWILIGHT trial, in which presence of chronic kidney disease was one of the enrichment factors according to the trial protocol [35]. In TWILIGHT, comparing ticagrelor monotherapy following 3-month event-free period of DAPT after PCI with DAPT strategy, a significant reduction of the composite primary endpoint of bleeding BARC 2, 3 or 5 type (HR 0.56, 95% CI 0.45–0.68, pfor superiority < 0.001) has been demonstrated in the experimental group versus the reference group 15 months after PCI (12 months after randomization) [36]. The trial also showed non-inferiority of the experimental treatment with regard to the composite secondary endpoint of all-cause death, non-fatal MI, or stroke (HR 0.99, 95% CI 0.78–1.25, pfor non-inferiority < 0.001), with the caveat of a higher than anticipated drop-out in the first 3 months after the index procedure, leading to a lower rate of this endpoint and potential bias of the results towards null hypothesis [36, 37].
Limitations
This study has several limitations. Given that two antiplatelet strategies did not differ significantly with respect to the rates of the primary endpoint in the overall trial [11], all presented findings have to be considered exploratory and hypothesis-generating. The randomization in GLOBAL LEADERS study was not stratified for renal function; thus some imbalance between the randomized groups may exist among patients with IRF. Importantly, this was a prespecified subgroup analysis based on prespecified cut-off points of renal function at admission. Creatinine data were not available in 85 patients (0.5%); however, this rate of missing creatinine data is significantly lower, compared to prior trials on antiplatelet agents in context of renal dysfunction [9].
GLOBAL LEADERS was an open label trial; however, to minimize bias, the primary endpoint included solid components of all-cause mortality—not requiring adjudication, and a core lab adjudicated new-Q wave MI. No central adjudication of investigator-reported secondary clinical outcomes was performed. Bias and misclassification can, therefore, not be excluded. This limitation should be considered in particular when interpreting bleeding event rates. However, the trial was monitored for event definition consistency and event under-reporting, with as many as seven on-site monitoring visits done at individual sites and one-fifth of events verified based on the source documentation [11, 38]. Use of site-reported endpoints is a valid methodology in clinical research, especially involving large cohorts and well-defined and restricted categories within a classification (e.g. BARC-defined bleeding type 3–5 as compared with type 1 and 2) are expected to provide higher concordance among sites than a central clinical event adjudication committee, as well as higher reproducibility.
Conclusions
IRF is associated with worse short- and long-term clinical outcomes after PCI. There were no differential treatment effects found with regard to all-cause death or new Q-wave MI after PCI in patients with IRF treated with ticagrelor monotherapy after 1-month dual therapy with aspirin. In ACS patients with IRF, the experimental strategy may be associated with less ischemic events and similar bleeding rates, compared to standard DAPT after PCI.
References
Dumaine RL, Montalescot G, Steg PG, Ohman EM, Eagle K, Bhatt DL, Investigators RR (2009) Renal function, atherothrombosis extent, and outcomes in high-risk patients. Am Heart J 158(1):141–148.e141. https://doi.org/10.1016/j.ahj.2009.05.011
Capodanno D, Angiolillo DJ (2012) Antithrombotic therapy in patients with chronic kidney disease. Circulation 125(21):2649–2661. https://doi.org/10.1161/CIRCULATIONAHA.111.084996
Bonello L, Angiolillo DJ, Aradi D, Sibbing D (2018) P2Y12-ADP receptor blockade in chronic kidney disease patients with acute coronary syndromes. Circulation 138(15):1582–1596. https://doi.org/10.1161/CIRCULATIONAHA.118.032078
Lee SY, Hong MK, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Kim HS, Valgimigli M, Palmerini T, Stone GW (2017) Clinical outcomes of dual antiplatelet therapy after implantation of drug-eluting stents in patients with different cardiovascular risk factors. Clin Res Cardiol 106(3):165–173. https://doi.org/10.1007/s00392-016-1035-4
Weidner K, Behnes M, Schupp T, Rusnak J, Reiser L, Taton G, Reichelt T, Ellguth D, Engelke N, Bollow A, El-Battrawy I, Ansari U, Hoppner J, Nienaber CA, Mashayekhi K, Weiss C, Akin M, Borggrefe M, Akin I (2019) Prognostic impact of chronic kidney disease and renal replacement therapy in ventricular tachyarrhythmias and aborted cardiac arrest. Clin Res Cardiol 108(6):669–682. https://doi.org/10.1007/s00392-018-1396-y
Marx N, Noels H, Jankowski J, Floege J, Fliser D, Bohm M (2018) Mechanisms of cardiovascular complications in chronic kidney disease: research focus of the transregional research consortium SFB TRR219 of the University Hospital Aachen (RWTH) and the Saarland University. Clin Res Cardiol 107(Suppl 2):120–126. https://doi.org/10.1007/s00392-018-1260-0
Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K (2014) Haemostasis in chronic kidney disease. Nephrol Dial Transplant 29(1):29–40. https://doi.org/10.1093/ndt/gft209
Franchi F, Rollini F, Angiolillo DJ (2015) Defining the link between chronic kidney disease, high platelet reactivity, and clinical outcomes in clopidogrel-treated patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 8(6):e002760. https://doi.org/10.1161/CIRCINTERVENTIONS.115.002760
James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, Harrington RA, Horrow J, Katus H, Keltai M, Lewis BS, Parikh K, Storey RF, Szummer K, Wojdyla D, Wallentin L (2010) Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the platelet inhibition and patient outcomes (PLATO) trial. Circulation 122(11):1056–1067. https://doi.org/10.1161/CIRCULATIONAHA.109.933796
Capodanno D, Mehran R, Valgimigli M, Baber U, Windecker S, Vranckx P, Dangas G, Rollini F, Kimura T, Collet JP, Gibson CM, Steg PG, Lopes RD, Gwon HC, Storey RF, Franchi F, Bhatt DL, Serruys PW, Angiolillo DJ (2018) Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol 15(8):480–496. https://doi.org/10.1038/s41569-018-0049-1
Vranckx P, Valgimigli M, Juni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, Chichareon P, Benit E, Mollmann H, Janssens L, Ferrario M, Moschovitis A, Zurakowski A, Dominici M, Van Geuns RJ, Huber K, Slagboom T, Serruys PW, Windecker S, Investigators GL (2018) Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. https://doi.org/10.1016/S0140-6736(18)31858-0
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158(11):825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Vranckx P, Valgimigli M, Windecker S, Steg PG, Hamm C, Juni P, Garcia-Garcia HM, van Es GA, Serruys PW (2016) Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation: rationale and design of the GLOBAL LEADERS trial. EuroIntervention 12(10):1239–1245. https://doi.org/10.4244/EIJY15M11_07
Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice M-C, di Mario C, Davies S, van Geuns R-J, Eerdmans P, van Es G-A, Meier B, Jüni P (2008) Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. The Lancet 372(9644):1163–1173. https://doi.org/10.1016/s0140-6736(08)61244-1
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354(23):2473–2483. https://doi.org/10.1056/NEJMra054415
Prineas R, Crow RS, Zhang Z (2010) The Minnesota code manual of electrocardiographic findings. Springer, London
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation 123(23):2736–2747. https://doi.org/10.1161/CIRCULATIONAHA.110.009449
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW, Academic Research C (2007) Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17):2344–2351. https://doi.org/10.1161/CIRCULATIONAHA.106.685313
Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW, Academic Research C (2018) Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Eur Heart J 39(23):2192–2207. https://doi.org/10.1093/eurheartj/ehy223
Tomaniak M, Chichareon P, Onuma Y, Deliargyris EN, Takahashi K, Kogame N, Modolo R, Chang CC, Rademaker-Havinga T, Storey RF, Dangas GD, Bhatt DL, Angiolillo DJ, Hamm C, Valgimigli M, Windecker S, Steg PG, Vranckx P, Serruys PW, Investigators tGLT (2019) Benefit and risks of aspirin on top of ticagrelor in acute coronary syndromes: a post hoc analysis of the randomized GLOBAL LEADERS trial. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2019.2255
Valgimigli M, Frigoli E, Leonardi S, Rothenbuhler M, Gagnor A, Calabro P, Garducci S, Rubartelli P, Briguori C, Ando G, Repetto A, Limbruno U, Garbo R, Sganzerla P, Russo F, Lupi A, Cortese B, Ausiello A, Ierna S, Esposito G, Presbitero P, Santarelli A, Sardella G, Varbella F, Tresoldi S, de Cesare N, Rigattieri S, Zingarelli A, Tosi P, t’ van Hof A, Boccuzzi G, Omerovic E, Sabate M, Heg D, Juni P, Vranckx P, Investigators M (2015) Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med 373(11):997–1009. https://doi.org/10.1056/NEJMoa1507854
Serruys PW, Takahashi K, Chichareon P, Kogame N, Tomaniak M, Modolo R, Chang CC, Komiyama H, Soliman O, Wykrzykowska JJ, de Winter RJ, Ferrario M, Dominici M, Buszman P, Bolognese L, Tumscitz C, Benit E, Stoll HP, Hamm C, Steg PG, Onuma Y, Juni P, Windecker S, Vranckx P, Colombo A, Valgimigli M (2019) Impact of long-term ticagrelor monotherapy following 1-month dual antiplatelet therapy in patients who underwent complex percutaneous coronary intervention: insights from the global leaders trial. Eur Heart J 40(31):2595–2604. https://doi.org/10.1093/eurheartj/ehz453
Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC (2019) Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the academic research consortium for high bleeding risk. Eur Heart J 40(31):2632–2653. https://doi.org/10.1093/eurheartj/ehz372
Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM (2007) Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med 357(21):2189–2194. https://doi.org/10.1056/NEJMsr077003
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361(11):1045–1057. https://doi.org/10.1056/NEJMoa0904327
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS, Committee P-TS, Investigators (2015) Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 372(19):1791–1800. https://doi.org/10.1056/NEJMoa1500857
Wolff G, Lin Y, Quade J, Bader S, Kosejian L, Brockmeyer M, Karathanos A, Parco C, Krieger T, Heinen Y, Perings S, Albert A, Icks A, Kelm M, Schulze V (2019) Validation of national cardiovascular data registry risk models for mortality, bleeding and acute kidney injury in interventional cardiology at a German heart center. Clin Res Cardiol. https://doi.org/10.1007/s00392-019-01506-x
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM, Investigators T-T (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357(20):2001–2015. https://doi.org/10.1056/NEJMoa0706482
Bonello L, Laine M, Lemesle G, Puymirat E, Dabry T, Thuny F, Paganelli F, Aradi D, Frere C, Burtey S, Sibbing D, Mancini J (2018) Meta-analysis of potent P2Y12-ADP receptor antagonist therapy compared to clopidogrel therapy in acute coronary syndrome patients with chronic kidney disease. Thromb Haemost 118(10):1839–1846. https://doi.org/10.1055/s-0038-1669426
Schupke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wohrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, Akin I, Bott-Flugel L, Fischer M, Landmesser U, Katus HA, Sibbing D, Seyfarth M, Janisch M, Boncompagni D, Hilz R, Rottbauer W, Okrojek R, Mollmann H, Hochholzer W, Migliorini A, Cassese S, Mollo P, Xhepa E, Kufner S, Strehle A, Leggewie S, Allali A, Ndrepepa G, Schuhlen H, Angiolillo DJ, Hamm CW, Hapfelmeier A, Tolg R, Trenk D, Schunkert H, Laugwitz KL, Kastrati A, Investigators I-RT (2019) Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. https://doi.org/10.1056/NEJMoa1908973
Qaderdan K, Ishak M, Heestermans AA, de Vrey E, Jukema JW, Voskuil M, de Boer MJ, van’t Hof AW, Groenemeijer BE, Vos GJ, Janssen PW, Bergmeijer TO, Kelder JC, Deneer VH, ten Berg JM (2015) Ticagrelor or prasugrel versus clopidogrel in elderly patients with an acute coronary syndrome: optimization of antiplatelet treatment in patients 70 years and older—rationale and design of the POPular AGE study. Am Heart J 170(5):981–985.e981. https://doi.org/10.1016/j.ahj.2015.07.030
Montalescot G, Silvain J (2010) Ticagrelor in the renal dysfunction subgroup: subjugated or substantiated? Circulation 122(11):1049–1052. https://doi.org/10.1161/CIRCULATIONAHA.110.974683
Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabate M, Ferreiro JL, Ueno M, Jimenez-Quevedo P, Alfonso F, Bass TA, Macaya C, Fernandez-Ortiz A (2010) Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol 55(11):1139–1146. https://doi.org/10.1016/j.jacc.2009.10.043
Baber U, Mehran R, Kirtane AJ, Gurbel PA, Christodoulidis G, Maehara A, Witzenbichler B, Weisz G, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Xu K, Parise H, Brodie BR, Stuckey TD, Stone GW (2015) Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the assessment of dual antiplatelet therapy with drug-eluting stents registry. Circ Cardiovasc Interv 8(6):e001683. https://doi.org/10.1161/CIRCINTERVENTIONS.115.001683
Baber U, Dangas G, Cohen DJ, Gibson CM, Mehta SR, Angiolillo DJ, Pocock SJ, Krucoff MW, Kastrati A, Ohman EM, Steg PG, Badimon J, Zafar MU, Chandrasekhar J, Sartori S, Aquino M, Mehran R (2016) Ticagrelor with aspirin or alone in high-risk patients after coronary intervention: rationale and design of the TWILIGHT study. Am Heart J 182:125–134. https://doi.org/10.1016/j.ahj.2016.09.006
Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Dzavik V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM (2019) Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. https://doi.org/10.1056/NEJMoa1908419
Tomaniak M, Storey RF, Serruys PW (2019) Aspirin-free antiplatelet regimens after PCI: when is it best to stop aspirin and who could ultimately benefit? EuroInterv: J EuroPCR Collab Work Group Interv Cardiol Eur Soc Cardiol. https://doi.org/10.4244/EIJY19M10_01
Serruys PW, Tomaniak M, Chichareon P, Modolo R, Kogame N, Takahashi K, Chang CC, Spitzer E, Walsh SJ, Adlam D, Hildick-Smith D, Edes I, van de Harst P, Krackhardt F, Tijssen JGP, Rademaker-Havinga T, Garg S, Steg PG, Hamm C, Juni P, Vranckx P, Onuma Y, Verheugt FWA (2019) Patient-oriented composite endpoints and net adverse clinical events with ticagrelor monotherapy following percutaneous coronary intervention: insights from the randomized GLOBAL LEADERS trial. EuroInter: J EuroPCR Collab Work Group Interv Cardiol Eur Soc Cardiol. https://doi.org/10.4244/EIJ-D-19-00202
Funding
This work was supported by the European Clinical Research Institute, which received unrestricted Grants from Biosensors International, AstraZeneca, and the Medicines Company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Tomaniak reports lecture fee from Astra Zeneca, outside the submitted work. Dr. Chichareon reports Grants from biosensons, outside the submitted work. Dr. Modolo reports Grants from Biosensors, outside the submitted work. Dr. Curzen reports Grants and personal fees from Boston Scientific, Grants and personal fees from Heartflow, Grants and personal fees from Haemonetics, outside the submitted work. Dr. Haude reports institutional Grant/research support from Biotronik AG, Abbott Vascular, Cardiac Dimensions, Volcano, Lilly and consultant/speaker´s bureau: Biotronik AG, Abbott Vascular, Cardiac Dimensions. Dr. Montalescot has received research Grants to the institution or consulting/lecture fees from Abbott, Amgen, Actelion, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, Cardiovascular Research Foundation, Daiichi-Sankyo, Idorsia, Lilly, Europa, Elsevier, Fédération Française de Cardiologie, ICAN, Medtronic, Journal of the American College of Cardiology, Lead-Up, Menarini, Merck Sharp & Dohme, Novo Nordisk, Pfizer, Sanofi, Servier, The Mount Sinai School, TIMI Study Group, and WebMD. Dr. Angiolillo reports Grants and personal fees from Amgen, Grants and personal fees from Aralez, Grants and personal fees from Bayer, Grants and personal fees from Biosensors, Grants and personal fees from Boehringer Ingelheim, Grants and personal fees from Bristol-Myers Squibb, Grants and personal fees from Chiesi, Grants and personal fees from Daiichi-Sankyo, Grants and personal fees from Eli Lilly, personal fees from Haemonetics, Grants and personal fees from Janssen, Grants and personal fees from Merck, personal fees from PhaseBio, personal fees from PLx Pharma, personal fees from Pfizer, Grants and personal fees from Sanofi, personal fees from The Medicines company, Grants and personal fees from CeloNova, personal fees from St Jude Medical, Grants from CSL Behring, Grants from Eisai, Grants from Gilead, Grants from Idorsia Pharmaceuticals Ltd, Grants from Matsutani Chemical Industry Co., Grants from Novartis, Grants from Osprey Medical, Grants from Renal Guard Solutions, Grants from Scott R. MacKenzie Foundation, Grants from NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01 HG007269, Grants and personal fees from Astra Zeneca, outside the submitted work. Dr. Capodanno reports personal fees from Bayer, personal fees from AstraZeneca, personal fees from Sanofi Aventis, personal fees from Baehringer, personal fees from Daiichi Sankyo, outside the submitted work. Dr. Storey reports personal fees from Bayer, personal fees from Bristol-Myers Squibb/Pfizer, Grants and personal fees from AstraZeneca, personal fees from Novartis, personal fees from Idorsia, Grants and personal fees from Thromboserin, personal fees from Haemonetics, personal fees from Amgen, Grants and personal fees from Glycardial Diagnostics, outside the submitted work. Dr. Hamm reports personal fees from AstraZeneca, outside the submitted work. Dr. Vranckx reports personal fees from AstraZeneca and the Medicines Company during the conduct of the study and personal fees from Bayer Health Care, Terumo, and Daiichi-Sankyo outside the submitted work. Dr. Valgimigli reports Grants and personal fees from Abbott, personal fees from Chiesi, personal fees from Bayer, personal fees from Daiichi Sankyo, personal fees from Amgen, Grants and personal fees from Terumo, personal fees from Alvimedica, Grants from Medicure, Grants and personal fees from AstraZeneca, personal fees from Biosensors, outside the submitted work. Dr. Windecker’s institution has research contracts with Abbott, Amgen, Bayer, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, St Jude Medical, Symetis SA, and Terumo outside the submitted work. Dr. Onuma has received consultancy fees from Abbott Vascular outside the submitted work. Dr. Serruys has received personal fees from Abbot Laboratories, AstraZeneca, Biotronik, Cardialysis, GLG Research, Medtronic, Sino Medical Sciences Technology, Société Europa Digital Publishing, Stentys France, Svelte Medical Systems, Philips/Volcano, St Jude Medical, Qualimed, and Xeltis, outside the submitted work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tomaniak, M., Chichareon, P., Klimczak-Tomaniak, D. et al. Impact of renal function on clinical outcomes after PCI in ACS and stable CAD patients treated with ticagrelor: a prespecified analysis of the GLOBAL LEADERS randomized clinical trial. Clin Res Cardiol 109, 930–943 (2020). https://doi.org/10.1007/s00392-019-01586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-019-01586-9